Abstract
Urinary tract infections (UTIs) inflict extreme pain and discomfort to those affected and have profound medical and socioeconomic impact. Although acute UTIs are often treatable with antibiotics, a large proportion of patients suffer from multiple recurrent infections. Here, we describe and provide a protocol for a robust murine UTI model that allows for the study of uropathogens in an ideal setting. The infections in the urinary tract can be monitored quantitatively by determining the bacterial loads at different times post-infection. In addition, the simple bladder architecture allows observation of disease progression and the uropathogenic virulence cascade using a variety of microscopic techniques. This mouse UTI model is extremely flexible, allowing the study of different bacterial strains and species of uropathogens in a broad range of mouse genetic backgrounds. We have used this protocol to identify important aspects of the host-pathogen interaction that determine the outcome of infection. The time required to complete the entire procedure will depend on the number of bacterial strains and mice included in the study. Nevertheless, one should expect 4 h of hands-on time, including inoculum preparation on the day of infection, transurethral inoculation, tissue harvest and post-harvest processing for a small group of mice (e.g., 5 mice).
INTRODUCTION
Urinary tract infections (UTIs) affect primarily women of all ages, although young boys and elderly men are also at relatively high risk. These infections can result in cystitis (bladder infections) or pyelonephritis (kidney infections). Besides the tremendous economic ramifications, the effects of various UTI sequelae, ranging from renal scarring in children, premature delivery in pregnant women and sepsis in the elderly, are of considerable socioeconomic consequence. Uropathogenic Escherichia coli (UPEC) is the primary etiological pathogen, accounting for over 80% of UTIs1,2. These infections are often recurrent, with nearly one-quarter of the patients experiencing multiple infections within 6 months of the initial episode. Although seldom fatal, UTIs are a debilitating disease for a large portion of the female population and a major health risk for individuals with other underlying illnesses, such as diabetes.
Structure of a healthy bladder
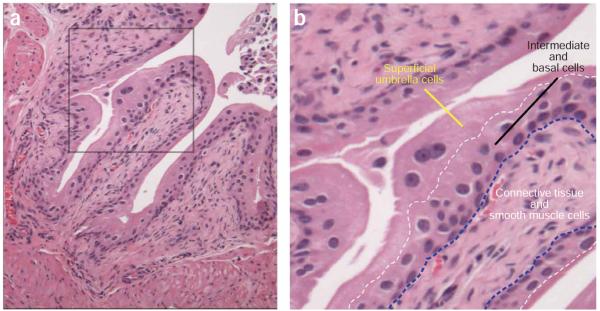
The bladder epithelium, the principal site of UPEC colonization, is a stratified transitional epithelium (urothelium) of 3–4 cell layers deep (Fig. 1). A thin basement membrane and lamina propria separate the epithelial cells from the smooth muscle and serous layers in the outer wall of the bladder. The urothelium is comprised of small, relatively undifferentiated basal and intermediate epithelial cells underlying a single layer of highly differentiated, large and often-binucleate superficial facet cells (Fig. 1a–b). These cells are coated on the apical side with semi-crystalline arrays of four integral membrane proteins known as uroplakins (UPIa, UPIb, UPII and UPIII). The primary sequence and biochemical properties of the uroplakins as well as the ultrastructures of the asymmetric unit membrane they form are highly conserved among many mammalian species, including human and mouse3. The uroplakin plaques provide an impermeable layer, preventing reabsorption of metabolic waste in the urine, which is collected in the bladder before being expelled. These plaques also provide the rigidity and strength required for the bladder to expand and contract without affecting its structural integrity.
Figure 1.
Mouse urothelium. Hematoxylin and eosin staining of 7-week-old female C3H/HeN mouse bladder sections allows easy visualization of the tissue architecture. (a) A relaxed mouse bladder displays multiple folds of tissues into the lumen. (b) Digital enlargement of the histological image in a (boxed region) reveals the three cellular layers comprising the urothelium-superficial umbrella cells, intermediate and basal cells and connective tissue and smooth muscle cells. All animal studies were conducted in accordance to the Guide for the Care and Use of Laboratory Animals43 under assurance number A3381-01. The Washington University School of Medicine Animal Study Committee approved all experimental procedures described here.
Biology of UTI
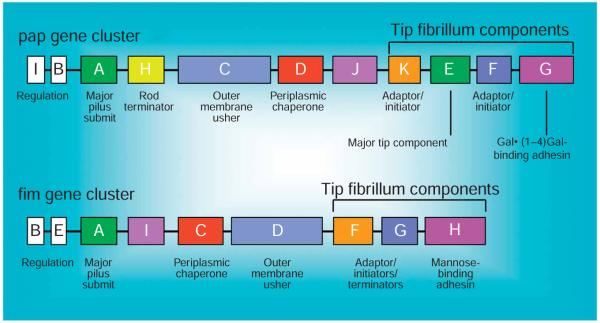
Adherence of pathogens to host cells has a critical role for efficient colonization and subsequent disease progression in most, if not all, mucosal infections. UPEC uses extracellular organelles, termed type 1 pili, for achieving an intimate contact with the urothelium4-7. Expression of type 1 pili in UPEC is correlated with enhanced virulence in human populations4,8. Further, a vaccine targeting the adhesin of type 1 pili, FimH, has been shown to be effective in preventing UTIs in animal models5,9. Type 1 pili, encoded by the fim operon, belong to a family of bacterial adhesive organelles termed chaperone–usher pili, of which type 1 and P pili have been extensively characterized (Fig. 2) (for detailed reviews, see Nuccio et al.10 and Thanassi et al.11). Type 1 pili are composite fibers consisting of short linear fibrillae joined to a long rigid helical rod. The assembly of type 1 pili requires the periplasmic chaperone, FimC, and the outer membrane usher, FimD. The adhesin, FimH, is located at the very distal tip of type 1 pilus and exhibits strong affinity for mannose residues12,13. Interaction of type 1 pili with cellular membrane receptors leads to a cascade of signaling events in mammalian cells resulting in the entry of UPEC by a membrane-zippering mechanism14,15. In vitro studies showed that UPIa derived from bladders of diverse mammalian species could serve as the principle receptor for E. coli type 1 pili16.
Figure 2.
Schematic of the pap and fim operons. The genetic organizations of two prototypic chaperone–usher mediated pili, P (encoded by pap operon) and type 1 (encoded by fim operon), are depicted. The gene names and their corresponding functions are denoted.
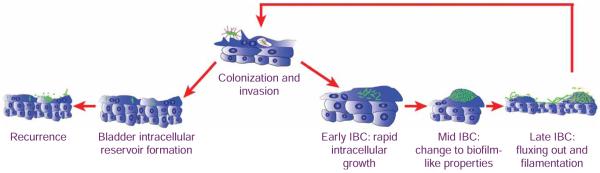
Fluorescence video microscopy was used to uncover a multi-step UPEC pathogenic cycle (Fig. 3) that occurs when UPEC enters the bladder17. This pathogenic cycle includes a series of developmental program changes in UPEC. First, UPEC binds to (colonization) and enters the bladder epithelial cells (invasion) in a type 1 pilus-dependent manner. Bacteria break out of the phagocytic vacuoles within 4 h after invasion and replicate rapidly (doubling time of 30 min) as an amorphous intracellular bacterial community (IBC) (early-IBC). Rapid intracellular replication provides UPEC an added advantage to evade the host immune response, as well as to multiply in numbers. A developmental program change occurs where intracellular bacteria continue to divide without increasing in length, resulting in coccoid-shaped bacteria. In addition, the bacterial colonies become very organized and tightly compact and show biofilm properties (mid-IBC). Bacteria within the mid-IBC continue to grow with a coccoid-shape, although with slower growth kinetics, eventually filling up a large portion of the epithelial cell cytoplasm. The IBCs grow so large that they extend the membrane of infected bladder’s superficial facet cells into the lumen of the bladder, giving the appearance of a pod structure as visualized by scanning-electron microscopy (SEM) (Fig. 4). At late stages, bacteria on the edge of the IBC convert back to a rod-shaped morphology, become motile and detach from the biofilm (late-IBC). This may be analogous to the detachment stage of bacterial biofilms on abiotic surfaces. The motile bacteria eventually exit the epithelial cell (fluxing). During this process a portion of the bacteria forms filaments (filamentation), likely in response to host factors. Filamentous bacteria survive neutrophil attacks better than rod-shaped ones, and thus this appears to be a critical step in pathogenesis18. Filamentous bacteria give rise to rod-shaped daughter cells, which invade ‘naïve’ superficial facet cells and re-initiate the cycle. At such points during the pathogenic cycle, some intracellular bacteria become dormant forming quiescent reservoirs (reservoir), which can serve as seeds for recurrent infection (recurrence).
Figure 3.
Uropathogenic Escherichia coli (UPEC) pathogenic cycle. The proposed model of the UPEC uropathogenic cycle is depicted in this schematic. See text for detail.
