Abstract
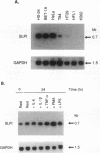
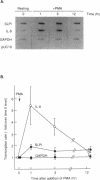
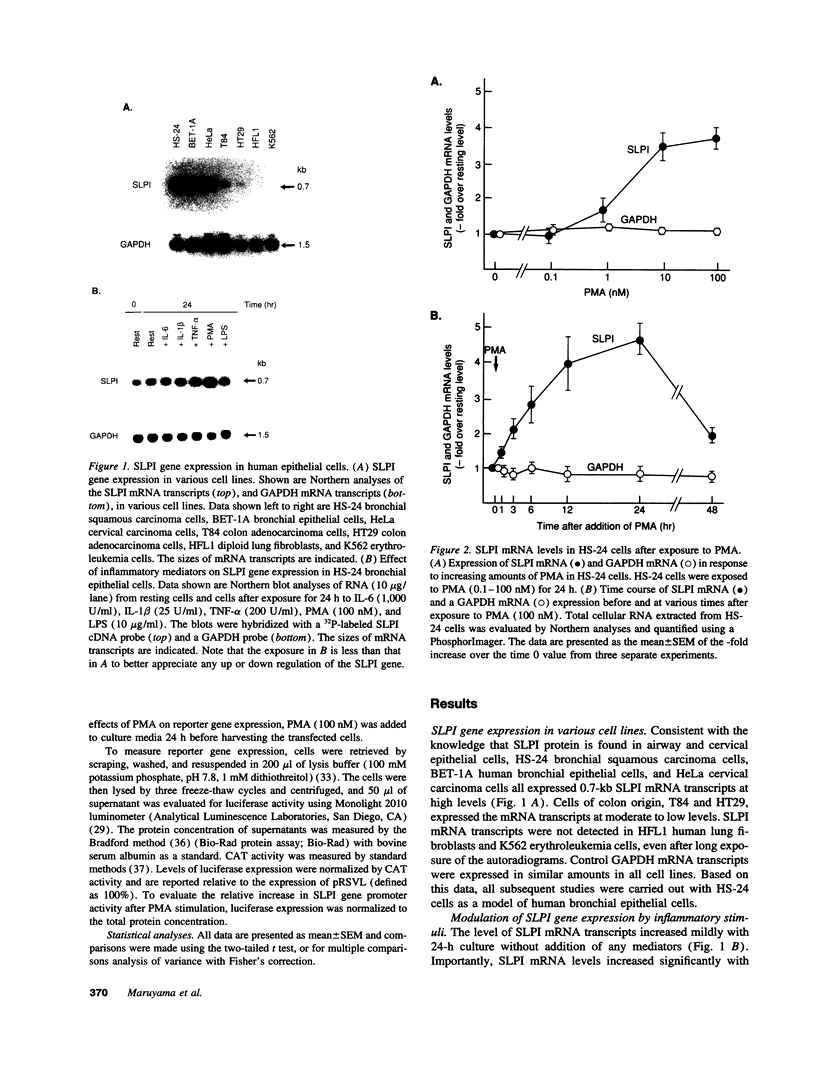
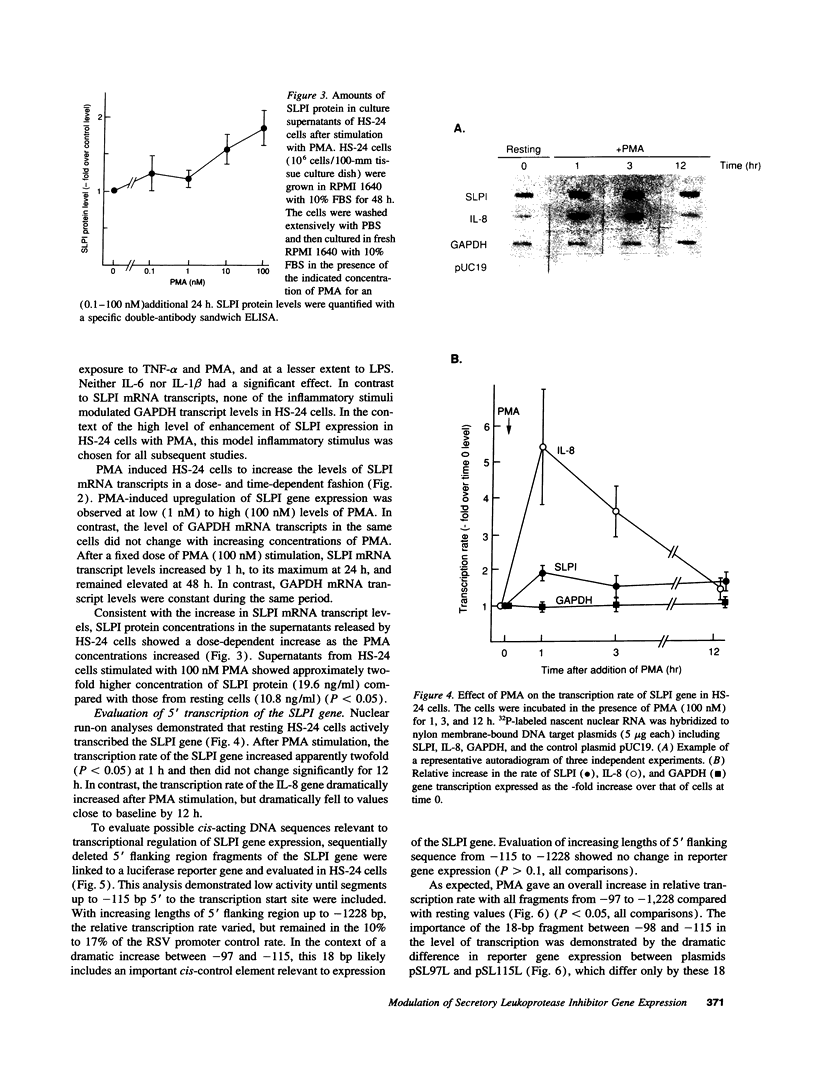
Secretory leukoprotease inhibitor (SLPI), a 12-kD nonglycosylated serine antiprotease, helps to protect the epithelial surface of the airways from the destructive capacity of neutrophil elastase. Based on the recognition that SLPI levels can increase in the presence of airway inflammation, we hypothesized that inflammatory stimuli should modulate the expression of the SLPI gene in airway epithelial cells. To evaluate this, the modulation of SLPI gene expression with various inflammatory stimuli was evaluated in the HS-24 human bronchial epithelial cell line. After preliminary studies showed that several inflammatory mediators enhanced SLPI messenger RNA (mRNA) levels, PMA was used as a model inflammatory stimulus. PMA significantly increased the level of 0.7-kb SLPI mRNA transcripts in HS-24 cells in a dose- and time-dependent fashion and increased the amount of SLPI protein in the culture supernatant. Nuclear run-on analyses showed that the SLPI gene transcription rate increased approximately twofold after PMA stimulation. Transfection studies using fusion genes composed of fragments of up to 1.2 kb of the 5' flanking sequence of the SLPI gene and a luciferase reporter gene demonstrated potent promoter activity in the 131-bp segment (-115 to +16 relative to the transcription start site), and all longer segments up to 1.2 kb, whereas smaller segments showed low promoter activity. An 18-bp element (-98 to -115), in a region with homology to PMA-responsive regions in the Moloney murine leukemia virus enhancer and the IL-8 gene, was shown to be of importance in the level of transcription of the SLPI gene. However, this element was not responsible for the upregulation of SLPI gene expression by PMA. Evaluation of HS-24 cells in the presence of actinomycin D demonstrated that SLPI mRNA transcripts were very stable and became more so in the presence of PMA. Thus, SLPI gene expression in airway epithelial cells can be upregulated by an inflammatory stimulus, and this modulation is regulated at both the transcriptional and posttranscriptional levels. These mechanisms of SLPI upregulation likely play a role in defending the epithelial surface in the local milieu of inflammatory lung diseases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Kobayashi N., Yoshimura K., Trapnell B. C., Kim H., Hubbard R. C., Brewer M. T., Thompson R. C., Crystal R. G. Expression of the secretory leukoprotease inhibitor gene in epithelial cells. J Clin Invest. 1991 Jun;87(6):2207–2215. doi: 10.1172/JCI115255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Appelhans B., Ender B., Sachse G., Nikiforov T., Appelhans H., Ebert W. Secretion of antileucoprotease from a human lung tumor cell line. FEBS Lett. 1987 Nov 16;224(1):14–18. doi: 10.1016/0014-5793(87)80413-1. [DOI] [PubMed] [Google Scholar]
