In both rats and dogs, the immunosuppressive drugs cyclosporine (CyA) and FK 506 augment liver regeneration and possess other hepatotrophic qualities. 1–4 In contrast, rapamycin (RPM), a powerful immunosuppressant that is chemically related to FK 506 but targeted to a different stage of T-cell activation,5,6 was recently shown to have antiproliferative properties, including inhibition of regeneration of the livers, as well as of the intestine and kidney.7,8 However, there is a paucity of information about the influence of other immunosuppressive drugs on growth and regeneration. Using in vivo (partial hepatectomy and portacaval shunt) and in vitro (tissue culture) experimental models described elsewhere,2–4,8–10 we have investigated the effect on hepatocyte proliferation of methylprednisolone (MP), mycophenolic acid (MPA), mizoribine (MZ), azathioprine (AZA), and prostaglandin E1 (PGE1).
MATERIALS AND METHODS
In Vitro Study
Hepatocytes in Primary Culture
The livers were removed from 7-week-old male rats (Fischer 344 purchased from Hilltop Lab Animals Inc, Scottdale, Pa) weighing 180 to 200 g. Hepatocytes were isolated by a modification of the in situ two-step collagenase perfusion technique of Seglen and Jirtle et al. 11,12 The hepatocytes were dispersed and washed twice with cold Ca2+ free perfusion buffer and resuspended in basal medium (MEM) supplemented with pyruvate (1 mmol/L), proline (0.26 mmol/L), insulin (10−7 mol/L) and 5% fetal calf serum. Viability was determined by Trypan blue exclusion, and only preparations having >90% viability at the outset were used. Cell number was determined with a hemocytometer. The cells were plated at a cell density of 6.5 × 104 per well in Corning 35 mm tissue culture dishes (Corning, NY) containing 1.5 mL medium and maintained at 37°C in a 5% CO2 atmosphere. After a 3-hour attachment period, the medium was aspirated and 1.5 mL MEM containing epidermal growth factor (EGF) and insulin at concentrations of 10 ng/mL and 10−7 mol/L, respectively, were added. The substances were dissolved in DMSO (MPA), saline (MZ), or ethanol (PGE1, MP, AZA) and added in the appropriate concentrations. The amount of alcohol or DMSO added to the medium was never more than 2 μL/mL, which does not affect hepatocyte proliferation.
In Vitro [3H] Thymidine Incorporation
To determine in vitro DNA synthesis, 3 μCi[3H]thymidine (Dupont New England Nuclear Research Products, Boston, Mass) was added to each well and maintained for 24–48 hours of the culture period. When the cells were harvested, DNA content was determined by the microfluorometric method of Setara and Morley,13 and DNA synthesis was measured by the method of Michelopoulos et al.14
In Vivo Study
70% Partial Hepatectomy: Rat Model
Rats similar to those used in the in vitro experiments were assigned to groups and treated for 4 days as controls or with drugs (Table 1). On the 4th day, between 0900 and 1030 hours, the rats received a standard 70% hepatectomy under light ether anesthesia. Food and drink were allowed immediately. Parenteral fluid and electrolyte support were not required.
Table 1.
Regimens
| Group | Drugs | Dose Used (mg/kg/dose) | Route | Vehicle |
|---|---|---|---|---|
| 1 (n = 5) | — | — | IM | Saline |
| 2 (n = 10) | MP | 1 | IM | Saline |
| 3 (n = 5) | — | — | IM | Saline |
| 4 (n = 10) | MZ | 20 | IM | Saline |
| 5 (n = 5) | — | — | PO | 0.5% CMC; 0.4% Tween 0.9 alcohol in saline |
| 6 (n = 10) | MPA | 15 | PO | 0.5% CMC; 0.4% Tween 0.9% alcohol in saline |
| 7 (n = 5) | — | — | IM | 1% CMC in saline |
| 8 (n = 10) | AZA | 6 | IM | 1% CMC in saline |
| 9 (n = 5) | — | — | IM | 10% ethanol in saline |
| 10 (n = 10) | PGE1 | 0.2 | IM | 10% ethanol in saline |
MP and MZ were dissolved in saline; MPA was dissolved in 0.5% carbossil-methil-cellulose (CMC), 0.4% Tween, and 0.9% alcohol in saline; AZA was mixed with olive oil or dissolved with 1% CMC in saline; PGE1 was dissolved in alcohol and diluted 10 times with saline.