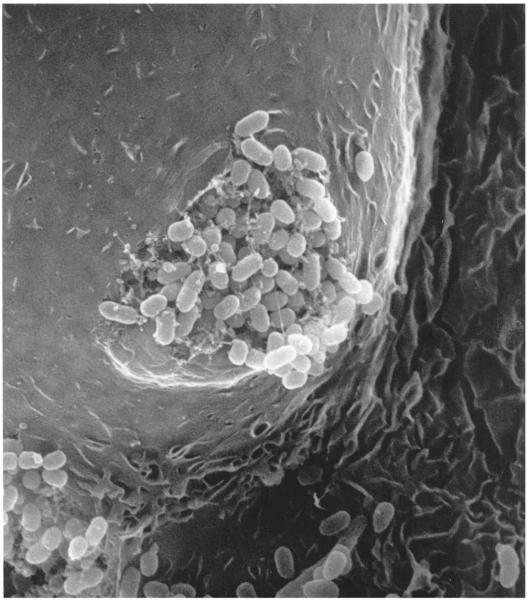
Figure 4.
The intracellular bacterial community (IBC). Scanning electron micrograph of an infected bladder superficial facet cell shows a protrusion of the cell surface into the lumen of the bladder. Bacteria fluxing out of the infected cell are visible. A 7-week-old female C3H/HeN mouse was infected with uropathogenic Escherichia coli (UPEC) strain UTI89 and its bladder harvested at 24 h after infection. All animal studies were conducted in accordance to the Guide for the Care and Use of Laboratory Animals43 under assurance number A3381-01. The Washington University School of Medicine Animal Study Committee approved all experimental procedures described here.
Murine models of UTI
There have been many published murine models of UTIs19-27. All these models use different permutations of intraurethral or transurethral inoculation, with, e.g., variations in the compositions of urinary catheters and inoculum sizes, to introduce bacteria into the mouse bladders. Using 108 colony forming units (CFUs) inoculum size, Hopkins et al.27 examined the efficiency of infection and induction of vesicoureteral reflux (VUR) with different inoculum volumes and routes of inoculation (intraurethral versus transurethral). The study showed that bacteria were efficiently introduced into the bladders with any of the inoculation volume–route combinations tested. With the exception of intraurethral inoculation of 10 μl bacteria, all other methods led to detectable kidney titers soon after infection, a result confirmed by Schaeffer et al.20. However, kidney infections occurred sporadically at low levels in this study group and resolved after 7 d post infection. The authors attributed the rapid presence of bacteria in the kidneys to VUR and recommended low volume-intraurethral inoculation as the preferred method to study ascending UTIs. It is interesting to note that ~20% of mice developed kidney infections in groups inoculated transurethrally into the bladders with either 10 or 50 μl bacterial suspensions. Others have later reported the absence or reduction of VUR rate with transurethral inoculation of 50 μl volume23. Many studies subsequently adopted transurethral delivery of 50 μl bacteria at 108 CFU per mouse19,21,23,24,28; some used as high as 109 CFU per mouse25 as the standard protocol. In most reported models, catheters are made with polyethylene plastic tubing of different sizes. The tubing is fitted over needles of appropriate sizes and bacteria in attached syringes are introduced into the animals through these catheters. Studies reported by Lane et al.25 and Gunther et al.22 presented a variation of the inoculation method. They inserted the flexible polyethylene tubing into the bladder of a mouse, attached the tubing to an infusion pump and slowly introduced the 50 μl inoculum over a period of 30 s. Although this method eliminates VUR, catheterizing mouse bladders with flexible tubing could prove to be technically challenging and time consuming.
Overview of the murine model of UTI presented here
To better understand the pathogenesis of UTI, our laboratory has optimized a murine model and established microscopic techniques to study host–pathogen interactions and disease progression6,17,29-32. UPEC grown under type 1 pilus-inducing conditions are introduced into the mouse bladder by transurethral catheterization. The urinary catheters are made out of polyethylene tubing covering 30-G hypodermic needles. The rigid inner metal support provides an easy way of inserting the catheter into the bladder, compared with using the polyethylene tubing alone22,25. Catheters are fitted onto sterile disposable syringes containing the inoculum. Disposable syringes ensure sterility of inoculum and avoid cross-contaminations when multiple bacterial strains are used. As the length of the female mouse urethra is estimated to be about 1 cm (ref. 27), full insertion of the catheter (~1.3 cm) deposits the inoculum directly into the bladder. The graduations on the disposable syringes allow 50 μl of bacterial suspension to be dispensed accurately. There have been concerns regarding VUR due to the pressure of injecting 50-μl liquid into the bladder20,27,33,34. Using bromophenol blue dye, we have found that slow instillation of 50 μl volume (at ~10 μl s−1) did not introduce the dye into the ureter and kidneys as a result of VUR (C.-S.H., unpublished observations). However, we could not rule out the possibility that a very small amount of inoculum, undetectable by the naked eye, reaches the upper urinary tract because of VUR. Once inside the bladder, UPEC can colonize the bladder epithelium as well as ascend through the ureter to colonize the kidneys. We have optimized the inoculum size to be 1–3 × 107 CFU per mouse using type 1 piliated UPEC. At this infectious dose, we are able to obtain consistent infections compared with lower inocula35. Furthermore, infection at higher dosage (i.e., 108 CFU per mouse) often results in an overt and rapid exfoliation response of the bladder epithelium, especially in the C57BL/6 mouse strain, which is not seen with an inoculum size of 107 CFU (refs. 6,36).
Bacterial load in the bladder as well as in the upper urinary tract organ—kidneys—can be determined quantitatively by plating tissue homogenate on suitable agar plates at defined time points post infection. However, enumeration of bacterial titers in infected organs provides only a limited view of the infection. In the optimization of the murine UTI model, we also strive to establish and perfect associated techniques to gain a more complete picture of UPEC pathogenesis. As shown by various microscopic methods17,29,30, bacteria in the bladder may reside in the bladder lumen, in IBC or as small collections in intracellular vesicles during different stages of infection. Therefore, it is important that visual examination be used to complement microbiological methods to assess the pathogenic process and the effects of mutations in the pathogen or host on the infection. This is evident in the case where temporal restriction of type 1 pili expression in a UPEC mutant reduced the bladder titers and, more importantly, dramatically affected both the numbers and the architecture of IBCs31. Because of the relatively simple architecture of the bladders, infected bladders can be visualized by SEM6,29,37, light microscopy18,35,37, immunofluorescence microscopy29,30,37,38 and confocal microscopy17,18,29,31 to assess the status of both the host and bacteria at defined time points post infection.
Implication and importance of the murine UTI model
The murine UTI model has proven to be a very powerful tool for advancing our understanding of UPEC pathogenesis in humans. The IBCs and phenotypes of filamentous bacteria observed in the mouse model have also been detected in urine sediments from UTI patients39. Such correlation strengthens the validity of the depicted UPEC pathogenic cascade and the relevance of this mouse model. Using the model and techniques described here, our laboratory continues to investigate the molecular details of both host and pathogen mechanisms involved throughout the different steps of the pathogenic cascade. This murine UTI model has also been established in C57BL/6, CBA/J, FVB/NJ and C3H/HeJ mouse genetic backgrounds, in addition to the C3H/HeN mouse strain described here36. UPEC IBC formation was observed in all the tested strains. However, it is important to note that there are mouse strain-specific differences in the degrees and kinetics of host response as well as the UPEC pathogenic cascade. For example, C57BL/6 mice mount a more robust inflammatory response and bladder exfoliation than other strains. Also, UPEC filamentation is not observed in C3H/HeJ mice until much later in the course of infection, likely due to the lack of host inflammatory response in the C3H/HeJ background18. In addition, mouse UTIs caused by other uropathogens, such as Enterococcus faecalis and Klebsiella pneumoniae, have been studied using the same methodologies40-42. Combining the powerful bacterial genetics and transgenic mouse technologies along with a myriad of experimental assessment techniques, the murine UTI model will continue to provide useful tools, thereby furthering our understanding of this debilitating disease and the search for future therapeutics.
Experimental design
Mouse infection controls
It is advisable to include mock-infection controls when a new researcher is learning and perfecting the infection technique. Inoculate a few mice with 50 μl sterile phosphate buffered saline (PBS) in parallel and harvest the tissues along with experimental groups. There should not be any bacteria present in the tissues of the mock-infected controls at any time point assayed. Mock-infection controls are generally not necessary once the technique has been perfected.
UPEC mutants study controls
Whenever bacterial mutants are examined in the study, it is vital to include the appropriate wild-type strains as positive controls for each time point in the experiments. These wild-type controls should be assayed in parallel to the experimental groups.
Anesthesia optimization
Anesthetics are critical to sedate mice and reduce discomfort during inoculation. Mice of different genetic backgrounds might have different susceptibilities to anesthesia induced hypoxia; therefore, the Isoflurane concentration should be determined empirically. We have optimized the Isoflurane concentration for C3H/HeN mice to 4% for the induction of anesthesia and 2.5%–3% for maintenance of the anesthetic state at an oxygen flow-rate of 0.5 l min−1. C57BL/6 mice, however, should not be maintained at Isoflurane concentrations higher than 2%. In general, the anesthetic state can be induced at high Isoflurane concentration (i.e., 4%), but the anesthetic level should be lowered once mice have been sedated. Optimization of maintenance concentration of Isoflurane for other mouse strains should start at 2% and be raised at small increments (0.5%, or the smallest increment possible according to the limitations of the instrument).
Optimization of inoculum size and volume
The experimental procedures described here have been optimized to use 1–2 × 107 CFU per mouse in 50 μl volume for inoculation. The 1-ml tuberculin syringes have marked graduations of 10 μl. However, due to the accuracy of graduation on the syringes, 50 μl (5 graduations) provides a reliable and accurate volume of the inocula being delivered.