- Aronsen K. F., Ekelund G., Kindmark C. O., Laurell C. B. Sequential changes of plasma proteins after surgical trauma. Scand J Clin Lab Invest Suppl. 1972;124:127–136. doi: 10.3109/00365517209102760. [DOI] [PubMed] [Google Scholar]
- Bargon J., Trapnell B. C., Yoshimura K., Dalemans W., Pavirani A., Lecocq J. P., Crystal R. G. Expression of the cystic fibrosis transmembrane conductance regulator gene can be regulated by protein kinase C. J Biol Chem. 1992 Aug 15;267(23):16056–16060. [PubMed] [Google Scholar]
- Berger M., Sorensen R. U., Tosi M. F., Dearborn D. G., Döring G. Complement receptor expression on neutrophils at an inflammatory site, the Pseudomonas-infected lung in cystic fibrosis. J Clin Invest. 1989 Oct;84(4):1302–1313. doi: 10.1172/JCI114298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cromwell O., Hamid Q., Corrigan C. J., Barkans J., Meng Q., Collins P. D., Kay A. B. Expression and generation of interleukin-8, IL-6 and granulocyte-macrophage colony-stimulating factor by bronchial epithelial cells and enhancement by IL-1 beta and tumour necrosis factor-alpha. Immunology. 1992 Nov;77(3):330–337. [PMC free article] [PubMed] [Google Scholar]
- Crystal R. G. Alpha 1-antitrypsin deficiency, emphysema, and liver disease. Genetic basis and strategies for therapy. J Clin Invest. 1990 May;85(5):1343–1352. doi: 10.1172/JCI114578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal R. G. The alpha 1-antitrypsin gene and its deficiency states. Trends Genet. 1989 Dec;5(12):411–417. doi: 10.1016/0168-9525(89)90200-x. [DOI] [PubMed] [Google Scholar]
- Deng W. P., Nickoloff J. A. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal Biochem. 1992 Jan;200(1):81–88. doi: 10.1016/0003-2697(92)90280-k. [DOI] [PubMed] [Google Scholar]
- Dickson I., Alper C. A. Changes in serum proteinase inhibitor levels following bone surgery. Clin Chim Acta. 1974 Aug 20;54(3):381–385. doi: 10.1016/0009-8981(74)90257-5. [DOI] [PubMed] [Google Scholar]
- Ercolani L., Florence B., Denaro M., Alexander M. Isolation and complete sequence of a functional human glyceraldehyde-3-phosphate dehydrogenase gene. J Biol Chem. 1988 Oct 25;263(30):15335–15341. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Franken C., Meijer C. J., Dijkman J. H. Tissue distribution of antileukoprotease and lysozyme in humans. J Histochem Cytochem. 1989 Apr;37(4):493–498. doi: 10.1177/37.4.2926127. [DOI] [PubMed] [Google Scholar]
- Fritz H. Human mucus proteinase inhibitor (human MPI). Human seminal inhibitor I (HUSI-I), antileukoprotease (ALP), secretory leukocyte protease inhibitor (SLPI). Biol Chem Hoppe Seyler. 1988 May;369 (Suppl):79–82. [PubMed] [Google Scholar]
- Fryksmark U., Ohlsson K., Polling A., Tegner H. Distribution of antileukoprotease in upper respiratory mucosa. Ann Otol Rhinol Laryngol. 1982 May-Jun;91(3 Pt 1):268–271. doi: 10.1177/000348948209100308. [DOI] [PubMed] [Google Scholar]