Twenty-four hours after the hepatectomies, 185 × 10−4 Bq [3H] thymidine was administered to all rats by intraperitoneal injection. The rats were killed 2 hours later by guillotine. Extraction and purification of hepatic DNA were accomplished by the method of Ove et al15 and DNA content was measured with calf thymus DNA (Sigma, St Louis, Mo) as the standard. 16 Specimens from each liver were prepared for histological examination with hematoxylin-eosin and the proportion of labeled hepatocytes was counted.
Portacaval Shunt: Dog Model
Conditioned female beagle dogs underwent a functional end-to-side portacaval shunt (PCS) as previously described.4,9,10 PGE1 was dissolved daily in vehicle solution (5 mmol/L ammonium acetate, 5 mg/L bovine serum albumin in saline) and infused for 4 days into the left branch of the portal vein.
At day 4, 0.2 mCi/kg of intravenous (IV) [3H] thymidine was given with a specific activity of 80–90 Ci/mmol. Two hours later, while the dogs were under sodium pentobarbital anesthesia, specimens were taken from left and right lobes of the liver and fixed in 10% normal buffered formalin. The dogs were killed with an IV bolus of potassium chloride.
Hepatocyte size and organelle structure were quantitated, and proliferation was estimated by nuclear thymidine incorporation (classical autoradiography). These parameters were compared in the left (treated) vs the right (untreated) lobes.
Statistical Analysis
Data were reported as mean ± SD. Student’s one-tailed t test was used to determine the significance of differences. A P value <.05 was considered significant.
RESULTS
In Vitro Hepatocyte Viability
MP A and MZ that were added after a 3-hour attachment period did not alter the DNA or AL T concentration in the medium after 48 hours of incubation (data not shown). In contrast, AZA killed the hepatocytes, making continuation of the experiment impossible.
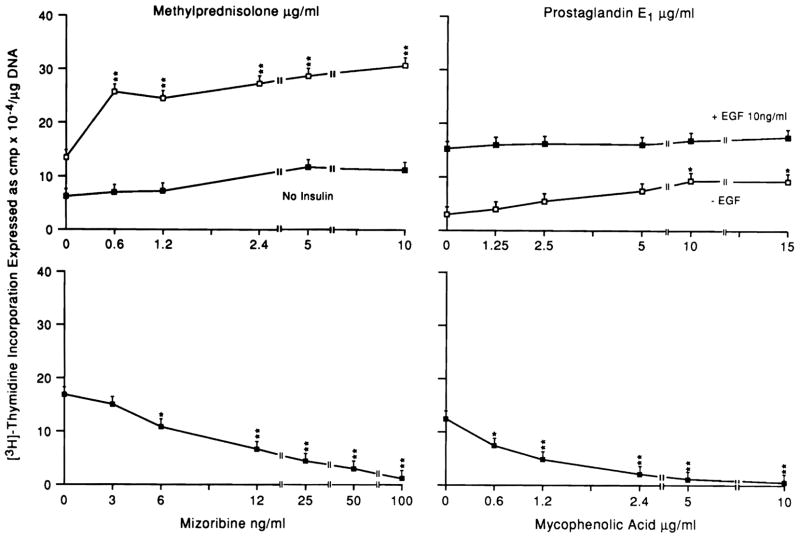
Figure 1 shows the effect in vitro on hepatocyte proliferation maintained for 48 hours in the presence of MP, MPA, MZ, and PGE1. MZ and MPA inhibited DNA synthesis, whereas MP caused a near doubling of thymine incorporation. PGE1 feebly stimulated hepatocyte proliferation at the higher concentration. This effect was overlapped by EGF stimulation.
Fig 1.
Effect of different doses of MP, PGE1 MZ, and MPA on DNA synthesis in hepatocytes cultured in the presence of EGF (10 ng/mL). In some experiments, MP was tested in the absence of insulin, and PGE1 in the absence of EGF. N = 9 for all data points, which represent three experiments with triplicate determinations. Data expressed as mean ± SD. *P <.05; **P <.01.
In Vivo Results
Table 2 reports the results obtained in the experiment in vivo using the 70% partial hepatectomy rat model.
Table 2.