Mouse strains
Successful infections of inbred mice from different genetic backgrounds, i.e., C3H/HeN, C3H/HeJ, C57BL/6, CBA/J and FVB/NJ, have been reported using our optimized method36. In addition, we have been able to successfully induce UTI in other mouse strains, such as BALB/c, C3H/HeOuJ, C3H/HeSnJ and Swiss Webster (outbred strain) (C.-S.H. and Thomas J. Hannan, unpublished observations). A study by Hopkins et al.19 suggested that mice of most genetic backgrounds could be successfully infected; however, the levels of bacterial load in the urinary tissues vary among these strains.
Mouse age and size
The mice used in our studies are typically 7–9 weeks old at the time of infection. Mice at this age range are considered to be adults. Although we have used older mice (10–14 weeks) in a limited manner earlier in our research, we were not able to obtain as consistent and high levels of infection with older C3H/HeN mice compared with younger ones (C.-S.H., unpublished observations). Nevertheless, 10–15-week-old C3H/HeJ mice could still be infected efficiently. However, we have limited our infection studies to mice no older than 10 weeks old, ideally younger than 9 weeks old. Mice at this age range showed little variations in their body weight (within 5 g). At this age range, we have not noticed any effects of mouse age and size in the susceptibility and levels of infection.
MATERIALS
REAGENTS
7–9-week-old female C3H/HeN mice (Harlan Sprague Dawley, cat. no. C3H/HeNHsd; http://www.harlan.com/research_models_and_services) ▲ CRITICAL 6–7-week-old mice are requested from vendor. Mice are allowed to recover from the stress of transport in our institutional animal facility for at least 5–7 d before experiments are carried out. We limit our infections to mice younger than 9 weeks old for consistent results (see Experimental design). ▲ CRITICAL All animal studies were conducted in accordance to the Guide for the Care and Use of Laboratory Animals43 under assurance number A3381-01. The Washington University School of Medicine Animal Study Committee approved all the experimental procedures described here. Mice are housed in micro-isolator cages in a BSL2 barrier animal facility and cared for by the facility personnel. Mice are fed autoclaved standard laboratory rodent diet (Purina) and autoclaved tap water ad libitum.
Clinical uropathogenic E. coli, cystitis isolate; UTI89 (available upon request from the laboratory of Dr Scott Hultgren)
Difco LB Broth, Miller (Fisher Scientific, cat. no. 244610)
Bacto-agar (Fisher Scientific, cat. no. 214030)
Aerrane (Isoflurane) (Baxter, cat. no. FDG9623) ▲ CRITICAL Isoflurane (http://www.umm.edu/altmed/drugs/isoflurane-073000.htm) is the generic name for the inhalation anaesthetic. It can be found under many trade names, including aerrane, floran, florane, isothane and so on, in other countries. Inhalation anesthetics provide a rapid and convenient means to anesthetize research animals. However, the anesthetic effect does not last long unless the agent is provided continuously. Animals that will experience prolonged exposure to anesthetics need to be regulated and monitored closely to prevent death. Injectable anesthetics, such as ketamine–xylazine mixture (also known as Mouse Cocktail) can be used for long-term (up to 30 min) sedation of mice. Ketamine is a controlled substance, and requires secured storage and detailed records of usage. Furthermore, the amount of drugs injected into each mouse varies based on the weight of the animal, making the usage of this anesthetic method impractical when a large number of mice are to be infected. ! CAUTION Isoflurane, a halogenated ether, is a colorless liquid anesthetic with a pungent odor. Isoflurane is not known to be carcinogenic or mutagenic, but has been demonstrated to have a fetal toxic effect when given to mice at high dose. Although the potential of fetal toxic effects in humans is not known, it is recommended that this agent should not to be used by pregnant women unless no other means of anesthesia are available. Only use Isoflurane with an approved anesthetic respirator system or in small amounts in a jar. Also work in well-ventilated area when using inhalation anesthetics such as Isoflurane.
Sterile lubricating jelly (Triad Disposables, cat. no. 10-8919)
10% (wt/vol) neutral buffered formalin solution (Sigma-Aldrich, cat. no. HT501128)
Ethanol, denatured (Fisher Scientific, cat. no. A407)
Paraformaldehyde, 20% (wt/vol) solution, EM Grade (Electron Microscopy Sciences, cat. no. 15713)
Fluorescence-conjugated (Alexa Fluor 594) wheat germ agglutinin (WGA) (Invitrogen, cat. no. W11262)
Fluorescent DNA dyes SYTO9 and SYTO83 (Invitrogen, cat. nos. S34854 and S11364)
ProLong Gold antifade reagent (Invitrogen, cat. no. P36930)
Phosphate buffered saline, pH 7.4 (Sigma-Aldrich, cat. no. P3813-10PAK) (see REAGENT SETUP)
LB plates (see REAGENT SETUP)
EQUIPMENT
Intramedic non-radiopaque polyethylene tubing, PE10 (inner dimension 0.28 mm (0.011 inch); outer dimension 0.61 mm (0.024 inch)) (BD Biosciences, cat. no. 427401)
30-G hypodermic needles, 1/2 inch (BD Biosciences, cat. no. 305106)
1-ml tuberculin Slip-Tip syringe (BD Biosciences, cat. no. 309602)
3-ml Luer-Lock syringe (BD Biosciences, cat. no. 309585)
Stainless steel tissue capsule (Electron Microscopy Sciences, cat. no. 62320-01)
Omni tissue homogenizer (TH) (Omni International, cat. no. TH115)
7 mm × 95 mm Saw Tooth (fine) generator probe (Omni International, cat. no. G7-95ST)
SYLGARD 184 Silicone elastomer kit (Dow Corning)
Minutien Pins, 0.2 mm base diameter (Fine Science Tools, cat. no. 26002-20)
Fisherbrand TRUFLOW tissue and biopsy cassettes (Fisher Scientific, cat. no. 15-200-403)
Fisherbrand biopsy foam pads (Fisher Scientific, cat. no. 22-038221)
Mobile induction system anesthetic respirator (Medrec) (see EQUIPMENT SETUP)
LSM510 META confocal fluorescence microscope (Carl Zeiss MicroImaging) (see EQUIPMENT SETUP)
LSM510 version 4.2 (Carl Zeiss MicroImaging)
Volocity 4 (Improvision) (see EQUIPMENT SETUP)
REAGENT SETUP
Phosphate buffered saline, pH 7.4
Pre-mixed phosphate buffered saline (PBS) powder can be purchased from Sigma-Aldrich (St. Louis, MO, USA) or other molecular biology reagent suppliers. PBS solutions are made by dissolving the pre-mixed powder in specified amount of water according to the manufacturer. Alternatively, PBS could be prepared by mixing all the necessary components: 0.138 M NaCl, 0.0027 M KCL, 0.01 M Na2HPO4 and 0.0017 M KH2PO4 in dH2O. PBS solutions used for resuspending bacteria and dilution of bacterial titers should be sterilized by filtration through 0.22 μm filters. PBS solution can be made ahead of time and stored on the benchtop for months. However, it is critical to ensure the sterility of the solution.
LB agar plates
Agar plates used for streaking bacteria from freezer stocks and for tissue titering should be made ahead of time. LB agar plates are made according to standard molecular biology protocols 25 g LB Broth (Miller) and 14 g Bacto-agar in 1 L dH2O. Autoclave using liquid cycle at 121 °C for 20 min to dissolve and sterilize. If antibiotics are needed for selection of particular strains, they should be added into autoclaved liquid LB agar solution after it has cooled to touch. LB agar plates should be stored at 4 °C and brought out to dry overnight at room temperature (23–26 °C) before use.
EQUIPMENT SETUP
Mobile induction system anesthetic respirator
A mobile anesthetic respirator for small animals typically includes an E-cylinder oxygen tank, an oxygen gas regulator, an anesthetic vaporizer with reservoir, a nosecone and/or an induction chamber and waste anesthetic gas scavenging canisters (Fig. 5). If both the nosecone and the induction chamber are present in the anesthetic machine, be sure to close the valve leading to the nosecone and open the induction chamber valve before operating. Turn on the oxygen tank and open the oxygen flow valve to 2 l min−1 to fill the induction chamber and tubing for about 2 min. Reduce the oxygen flow-rate down to 0.5 l min−1. Put mice in the induction chamber and turn on Isoflurane to 4%. The maximum number of mice able to be put into the induction chamber depends on the size of the induction chamber. When the animals stop moving and their breathing slows down, reduce Isoflurane flow-rate down to 2.5%–3%. Different mouse strains have different sensitivity to anesthesia. The Isoflurane concentration stated here is optimized for C3H/HeN mice. Mice of different genetic backgrounds might have different susceptibilities to anesthesia-induced hypoxia; therefore, the Isoflurane concentration should be determined empirically. Open the anesthetic gas flow to the nosecone if it is used on mice during infection procedure. Mice can be maintained anesthetized in the induction chamber before they are infected. Follow the manufacturers’ operating instructions if they are different from what is stated here.
Figure 5.