- Fryksmark U., Prellner T., Tegner H., Ohlsson K. Studies on the role of antileukoprotease in respiratory tract diseases. Eur J Respir Dis. 1984 Apr;65(3):201–209. [PubMed] [Google Scholar]
- Gauthier F., Fryksmark U., Ohlsson K., Bieth J. G. Kinetics of the inhibition of leukocyte elastase by the bronchial inhibitor. Biochim Biophys Acta. 1982 Jan 18;700(2):178–183. doi: 10.1016/0167-4838(82)90095-4. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hansen S. K., Nerlov C., Zabel U., Verde P., Johnsen M., Baeuerle P. A., Blasi F. A novel complex between the p65 subunit of NF-kappa B and c-Rel binds to a DNA element involved in the phorbol ester induction of the human urokinase gene. EMBO J. 1992 Jan;11(1):205–213. doi: 10.1002/j.1460-2075.1992.tb05043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Kida K., Mizuuchi T., Takeyama K., Hiratsuka T., Jinno S., Hosoda K., Imaizumi A., Suzuki Y. Serum secretory leukoprotease inhibitor levels to diagnose pneumonia in the elderly. Am Rev Respir Dis. 1992 Dec;146(6):1426–1429. doi: 10.1164/ajrccm/146.6.1426. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Liu J. M., Fujii H., Green S. W., Komatsu N., Young N. S., Shimada T. Indiscriminate activity from the B19 parvovirus p6 promoter in nonpermissive cells. Virology. 1991 May;182(1):361–364. doi: 10.1016/0042-6822(91)90682-2. [DOI] [PubMed] [Google Scholar]
- Lund L. R., Rønne E., Roldan A. L., Behrendt N., Rømer J., Blasi F., Danø K. Urokinase receptor mRNA level and gene transcription are strongly and rapidly increased by phorbol myristate acetate in human monocyte-like U937 cells. J Biol Chem. 1991 Mar 15;266(8):5177–5181. [PubMed] [Google Scholar]
- Mattoli S., Colotta F., Fincato G., Mezzetti M., Mantovani A., Patalano F., Fasoli A. Time course of IL1 and IL6 synthesis and release in human bronchial epithelial cell cultures exposed to toluene diisocyanate. J Cell Physiol. 1991 Nov;149(2):260–268. doi: 10.1002/jcp.1041490212. [DOI] [PubMed] [Google Scholar]
- McElvaney N. G., Hubbard R. C., Birrer P., Chernick M. S., Caplan D. B., Frank M. M., Crystal R. G. Aerosol alpha 1-antitrypsin treatment for cystic fibrosis. Lancet. 1991 Feb 16;337(8738):392–394. doi: 10.1016/0140-6736(91)91167-s. [DOI] [PubMed] [Google Scholar]
- McElvaney N. G., Nakamura H., Birrer P., Hébert C. A., Wong W. L., Alphonso M., Baker J. B., Catalano M. A., Crystal R. G. Modulation of airway inflammation in cystic fibrosis. In vivo suppression of interleukin-8 levels on the respiratory epithelial surface by aerosolization of recombinant secretory leukoprotease inhibitor. J Clin Invest. 1992 Oct;90(4):1296–1301. doi: 10.1172/JCI115994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooren H. W., Kramps J. A., Franken C., Meijer C. J., Dijkman J. A. Localisation of a low-molecular-weight bronchial protease inhibitor in the peripheral human lung. Thorax. 1983 Mar;38(3):180–183. doi: 10.1136/thx.38.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaida N., Mahe Y., Matsushima K. Cooperative interaction of nuclear factor-kappa B- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J Biol Chem. 1990 Dec 5;265(34):21128–21133. [PubMed] [Google Scholar]