Effects of MP, MZ, MPA, AZA, and PGE1 on Liver Regeneration in 70% Partial Hepatectomy Rat Model
| Group | Substances | Dose Used (mg/kg/dose) | Route | DNA (cpm/mg × 10−3) | Percent of Labeled Nuclei |
|---|---|---|---|---|---|
| 1 (n = 5) | Vehicle | — | IM | 91 ± 8 | 18 ± 3 |
| 2 (n = 10) | MP | 1 | IM | 153 ± 11* | 39 ± 4* |
| 3 (n = 5) | Vehicle | — | IM | 96 ± 9 | 18 ± 2 |
| 4 (n = 10) | MZ | 20 | IM | 17 ± 4* | 6 ± 2* |
| 5 (n = 5) | Vehicle | — | PO | 66 ± 5 | 17 ± 3 |
| 6 (n = 10) | MPA | 15 | PO | 63 ± 6 | 16 ± 4 |
| 7 (n = 5) | Vehicle | — | IM | 59 ± 4 | 16 ± 3 |
| 8 (n = 10) | AZA | 6 | IM | 56 ± 5 | 15 ± 3 |
| 9 (n = 5) | Vehicle | — | IM | 88 ± 7 | 17 ± 2 |
| 10 (n = 10) | PGE1 | 0.2 | IM | 96 ± 10 | 18 ± 3 |
P <.05 vs their own control group.
After partial hepatectomy, the different vehicle solutions showed considerable variability in liver regeneration. It suggested the need for concurrent controls for each test group.
The only drug that significantly augmented regeneration relative to the controls was MP. The only drug that significantly inhibited regeneration was MZ (Table 2). The MZ inhibition was profound, reducing DNA synthesis and the labeled nuclei rate. MPA, AZA, and PGE1 had no effect on regeneration in the whole animal (Table 2).
In contrast, PGE1 was found profoundly active when infused continuously for 4 days into the left branch of the portal vein in the PCS dog model (Table 3).
Table 3.
Hepatocyte Size and Autoradiographic Labeling After Continuous Infusion of Different Doses of PGE1 Into the Left Portal Vein Branch of Dogs With Eck’s Fistula
| Group | N | Dose (μg/d) | No. of Labeled Hepatocytes per 1,000 Hepatocytes |
Cell Size (U) |
||
|---|---|---|---|---|---|---|
| Left lobe | Right lobe | Left lobe | Right lobe | |||
| 1 | 2 | 4.8 | 12.5 ± 0.5* | 5.1 ± 0.7 | 0.158 ± 0.01* | 0.087 ± 0.007 |
| 2 | 2 | 0.48 | 10 ± 0.3* | 3.9 ± 0.3 | 0.161 ± 0.005* | 0.100 ± 0.005 |
| 3 | 2 | 0.24 | 6 ± 0.2* | 3.8 ± 0.1 | 0.131 ± 0.004* | 0.095 ± 0.009 |
| 4 | 2 | 0.048 | 5.1 ± 0.3 | 4.6 ± 0.4 | 0.104 ± 0.002 | 0.099 ± 0.004 |
P <.05, left lobe vs right lobe.
CONSIDERATIONS
AZA, MPA, and MZ are cytotoxic agents. They act by selectively inhibiting the synthesis of purine nucleotides (adenine for AZA and guanine for MPA and MZ), thereby reducing DNA synthesis of a variety of immunologic and other specialized cells, including hepatocytes.
In accord with this, our in vitro results show both an inhibitory effect of MPA and MZ and a toxic one of AZA. In in vivo experiments, only MZ confirmed the inhibition found in vitro, whereas AZA and MPA at the doses used did not affect liver regeneration in rats after 70% partial hepatectomy.
The inhibition of hepatocyte proliferation by cytotoxic drugs is consistent with previous reports about AZA.17 A seemingly obvious explanation could be that AZA, MPA, and MZ selectively inhibit synthesis of purine nucleotides, which are required for DNA synthesis.
MP augmented liver regeneration in intact animals, and caused a striking increase in hepatocyte proliferation in culture. The absence of insulin in the medium drastically reduces MP stimulation to a level that is no longer significant (Figure 1).
MP is known to inhibit the synthesis and expression of multiple cytokines, including IL-1, IL-2, and migration inhibitor factor.18,19 Although IL-1 and IL-2 are thought from reported in vitro experiments to be growth suppres-sors,20 this could not be demonstrated by our laboratory-sensitive in vivo test system, in which the recombinant cytokines in question were infused directly into the tied off portal vein of the Eck fistula liver.21 Thus, the proliferative response to MP reported herein both in vivo and in vitro cannot be explained with what is currently known about steroid actions.