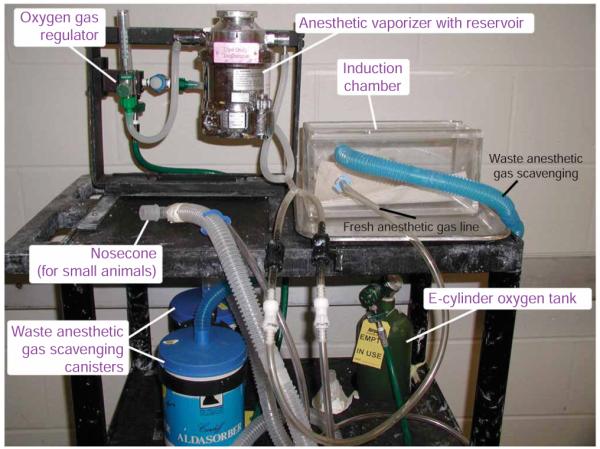
Typical make-up of a mobile induction system. A mobile induction system uses many components to deliver gaseous anesthetics in a controlled fashion. Different parts of the induction system are indicated.
Alternative anesthesia setup
If respirators are not available, a clear glass jar with Isoflurane-wetted cotton balls could be used as a substitute. Do not over-saturate the cotton balls with Isoflurane. Keep the cotton balls in a stainless steel tissue capsule or a consumer-type metal tea-infuser ball (generally available in local grocery stores) to prevent direct contact of the anesthetics with the animals, as it is harmful to the animals when in direct contact with the skin. As no oxygen is supplied in this alternative method, the progress of anesthesia needs to be monitored very carefully to prevent asphyxiation. Remove the animals from anesthesia and proceed with the inoculation procedure immediately when their breathing has slowed down to ~1 breath per s.
LSM510 META confocal microscope
The LSM510 META confocal microscope can be equipped with multiple light sources (a tunable Argon laser and 2 HeNe lasers of different wavelength) and can be used with virtually all commercially available fluorophores. The LSM510 image software is used for controlling both the laser system and the microscope, as well as for image acquisition. The output power of laser sources and acquisition gain of individual emission channels should be determined empirically for each experiment. A series of images (Z-stack) along the vertical axis of the sample can be collected using the Z-stack function. When collecting Z-stack images, be sure to optimize the pinhole size for each light source, which determines the thickness of each optical section and the intervals between each optical section. Images acquired through LSM510 software are stored in a database that can be exported as common graphical file formats or manipulated with other image analysis software such as Volocity, LSM image browser or ImageJ.
Volocity 4
Three-dimensional (3D) reconstruction of Z-stack images can be achieved with Volocity 4 image analysis software. Create a new library after opening the application and import the Z-stack image file (.lsm file) acquired from LSM510 into the library. Open the imported LSM file and select the ‘3D’ tab to generate a reconstructed image.
Preparing silicone bladder pinning pad
Silicone pads are made out of silicone elastomer (SYLGARD 184) poured in a 6-well plate. Making silicone pads in a 6-well plate provides a convenient way of pinning and processing multiple bladders. Combine silicone elastomer base with the curing agent in a 50-ml conical tube according to manufacturer’s specification. Slowly invert the conical tube for several minutes to ensure even mixing of the reagents without creating too many air bubbles. Pour liquid silicone elastomer into wells to about half the height. Allow silicone to cure at room temperature for 3–4 d. Silicone pads could also be poured in different sizes of multi-well plates or petri dishes depending on the need and ease of use. The bladder pinning pads could be used repeatedly for other experiments. There are no special storage requirements for these silicone pads.
Making urinary catheters
BOX 1 | MAKING URINARY CATHETERS ● TIMING 30 SEC PER CATHETER.
▲ CRITICAL Although the polyethylene tubing used to make urinary catheter is not sterile, the working surface and instruments used to make catheters should be thoroughly cleaned with ethanol before use. A laminar flow hood equipped with UV lamps provides an ideal catheter production and sterilization station.
! CAUTION The UV lamps in the laminar flow hood should be turned off whenever work is being carried out. Do not position any part of the body in the hood or look directly into the UV lamps while the lamps are still on.
Cut a segment of polyethylene tubing of about 12 inches.
Put a sterile 30-G hypodermic needle (1/2 inch long needle) on a sterile 3 ml syringe.
Pick up one end of the polyethylene tubing with a clean pair of flat-head tweezers (such as stamp-holding tweezers).
Slip polyethylene tubing onto the needle all the way to the hub.
Cut the polyethylene tubing, leaving extra tubing of about a full needle length (Fig. 6a).
Remove the catheter from the syringe and place it in a sterile petri dish.
Repeat steps 2–6 for additional catheters. One catheter is required per experimental group.
Once all catheters are made, expose catheters in the uncovered petri dish to UV irradiation for at least 30 min to sterilize.
Enclose the catheters in the petri dish after UV irradiation.
■ PAUSE POINT UV-irradiated catheters can be stored in the sterile petri dish at room temperature for later use. These catheters can be stored indefinitely as long as they stay enclosed in the sterile petri dishes.
Figure 6.
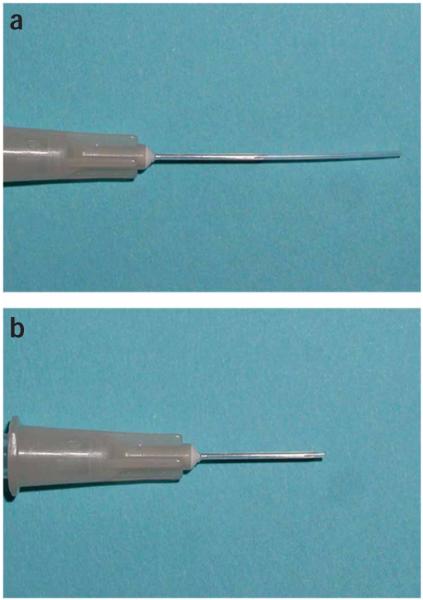
Urinary catheter. Urinary catheters are made out of polyethylene tubing on a 30-G hypodermic needle. An unused catheter (a) has extra tubing hanging off the end of the needle. The extra tubing is cut off near the bevel of the needle right before use (b).
PROCEDURE
Culturing type 1-piliated bacteria ● TIMING 3 d
1| Day 1: streak out E. coli on an LB plate to obtain single colonies. Incubate the plate overnight at 37 °C.
2| Day 2: pick a single bacterial colony and use it to inoculate 10 ml of LB broth in a 125-ml Erlenmeyer flask.
3| Incubate the bacterial culture statically overnight at 37 °C.
▲ CRITICAL STEP Type 1-piliation levels in E. coli are affected by the growth conditions. Static growth of bacteria in LB broth at 37 °C ensures an optimal condition for type 1-pilus expression.
4| Day 3: After the first overnight growth in liquid broth, subculture the bacteria into fresh LB media by diluting 25 μl of the overnight culture into 25 ml of LB media in a 250-ml Erlenmeyer flask.
5| Incubate the bacterial culture statically overnight at 37 °C.
▲ CRITICAL STEP Type 1-pili expression is critical for UTIs. Serial passages of static overnight UPEC cultures will ensure type 1-pili expression in most, if not all, bacteria.
Preparation of Type 1-piliated bacterial inoculum ● TIMING 1 h
6| Day 4: Spin down the entire 25-ml bacterial culture in a sterile tube at 5,000g for 5 min at 4 °C.
7| Decant the supernatant and resuspend the bacterial pellet in 10 ml of sterile PBS.
8| Add 1 ml of resuspended bacterial culture into 9 ml of sterile PBS (1:10 dilution). Check the OD600 nm value of diluted culture.
9| Adjust the diluted bacterial culture with either sterile PBS or concentrated bacterial suspension to OD600 nm of 0.4–0.5. This is the inoculum to be used for mouse infection.
▲ CRITICAL STEP The inoculum OD value corresponds to 1–2 × 107 CFUs per 50 μl for the prototypic UPEC, UTI89, we routinely use. As the OD600 nm-viable CFU titer correlation varies among bacterial strains, the desired OD600 nm level should be determined empirically.
10| Titer an aliquot of the inoculum to verify the number of viable CFU in the inoculum by microtiter-plate dilution, as described in Box 2.
BOX 2 | TITERING VIABLE BACTERIAL POPULATIONS ● TIMING 15 MIN PER DILUTION PLATE.
-
Prepare dilution plates with 180-μl PBS in each well of rows B–H. Transfer 200 μl of each bacterial inoculum or homogenate to the top row (row A) of the dilution plate. Use 1 well per sample.
? TROUBLESHOOTING
-
Carry out a tenfold serial dilution down the column starting from the top row (row A) (ending up with 180 μl final volume in each well, except the last row (row H), which would contain 200 μl). In each column, there should be serial-diluted samples ranging from no dilution down to 107-fold dilution.
▲ CRITICAL STEP Multi-channel pipettes are essential for microtiter dilution. Owing to the small volumes involved, these pipettes need to be accurate and calibrated frequently. Microtiter dilution method coupled with spotting requires fewer LB plates compared with plating out 100 μl of diluted sample on LB plates with standard large volume serial dilution.
-
Spot 5 individual 10-μl spots for each dilution onto an LB plate. The total volume spotted equals to 50 μl. The volume value is used in calculation of bacterial titers. Incubate the LB titering plates at 37 °C overnight, or on benchtop at room temperature overnight if the infecting strain grows very rapidly at 37 °C.
▲ CRITICAL STEP Multi-channel pipettes are essential for spotting dilutions on plates. Using a multi-channel pipette, the eight dilutions of a sample (a single column on the dilution plate) could be picked up and spotted at the same time (10 μl each) on the same LB plate. Repeat the process five times to spot the dilutions onto the same LB plate.
■ PAUSE POINT Titering plates could be stored at 4 °C for ~1 week once bacterial colonies appear.