- Nakamura H., Yoshimura K., Jaffe H. A., Crystal R. G. Interleukin-8 gene expression in human bronchial epithelial cells. J Biol Chem. 1991 Oct 15;266(29):19611–19617. [PubMed] [Google Scholar]
- Nakamura H., Yoshimura K., McElvaney N. G., Crystal R. G. Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. J Clin Invest. 1992 May;89(5):1478–1484. doi: 10.1172/JCI115738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson K., Sveger T., Svenningsen N. Protease inhibitors in bronchoalveolar lavage fluid from neonates with special reference to secretory leukocyte protease inhibitor. Acta Paediatr. 1992 Oct;81(10):757–759. doi: 10.1111/j.1651-2227.1992.tb12097.x. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Dinarello C. A., Punsal P. I., Colten H. R. Cachectin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986 Nov;78(5):1349–1354. doi: 10.1172/JCI112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., May L. T., Sehgal P. B. Interferon beta 2/interleukin 6 modulates synthesis of alpha 1-antitrypsin in human mononuclear phagocytes and in human hepatoma cells. J Clin Invest. 1989 Jul;84(1):138–144. doi: 10.1172/JCI114133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddel R. R., Ke Y., Gerwin B. I., McMenamin M. G., Lechner J. F., Su R. T., Brash D. E., Park J. B., Rhim J. S., Harris C. C. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988 Apr 1;48(7):1904–1909. [PubMed] [Google Scholar]
- Rice W. G., Weiss S. J. Regulation of proteolysis at the neutrophil-substrate interface by secretory leukoprotease inhibitor. Science. 1990 Jul 13;249(4965):178–181. doi: 10.1126/science.2371565. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröter H., Shaw P. E., Nordheim A. Purification of intercalator-released p67, a polypeptide that interacts specifically with the c-fos serum response element. Nucleic Acids Res. 1987 Dec 23;15(24):10145–10158. doi: 10.1093/nar/15.24.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Smith S. F., Guz A., Winning A. J., Cooke N. T., Burton G. H., Tetley T. D. Comparison of human lung surface protein profiles from the central and peripheral airways sampled using two regional lavage techniques. Eur Respir J. 1988 Oct;1(9):792–800. [PubMed] [Google Scholar]
- Speck N. A., Renjifo B., Hopkins N. Point mutations in the Moloney murine leukemia virus enhancer identify a lymphoid-specific viral core motif and 1,3-phorbol myristate acetate-inducible element. J Virol. 1990 Feb;64(2):543–550. doi: 10.1128/jvi.64.2.543-550.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler G., Brewer M. T., Thompson R. C. Isolation and sequence of a human gene encoding a potent inhibitor of leukocyte proteases. Nucleic Acids Res. 1986 Oct 24;14(20):7883–7896. doi: 10.1093/nar/14.20.7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. C., Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6692–6696. doi: 10.1073/pnas.83.18.