-
Count the number of bacterial colonies on the LB titering plates when they are large enough but still separated. Calculate the total colony forming unit (CFU) per bladder (or per 2 kidneys if they are assayed) or per ml for innoculum with the following formula:
n = CFU counted
d = dilution at which the CFU was counted
v = total volume spotted
CFU per tissue or CFU per ml (for inoculum) = n × 10d × (1,000 per v)
Preparation of urinary catheters for transurethral inoculation ● TIMING 3 min
11| Draw sufficient inoculum for the number of mice to be infected into a 1-ml tuberculin syringe.
▲ CRITICAL STEP A standard inoculum size is 50 μl per mouse. It is, however, advisable to draw excess bacterial suspension into the syringe. A slip-tip tuberculin syringe with 10-μl graduations is ideal for inoculation.
▲ CRITICAL STEP The Institutional Animal Care and Use Committees (IACUC) from most institutions allow housing of experimental mice in their micro-isolator caging outside of animal housing facilities for 24 h or less. Therefore, if tissues were to be harvested within 24 h post infection, the subsequent steps could be performed outside of animal facilities. It is important to verify with one’s own IACUC before animals are taken out of the animal facility to fully comply with animal research policies. Otherwise, prepare the urinary catheters and carry out infection experiments inside animal facilities according to the IACUC policies.
12| Insert a sterile urinary catheter onto the syringe.
13| Wipe clean a pair of scissors with 95% ethanol or alcohol pads.
14| Aseptically cut-off the excess polyethylene tubing with scissors leaving about 1–2 mm tubing at the tip of the needle (Fig. 6b).
15| While holding the syringe with the tip up, gently tap the syringe to force air bubbles to rise to the top. Push all the air out of the syringe through the catheter and prime the catheter.
16| Put a dab of medical grade sterile lubricating jelly onto a piece of parafilm or the plastic wrapper of the syringe.
17| Set the syringe down on the working surface, such that the catheter is immersed in lubricating jelly.
Inoculation of bacteria into mouse bladders ● TIMING 2 min per mouse
18| Anesthetize a mouse, as described in Equipment setup, to immobilize the animal before catheterization.
▲ CRITICAL STEP Only anesthetize multiple animals with an anesthesia respirator, as they need to be maintained under an anesthetic state for a long period of time.
! CAUTION Work in well-ventilated areas and take extra precautions to prevent breathing in too much Isoflurane throughout the infection process.
19| After the mice have been anesthetized, take one mouse out of the induction chamber and lay it on its back, flat on the working surface. To maintain the mouse under anesthesia, an anesthetic nosecone can be placed over most of its head.
▲ CRITICAL STEP A 50-ml conical tube with Isoflurane-wetted cotton balls could be used as a convenient substitute for nosecones connected to a respirator (Fig. 7a). Be sure not to over-saturate the nosecone with anesthetics and pay close attention to the animal’s breathing during catheterization. When their breathing intervals slow down to more than 4 s between breaths, remove the nosecone from the mouse temporarily to prevent anesthesia-induced hypoxia and death. At this deep state of anesthesia, there should be enough time to complete the inoculation process. However, if the mouse shows signs of rapid breathing, which is a sign of waking up, reposition the nosecone back over the animal’s nose to keep it sedated.
Figure 7.
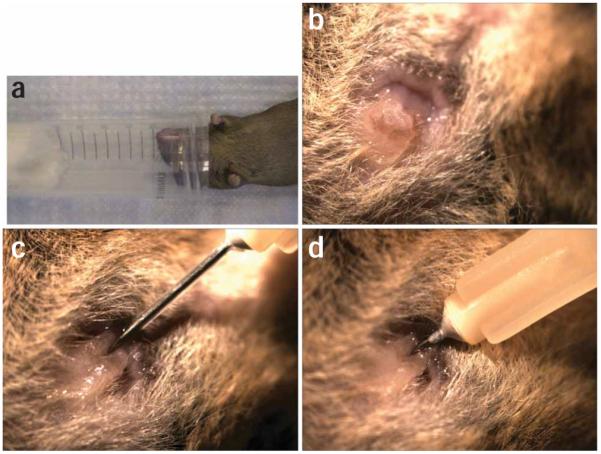
Catheterization process. (a) A 50-ml conical tube with Isoflurane-wetted cotton ball is used as a nosecone to maintain a mouse under anesthesia during catheterization. (b) The exterior portion of the mouse urethra is visible as a small mount of tissue above the vaginal opening. (c) A catheter is inserted into the exterior portion of the mouse urethra and lowered before being fully inserted (d) into the bladder. All animal studies were conducted in accordance to the Guide for the Care and Use of Laboratory Animals43 under assurance number A3381-01. The Washington University School of Medicine Animal Study Committee approved all experimental procedures described here.
20| Locate the urogenital opening of the mouse and place a finger next to the mound of tissue forming the urethral opening (Fig. 7b).
21| With fingers from the other hand, locate the bladder by feeling for an enlarged bump in the lower abdomen area. Gently massage and push down on the bladder to expel urine. Wipe off any residual urine on the urethral opening.
22| Hold the inoculating syringe vertically over the urethral opening. Insert lubricating jelly-coated catheter slowly into the urethra until it does not go in freely, which is about 3–4 mm from the opening.
23| Slowly lower the syringe toward you until it is nearly at the level of the mouse urethra (Fig. 7c).
24| Push the catheter further into the urethra until the hub of the catheter reaches the urethral opening (Fig. 7d).
▲ CRITICAL STEP This is the most challenging part of the experiment and takes a lot of patience and practice to perfect the technique. Holding the catheter vertically initially allows its proper insertion into the urethra. Slowly twirling the syringe could help guide the catheter through folded tissues inside the urethra. Lowering the syringe in Step 23 positions the catheter beneath the pelvic bone, which often hinders the catheterization process, and allows further insertion of the catheter into the bladder. If resistance is encountered before the catheter is fully inserted, moving the syringe slightly back and forth or twirling the syringe could help alleviate the problem.
25| Once the catheter is fully inserted, push the syringe plunger slowly to deposit 50-μl inoculum (at ~ 10 μls−1) into the bladder.
? TROUBLESHOOTING
26| Slowly withdraw the syringe and catheter out of the animal.
27| Rest the syringe back on the working surface with the catheter immersed in lubricating jelly.
▲ CRITICAL STEP Periodically monitor the breathing of the animal during catheterization. If the process of catheterization has gone on longer than expected and a conical tube nosecone is used, the nosecone could be removed from the animal and replaced as needed to prevent asphyxiation.
28| Place the infected animal back into a mouse cage.
29| Repeat Steps 19–28 for the infection of other animals in the same experimental group. The same catheter can be used for all the animals in the same group. Ensure that the catheter is lubricated each time before inserting it into an animal to minimize injury and discomfort.
■ PAUSE POINT Infected mice are returned to their respective cages with food and water. The animals should be maintained in the animal facilities until the day of tissue harvesting.
▲ CRITICAL STEP The IACUC from most institutions allows housing of experimental mice in their micro-isolator caging outside of animal housing facilities for 24 h or less. However, it is important to verify with one’s own IACUC before animals are taken out of the animal facility to fully comply with animal research policies.
Tissue harvest and processing ● TIMING 2 min per mouse
30| Bladders and kidneys can be harvested at defined time points to assess the degree and consequence of infection by many different methods. Animals are first killed by CO2-asphyxation or anesthetic agent inhalation (Isoflurane) followed by cervical dislocation.
! CAUTION Cervical dislocation, when carried out correctly, is considered to be a quick, efficient and humane way of killing mice. However, perfection of this technique requires training by individuals who are proficient in this technique. It is required in most institutions that animals are anesthetized by deep inhalation of anesthetic agent, such as Isoflurane, before cervical dislocation is carried out. Only CO2 gas from compressed gas cylinders or in-house CO2 gas lines are recommended for killing as per American Veterinary Medical Association guidelines (AVMA Guidelines on Euthanasia, June 2007; http://www.avma.org/resources/euthanasia.pdf). Other forms of CO2 generation, such as dry ice, fire extinguisher or chemical means, are not acceptable.
31| Lay the animals flat on their backs and spray the abdominal area with 95% ethanol to sterilize the area.
32| Cut open the lower abdomen to expose the internal organs (Fig. 8). The bladder appears as a round ball of tissue protruding outward, but in some cases could be buried underneath adipose tissues. Move the stomach, intestines and spleen out of the way to expose the kidneys, which are located near the back of the animals.
Figure 8.
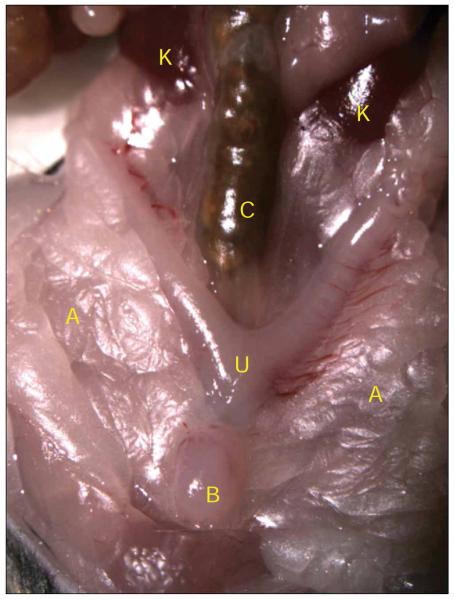
Mouse urinary-tract anatomy. The mouse bladder is visible as a small round ball of tissue at the very lower portion of the abdomen (B), on top of the uterus (U). It is sometimes hidden underneath layers of adipose tissue (A), which are pushed aside for this image. The mouse intestines cover up the upper urinary tract. Once the intestines are moved aside, the kidneys (K) should be visible along side the colon (C). All animal studies were conducted in accordance to the Guide for the Care and Use of Laboratory Animals43 under assurance number A3381-01. The Washington University School of Medicine Animal Study Committee approved all experimental procedures described here.