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi M. F., Zakem H., Berger M. Neutrophil elastase cleaves C3bi on opsonized pseudomonas as well as CR1 on neutrophils to create a functionally important opsonin receptor mismatch. J Clin Invest. 1990 Jul;86(1):300–308. doi: 10.1172/JCI114699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban M. B., Schreck R., Baeuerle P. A. NF-kappa B contacts DNA by a heterodimer of the p50 and p65 subunit. EMBO J. 1991 Jul;10(7):1817–1825. doi: 10.1002/j.1460-2075.1991.tb07707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakalopoulou E., Schaack J., Shenk T. A 32-kilodalton protein binds to AU-rich domains in the 3' untranslated regions of rapidly degraded mRNAs. Mol Cell Biol. 1991 Jun;11(6):3355–3364. doi: 10.1128/mcb.11.6.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelmeier C., Buhl R., Hoyt R. F., Wilson E., Fells G. A., Hubbard R. C., Schnebli H. P., Thompson R. C., Crystal R. G. Aerosolization of recombinant SLPI to augment antineutrophil elastase protection of pulmonary epithelium. J Appl Physiol (1985) 1990 Nov;69(5):1843–1848. doi: 10.1152/jappl.1990.69.5.1843. [DOI] [PubMed] [Google Scholar]
- Vogelmeier C., Hubbard R. C., Fells G. A., Schnebli H. P., Thompson R. C., Fritz H., Crystal R. G. Anti-neutrophil elastase defense of the normal human respiratory epithelial surface provided by the secretory leukoprotease inhibitor. J Clin Invest. 1991 Feb;87(2):482–488. doi: 10.1172/JCI115021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B., Horiguchi J., Luebbers R., Sherman M., Kufe D. Posttranscriptional stabilization of c-fms mRNA by a labile protein during human monocytic differentiation. Mol Cell Biol. 1989 Feb;9(2):769–775. doi: 10.1128/mcb.9.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Wertheimer S. J., Myers C. L., Wallace R. W., Parks T. P. Intercellular adhesion molecule-1 gene expression in human endothelial cells. Differential regulation by tumor necrosis factor-alpha and phorbol myristate acetate. J Biol Chem. 1992 Jun 15;267(17):12030–12035. [PubMed] [Google Scholar]
- Yamato K., el-Hajjaoui Z., Koeffler H. P. Regulation of levels of IL-1 mRNA in human fibroblasts. J Cell Physiol. 1989 Jun;139(3):610–616. doi: 10.1002/jcp.1041390322. [DOI] [PubMed] [Google Scholar]
- Yasumoto K., Okamoto S., Mukaida N., Murakami S., Mai M., Matsushima K. Tumor necrosis factor alpha and interferon gamma synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-kB-like binding sites of the interleukin 8 gene. J Biol Chem. 1992 Nov 5;267(31):22506–22511. [PubMed] [Google Scholar]
- Yoshimura K., Nakamura H., Trapnell B. C., Dalemans W., Pavirani A., Lecocq J. P., Crystal R. G. The cystic fibrosis gene has a "housekeeping"-type promoter and is expressed at low levels in cells of epithelial origin. J Biol Chem. 1991 May 15;266(14):9140–9144. [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]