33| At this point, different analyses can be carried out depending on the aim of the experiment. To enumerate bacterial loads in the tissues, follow option A. To carry out fluorescence imaging of bacteria in the bladder, follow option B. To carry out a histological examination of bladders and kidneys, follow option C. As tissues used for bacterial load determination (option A) are homogenized, image analyses cannot be carried out on these bladders. However, the tissue homogenates could be used for the determination of host cytokine expressions by ELISA (experiments not described in this protocol). Bladders imaged by fluorescence microscopy (option B) can be further processed for histological examination (option C). In such cases, it is critical that fluorescence microscopy (option B) is carried out first.
- Bacterial load quantification ● TIMING 5 min per mouse
- Lift the bladder from its base where it is attached next to the uterus.
- Aseptically remove the bladder and put it into a tube containing 1-ml sterile PBS. Keep tissues on ice until they are ready to be processed.
- If the kidneys are to be assayed, aseptically remove them and put them into a tube containing 0.8-ml sterile PBS. Both kidneys from a mouse, when homogenized, account for about 0.2 ml in volume. Therefore, homogenizing two kidneys in 0.8 ml PBS would allow for easier calculation of bacterial titers. Keep tissues on ice until they are ready to be processed.
- Homogenize the tissues with a mechanical tissue homogenizer. Keep the homogenates on ice.
- Titer the viable bacterial population in the homogenates by microtiter-plate dilution, as described in Box 2.
- Processing of whole mount bladders for fluorescence microscopy imaging ● TIMING 2 h for all the bladders
- Remove the bladder as described in Steps 33A(i),(ii) above.
- Bisect the bladder longitudinally and place the bladder halves onto a silicone bladder pinning pad (prepared as described in Equipment setup) covered with PBS.
- Hold one bladder half with a pair of forceps and pin down one edge of the bladder with a small minutien pin. Be sure to have the luminal side of the bladder facing up.
- Pull and stretch the bladder from the opposing side of the first pin. Secure the other end of the bladder with another pin.
- Repeat the stretching/pinning process until the bladder half is stretched out and flattened (Fig. 9).
- (Optional) If visualization of the bladder surface is desired, decant PBS in the wells containing stretched bladders and stain the bladders with 0.5 ml 2 μg ml−1 fluorescence-conjugated WGA solution (diluted in PBS) on benchtop at room temperature for 20 min. Wash the bladders three times with PBS after WGA staining.
- Fix bladders in 0.5 ml 3% paraformaldehyde (diluted in PBS) on benchtop at room temperature for 45 min.
- Wash the bladders three times with PBS.
-
To stain bacterial DNA and bladder cell nuclei, incubate in 5 μM cell-permeant fluorescent nucleic acid dyes (SYTO9 or SYTO83) in PBS for 15 min on benchtop at room temperature.▲ CRITICAL STEP Fluorescent WGA and nucleic acid dyes are available in a wide fluorescence spectrum. Thus, selections of these reagents can be varied to accommodate multi-color imaging. In addition, bacteria containing fluorescent protein reporter constructs can be used in conjunction with dyes in these experiments to assess the expression profiles of genes of interest.
-
Wash the bladders three times with PBS.■ PAUSE POINT If stained bladders will not be examined within 24 h, they should be stored in PBS at 4 °C in the dark and mounted onto microscope slides no longer than 24 h before microscopic examination. Stained bladders should not be stored for more than 48 h to prevent loss of fluorescence.
- Remove pins from the bladders and place the bladders on a microscope slide with the luminal side facing up.
-
Mount the bladders with 50-μl ProLong Gold antifade and cover them with a glass coverslip.▲ CRITICAL STEP ProLong Gold antifade should be warmed up to room temperature before use. Mix the viscous solution with a clean pipette tip rather than vortex mixing to avoid creation of bubbles.
-
Keep the microscope slides away from light. Visualize and image bladders under confocal laser scanning microscopes.? TROUBLESHOOTING
- (Optional) Bladders prepared for fluorescence microscopy could still be processed for histology. Remove the bladders from microscope slides and rinse them briefly in PBS. Place the bladders into tissue-embedding cassettes with biopsy foam pads to secure them in the cassettes. Bladders from multiple mice of the same study group could be put in the same cassette and embedded together. Continue from Step 33C(vii).
- Processing of bladders for histology ● TIMING 7 h to overnight, depending on fixation time allocated
- Remove the bladder and the kidneys as described in Steps 33A(i)–(iii).
- Put tissues in 10% neutral buffered formalin solution (NBF) in a 15-ml conical tube. In general, 5 ml of NBF is sufficient for a bladder and two kidneys from the same mouse.
- Fix the tissues for at least 6 h on benchtop at room temperature or overnight at 4 °C.
- Rinse the tissues three times in PBS.
- Bisect the bladder longitudinally and place the two halves in a tissue-embedding cassette.
- Kidneys are larger organs with complex architectures compared with the bladders; bisect them longitudinally or cross-section them into multiple slices. Place the kidney slices in a tissue-embedding cassette.
-
Store tissues in 70% ethanol (vol/vol in dH20) at 4 °C.■ PAUSE POINT Tissues could be stored for months at 4 °C before embedding process.▲ CRITICAL STEP Many institutions are equipped with histology cores. Paraffin embedding and sectioning of tissues as well as hematoxylin–eosin (H&E) staining of tissue sections are best handled by these cores, as specialized equipment is needed for these procedures. Tissues embedded in paraffin (tissue blocks) could be stored indefinitely at room temperature in the laboratory. Additional tissue sections could be generated from these tissue blocks in the future whenever necessary.
Figure 9.

Stretched bladder on silicone pad. This image shows a stretched and flattened bladder half. Pins holding down the bladder are visible around the tissue. Some adipose tissues are also visible on the left. All animal studies were conducted in accordance to the Guide for the Care and Use of Laboratory Animals43 under assurance number A3381-01. The Washington University School of Medicine Animal Study Committee approved all experimental procedures described here.
34| Once desired tissues have been harvested from the mice, dispose of animal carcasses as biohazard waste according to procedures established by the IACUC.
● TIMING
A complete mouse-infection experiment with the UTI model should take at least 4 d to complete. This estimation, however, does not take into account the additional time required for prolonged infection experiments (i.e., 24 h or longer infections). The first 3 d of the protocol described here is required to grow bacteria for inoculation. The actual mouse inoculation step could be accomplished within a few hours. Tissue harvesting and post-harvesting procedures could be accomplished within a day. With the exception of Steps 1–5, all the estimated timing to complete each step was given as actual hands-on time. In some cases (Steps 18–29, Steps 30–32 and Step 33A), the estimated time was given as time per mouse. The actual timing for these steps will depend on the number of mice being processed.
Summary of timing
Steps 1–5: 3 d
Steps 6–10: 1 h
Steps 11–17: 3 min
Steps 18–29: 2 min per mouse
Steps 30–32: 2 min per mouse
Step 33A: 5 min per mouse (this step could be carried out in parallel with Step 33C with different bladder samples)
Step 33B: 2 h (this step could be carried out in parallel with Step 33C with different bladder samples; in addition, samples completed in this step could also be further process in Step 33C).
Step 33C: 7 h to overnight.
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 1.
TABLE 1.
Troubleshooting table
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 25 | Leakage of a small amount of inoculum right after inoculation |
Bladder still contains a large amount of urine |
Do not re-inoculate the mouse Try to push out as much urine as possible in Step 21 for other mice |
| Catheter was inserted into the vagina rather than the urethra |
Carefully insert the catheter into urethra and re-inoculate the mouse |
||
| 33B(xiii) | Fluorescent staining appeared dim or not visible under microscope |
Tissue samples not stained well | Increase the concentration of fluorescent staining agents or staining time |
| Box 2, step (i) | Pipette tips clogged with tissue pieces | Tissues were not homogenized well | Re-pipette and try to avoid picking up tissue pieces If problem persists, re-homogenize the sample |
ANTICIPATED RESULTS
Within 1 h after infection, a large portion of the inoculum is lost from the animal (~1,000-fold reduction)—presumably excreted by micturition. Using an ex vivo gentamicin protection assay32, it was revealed that ~50% of bacteria remaining in the bladder were located within an intracellular niche. The ability to successfully establish intracellular and prolonged colonization requires the interaction of the FimH adhesin with cellular receptors31,44. Bacterial loads in the bladder increase from 1 h after infection. This increase coincides with intracellular replication and IBC formation observed by time-lapse fluorescence video microscopy of infected bladders17. Enumeration of viable bacteria within the mouse bladder and the kidney tissues reveals high levels of bacteria at 6 h and 1 d after infection (Fig. 10). Bacteria can persist in the mouse urinary tracts for at least 7 d, albeit at different levels, depending on the mouse background and bacterial strain used—a phenomenon also noted by Hopkins et al.19. Nevertheless, it should be noted that mouse-to-mouse variability of tissue titers exists even among mice of the same genetic background (Fig. 10). Robust host immune response including epithelial exfoliation and immune cell influx may account for the decrease in bacterial titer at later time points. Using this murine UTI model, several bacterial genes critical for various steps in the developmental process have been identified18,31,32,45,46. Laser scanning confocal fluorescence microscopy imaging provides an additional dimension for visualizing bacteria in infected bladders. Large compact IBCs with coccoid-shape bacteria reside within the lumen of bladder superficial umbrella cells (Fig. 11a–c). Long filamentous bacteria are also visible (Fig. 11d–e). At the late stage of IBC development, bacteria disperse from the dense aggregate and occupy the cytoplasm, but are confined by the membrane of infected cells (Fig. 11f). These morphological phenotypes exist for many different clinical isolates of UPEC and occur in all mouse strain backgrounds tested thus far, including C3H/HeN, C57BL/6, CBA/J, FVB/NJ and C3H/HeJ36. When coupled with fluorescence protein reporters, the temporal and spatial expression of bacterial genes can also be assessed18,29,31,47.
Figure 10.
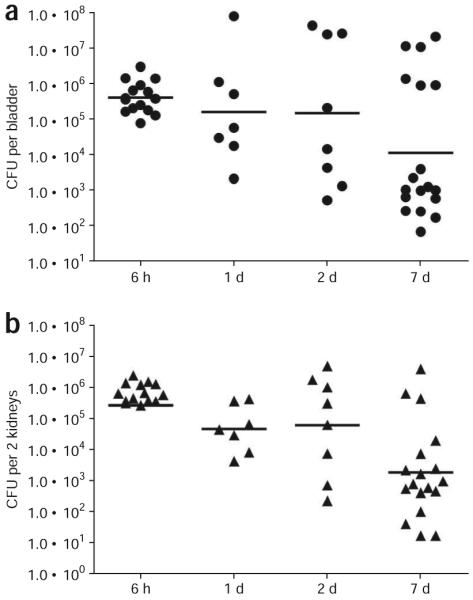
Sample tissue bacterial titers. Enumerating viable bacterial titers in infected tissues can assess the success of bacterial colonization. 7–8-week-old female C3H/HeN mice were infected with uropathogenic Escherichia coli (UPEC) strain UTI89. The bacterial titers in bladders (a) and the kidneys (b) were determined at indicated time points post infection. Each symbol in each graph represents values from a single mouse. The horizontal bar denotes the Geometric Mean of each data group. All animal studies were conducted in accordance to the Guide for the Care and Use of Laboratory Animals43 under assurance number A3381-01. The Washington University School of Medicine Animal Study Committee approved all experimental procedures described here.
Figure 11.
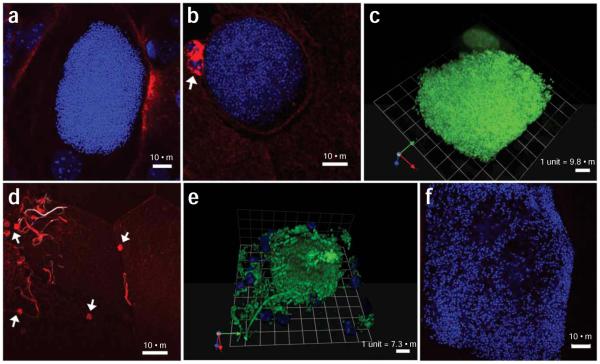
Confocal fluorescence microscopy images of intracellular bacterial communities (IBCs) and filamentous bacteria. 7–8-week-old female C3H/HeN mice were infected with uropathogenic Escherichia coli (UPEC) strain UTI89. Confocal microscopy analysis of infected bladders reveals UPEC at different stages of the pathogenic cycle. (a,b) Optical sections of compact IBCs (DNA stained and pseudo-colored blue) are visible at 6 h after infection. Bladder surfaces are stained with fluorescent wheat germ agglutinin (WGA) (pseudo-colored red). Two polymorphonuclears (PMNs) homed to the UPEC infected cell in panel (b) appear as clusters of WGA-rich clumps (white arrow). (c) Three-dimensional reconstruction of an IBC from a 6-h infected bladder (DNA stained with SYTO9 and pseudocolored green) generated in Volocity shows a compact cluster of bacteria. Bladder epithelial cell nucleus (also pseudo-colored green due to the same DNA stain) is visible next to the IBC. (d) Filamentous bacteria (DNA stained and pseudo-colored white) can be seen on the surface of infected bladder (stained with WGA and pseudo-colored red) starting at 6 h after infection. In some instances, filamentous bacteria are stained with WGA, thus appearing red or light red. The source of WGA recognition sites on filamentous bacteria are currently unknown. PMNs (white arrows) congregating near surface bacteria appear as small red clumps due to cellular membrane stained with WGA (pseudo-colored red). (e) Three-dimensional reconstruction of an IBC (bacteria expressing green fluorescent protein (GFP) and pseudo-colored green) generated in Volocity shows a compact cluster of bacteria with some rod-shaped bacteria on the edge and away from the IBC. A filamentous bacteria coming out of the IBC is also visible. The nuclei of PMNs (pseudo-colored blue) attacking loose bacteria can be seen in the picture. (f) At late IBC stage, loose bacteria inside a superficial umbrella cell (DNA stained and pseudo-colored blue) fill most of the cytoplasmic space, revealing the outline of the bladder cell. The WGA-stained bladder surface is not visible at this confocal optical section. Scale bars: a, b, d and f, 10 μm; c, 9.8 μm and e, 7.3 μm. All animal studies were conducted in accordance to the Guide for the Care and Use of Laboratory Animals43 under assurance number A3381-01. The Washington University School of Medicine Animal Study Committee approved all experimental procedures described here.
In addition to the examination of bacterial virulence factors, host responses to infection and factors involved in such responses have been studied30,37,38,48,49. H&E staining of tissue sections have provided a general view of host responses in infected bladders and kidneys, i.e., tissue edema, bladder epithelium damage/exfoliation (Fig. 12a), inflammatory cell-infiltration (Fig. 12b-d) and IBCs (Fig. 12b,c). Polymorphonuclear (PMN) cell-influx into infected bladders is evident as early as 6 h after infection (Figs. 11b and 12b,c). Many of these PMNs home directly to infected bladder epithelial cells containing IBCs. Unstained paraffin-embedded tissue sections can also be used for immunofluorescence staining of specific host or bacterial markers30,31.
Figure 12.
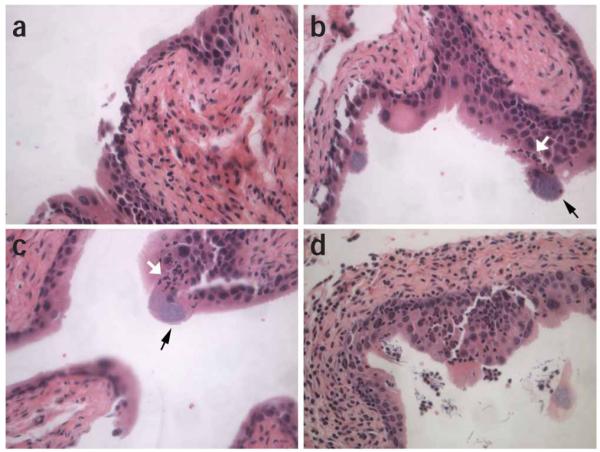
Histological images of infected bladder. Histological examinations of H&E stained tissue sections allows visualization of infection progression and host inflammatory response. 7–8-week-old female C3H/HeN mice were infected with uropathogenic Escherichia coli (UPEC) strain UTI89. (a) Bladders harvested at 6 h after infection reveal the state of exfoliation, slight neoplasia of the transitional epithelium and edema of the connective tissue layer. (b-c) Polymorphonuclears (PMNs) infiltrating into the bladder (white arrows) can be seen homing towards intracellular bacterial communities (IBCs) (black arrows) in the bladders at 6 h after infection. (d) At 1 d after infection, extensive edema, hyperproliferation of the transitional epithelium, PMN infiltration, filamentous bacteria and exfoliation of IBC-containing superficial umbrella cells are visible. Magnification, ×40. All animal studies were conducted in accordance to the Guide for the Care and Use of Laboratory Animals43 under assurance number A3381-01. The Washington University School of Medicine Animal Study Committee approved all experimental procedures described here.
ACKNOWLEDGMENTS
We thank all current and former Hultgren lab members in optimizing and perfecting the murine UTI model. We thank Dr. Wandy Beatty in the Molecular Microbiology Imaging Facility at Washington University School of Medicine for her excellent technical assistance with fluorescence microscopy. C.-S.H., K.W.D. and S.J.H. are supported by National Institutes of Health Office of Research on Women’s Health: Specialized Center of Research on Sex and Gender Factors Affecting Women’s Health, grant R01 DK64540, National Institute of Diabetes and Digestive and Kidney Diseases, grants R01 DK051406 and National Institute of Allergy and Infectious Diseases, grants R01 AI29549, R01 AI48689 and R01 AI50011.
References
- 1.Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect. Dis. Clin. North Am. 1997;11:551–581. doi: 10.1016/s0891-5520(05)70373-1. [DOI] [PubMed] [Google Scholar]
- 2.Svanborg C, Godaly G. Bacterial virulence in urinary tract infection. Infect. Dis. Clin. North Am. 1997;11:513–529. doi: 10.1016/s0891-5520(05)70371-8. [DOI] [PubMed] [Google Scholar]
- 3.Wu XR, et al. Mammalian uroplakins. A group of highly conserved urothelial differentiation-related membrane proteins. J Biol Chem. 1994;269:13716–13724. [PubMed] [Google Scholar]
- 4.Connell H, et al. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl. Acad. Sci. USA. 1996;93:9827–9832. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langermann S, et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 6.Mulvey MA, et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 7.Thankavel K, et al. Localization of a domain in the FimH adhesin of Escherichia coli type 1 fimbriae capable of receptor recognition and use of a domain-specific antibody to confer protection against experimental urinary tract infection. J. Clin. Invest. 1997;100:1123–1126. doi: 10.1172/JCI119623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kisielius PV, Schwan WR, Amundsen SK, Duncan JL, Schaeffer AJ. In vivo expression and variation of Escherichia coli type 1 and P pili in the urine of adults with acute urinary tract infections. Infect. Immun. 1989;57:1656–1662. doi: 10.1128/iai.57.6.1656-1662.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langermann S, et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J. Infect. Dis. 2000;181:774–778. doi: 10.1086/315258. [DOI] [PubMed] [Google Scholar]
- 10.Nuccio SP, Baumler AJ. Evolution of the chaperone/usher assembly pathway: fimbrial classification goes Greek. Microbiol. Mol. Biol. Rev. 2007;71:551–575. doi: 10.1128/MMBR.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thanassi DG, Hultgren SJ. Assembly of complex organelles: pilus biogenesis in gram-negative bacteria as a model system. Methods. 2000;20:111–126. doi: 10.1006/meth.1999.0910. [DOI] [PubMed] [Google Scholar]
- 12.Abraham SN, Sun D, Dale JB, Beachey EH. Conservation of the D-mannose-adhesion protein among type 1 fimbriated members of the family Enterobacteriaceae. Nature. 1988;336:682–684. doi: 10.1038/336682a0. [DOI] [PubMed] [Google Scholar]
- 13.Krogfelt KA, Bergmans H, Klemm P. Direct evidence that the FimH protein is the mannose-specific adhesin of Escherichia coli type 1 fimbriae. Infect. Immun. 1990;58:1995–1998. doi: 10.1128/iai.58.6.1995-1998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez JJ, Hultgren SJ. Requirement of Rho-family GTPases in the invasion of Type 1-piliated uropathogenic Escherichia coli. Cell Microbiol. 2002;4:19–28. doi: 10.1046/j.1462-5822.2002.00166.x. [DOI] [PubMed] [Google Scholar]
- 15.Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 2000;19:2803–2812. doi: 10.1093/emboj/19.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou G, et al. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. J. Cell Sci. 2001;114(Pt 22):4095–4103. doi: 10.1242/jcs.114.22.4095. [DOI] [PubMed] [Google Scholar]
- 17.Justice SS, et al. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. USA. 2004;101:1333–1338. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Justice SS, Hunstad DA, Seed PC, Hultgren SJ. Filamentation by Escherichia coli subverts innate defenses during urinary tract infection. Proc. Natl. Acad. Sci. USA. 2006;103:19884–19889. doi: 10.1073/pnas.0606329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopkins WJ, Gendron-Fitzpatrick A, Balish E, Uehling DT. Time course and host responses to Escherichia coli urinary tract infection in genetically distinct mouse strains. Infect. Immun. 1998;66:2798–2802. doi: 10.1128/iai.66.6.2798-2802.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaeffer AJ, Schwan WR, Hultgren SJ, Duncan JL. Relationship of type 1 pilus expression in Escherichia coli to ascending urinary tract infections in mice. Infect. Immun. 1987;55:373–380. doi: 10.1128/iai.55.2.373-380.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bishop BL, et al. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat. Med. 2007;13:625–630. doi: 10.1038/nm1572. [DOI] [PubMed] [Google Scholar]
- 22.Gunther N.W.t., Lockatell V, Johnson DE, Mobley HL. In vivo dynamics of type 1 fimbria regulation in uropathogenic Escherichia coli during experimental urinary tract infection. Infect. Immun. 2001;69:2838–2846. doi: 10.1128/IAI.69.5.2838-2846.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagberg L, et al. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritic E. coli of human origin. Infect. Immun. 1983;40:273–280. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hvidberg H, et al. Development of a long-term ascending urinary tract infection mouse model for antibiotic treatment studies. Antimicrob. Agents Chemother. 2000;44:156–163. doi: 10.1128/aac.44.1.156-163.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lane MC, Alteri CJ, Smith SN, Mobley HL. Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc. Natl. Acad. Sci. USA. 2007;104:16669–16674. doi: 10.1073/pnas.0607898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malaviya R, Ikeda T, Abraham SN, Malaviya R. Contribution of mast cells to bacterial clearance and their proliferation during experimental cystitis induced by type 1 fimbriated E. coli. Immunol. Lett. 2004;91:103–111. doi: 10.1016/j.imlet.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Hopkins WJ, Hall JA, Conway BP, Uehling DT. Induction of urinary tract infection by intraurethral inoculation with Escherichia coli: refining the murine model. J. Infect. Dis. 1995;171:462–465. doi: 10.1093/infdis/171.2.462. [DOI] [PubMed] [Google Scholar]
- 28.Rouschop KM, et al. Urothelial CD44 facilitates Escherichia coli infection of the murine urinary tract. J. Immunol. 2006;177:7225–7232. doi: 10.4049/jimmunol.177.10.7225. [DOI] [PubMed] [Google Scholar]
- 29.Anderson GG, et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 30.Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl. Acad. Sci. USA. 2006;103:14170–14175. doi: 10.1073/pnas.0602136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright KJ, Seed PC, Hultgren SJ. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell. Microbiol. 2007;9:2230–2241. doi: 10.1111/j.1462-5822.2007.00952.x. [DOI] [PubMed] [Google Scholar]
- 32.Justice SS, Lauer SR, Hultgren SJ, Hunstad DA. Maturation of intracellular Escherichia coli communities requires SurA. Infect. Immun. 2006;74:4793–4800. doi: 10.1128/IAI.00355-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson JR. Reflux in the mouse model of urinary tract infection. Infect. Immun. 1998;66:6063–6064. doi: 10.1128/iai.66.12.6063-6064.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson JR, Brown JJ. Defining inoculation conditions for the mouse model of ascending urinary tract infection that avoid immediate vesicoureteral reflux yet produce renal and bladder infection. J. Infect. Dis. 1996;173:746–749. doi: 10.1093/infdis/173.3.746. [DOI] [PubMed] [Google Scholar]
- 35.Rosen DA, Hung CS, Kline KA, Hultgren SJ. Streptozocin-induced diabetic mouse model of urinary tract infection. Infect. Immun. 2008;76:4290–4298. doi: 10.1128/IAI.00255-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garofalo CK, et al. Escherichia coli from urine of female patients with urinary tract infections is competent for intracellular bacterial community formation. Infect. Immun. 2007;75:52–60. doi: 10.1128/IAI.01123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 2001;69:4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schilling JD, Martin SM, Hung CS, Lorenz RG, Hultgren SJ. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA. 2003;100:4203–4208. doi: 10.1073/pnas.0736473100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kau AL, Hunstad DA, Hultgren SJ. Interaction of uropathogenic Escherichia coli with host uroepithelium. Curr. Opin. Microbiol. 2005;8:54–59. doi: 10.1016/j.mib.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Rosen DA, et al. Molecular variations in Klebsiella pneumoniae and Escherichia coli FimH affect function and pathogenesis in the urinary tract. Infect. Immun. 2008;76:3346–3356. doi: 10.1128/IAI.00340-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosen DA, et al. Utilization of an intracellular bacterial community pathway in Klebsiella pneumoniae urinary tract infection and the effects of FimK on type 1 pilus expression. Infect. Immun. 2008;76:3337–3345. doi: 10.1128/IAI.00090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Institute of Laboratory Animal Resources. Commission on Life Sciences. National research Council . Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC, USA: 1996. [Google Scholar]
- 44.Wellens A, et al. Intervening with urinary tract infections using anti-adhesives based on the crystal structure of the FimH-oligomannose-3 complex. PLoS ONE. 2008;3:e2040. doi: 10.1371/journal.pone.0002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kulesus RR, Diaz-Perez K, Slechta ES, Eto DS, Mulvey MA. Impact of the RNA chaperone Hfq on the fitness and virulence potential of uropathogenic Escherichia coli. Infect. Immun. 2008;76:3019–3026. doi: 10.1128/IAI.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hannan TJ, et al. LeuX tRNA-dependent and -independent mechanisms of Escherichia coli pathogenesis in acute cystitis. Mol. Microbiol. 2008;67:116–128. doi: 10.1111/j.1365-2958.2007.06025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright KJ, Seed PC, Hultgren SJ. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect. Immun. 2005;73:7657–7668. doi: 10.1128/IAI.73.11.7657-7668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mysorekar IU, Mulvey MA, Hultgren SJ, Gordon JI. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J. Biol. Chem. 2002;277:7412–7419. doi: 10.1074/jbc.M110560200. [DOI] [PubMed] [Google Scholar]
- 49.Schilling JD, Lorenz RG, Hultgren SJ. Effect of trimethoprim–sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect. Immun. 2002;70:7042–7049. doi: 10.1128/IAI.70.12.7042-7049.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]