PGE1 has been successfully used in the therapy of posttransplant patients, as well as in the therapy of fulminant hepatic failure (FHF).22–24 The administration of this drug after transplantation may reduce immunorejection22,25 and drastically reverses primary graft nonfunction after orthotopic liver transplantation.23
We demonstrated that PGE1 has hepatotrophic qualities, as is well known for other PGS.26–28 It stimulated hepatocyte proliferation in both in vitro and in vivo models. However, a continuous and topic infusion of PGE1 seems necessary to stimulate liver regeneration. In fact, we were not able to obtain any proliferation in rats treated with only one daily injection for 4 days. Instead, when the drug was injected continuously in one lobe of the liver, we noted a stimulation just of the infused lobe. It could mean that after the PGE1 passes through the liver it is much too diluted to stimulate the noninfused lobe or that it is promptly degradated as soon as it arrives at the lung.29
It is not possible to explain the growth effects of PGE1 by the well-known properties of this drug. Recently, a linkage has been proposed between TGFα and PG.28 It seems that TGFα may induce hepatocyte proliferation in vitro by regulating the metabolism of arachidonic acid and the formation of prostaglandins. However, it is unlikely that the beneficial effect of PGE1 in the therapy of FHF is due to its growth qualities; in fact, as we described elsewhere30 it is not possible to reverse FHF by administration of liver growth factors. A more likely explanation for the therapeutic action of PGE1 could be the protection of the endothelial cells’ integrity and an improvement of liver blood flow.31,32
Acknowledgments
Supported by Research grants from the Veterans Administration and Project Grants DK 29961 and CA 35373 from the National Institutes of Health, Bethesda, Maryland.
References
- 1.Makowka L, Svanas G, Esquivel CO, et al. Surg Forum. 1986;37:353. [PMC free article] [PubMed] [Google Scholar]
- 2.Francavilla A, Barone M, Todo S, et al. Lancet. 1989;2:1248. doi: 10.1016/s0140-6736(89)91853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francavilla A, Starzl TE, Barone M, et al. Hepatology. 1991;14:140. doi: 10.1002/hep.1840140123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Porter KA, Mazzaferro V, et al. Transplantation. 1991;51:67. doi: 10.1097/00007890-199101000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dumont FJ, Staruch MK, Koprak SL, et al. J Immunol. 1990;144:251. [PubMed] [Google Scholar]
- 6.Bierer BE, Mattila PS, Standaert RF, et al. Proc Natl Acad Sci USA. 1990;87:9231. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francavilla A, Starzl TE, Scotti C, et al. Transplantation. 1992;53:496. doi: 10.1097/00007890-199202010-00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francavilla A, Carr B, Starzl TE, et al. Hepatology. 1992;15:871. doi: 10.1002/hep.1840150520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Porter KA, Putnam CW. Lancet. 1975;2:1241. doi: 10.1016/s0140-6736(75)92076-0. [DOI] [PubMed] [Google Scholar]
- 10.Starzl TE, Watanabe K, Porter KA, et al. Lancet. 1976;1:821. doi: 10.1016/s0140-6736(76)90477-3. [DOI] [PubMed] [Google Scholar]
- 11.Seglen PO. Methods Cell Biol. 1976;13:29. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 12.Jirtle RL, Michalopoulos G, McLaine JR, et al. Cancer Res. 1981;41:3512. [PubMed] [Google Scholar]
- 13.Setaro F, Morley CGD. Anal Biochem. 1976;71:313. doi: 10.1016/0003-2697(76)90043-9. [DOI] [PubMed] [Google Scholar]
- 14.Michalopoulos G, Cianciulli HD, Novotny R, et al. Cancer Res. 1982;42:4673. [PubMed] [Google Scholar]
- 15.Ove P, Francavilla A, Coetzee M, et al. Liver and Hormones. New York: Raven; 1987. p. 265. [Google Scholar]
- 16.Burton K. Methods Enzymol. 1968;12:163. [Google Scholar]
- 17.Gonzalez EM, Krejczy K, Malt R. Surgery. 1970;68:254. [PubMed] [Google Scholar]
- 18.Kern JA, Lamb RJ, Reed JC, et al. J Clin Invest. 1988;81:237. doi: 10.1172/JCI113301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horst HJ, Flad HD. Exp Immunol. 1987;68:156. [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura T, Arakaki R, Ichihara A. Exp Cell Res. 1988;179:488. doi: 10.1016/0014-4827(88)90286-8. [DOI] [PubMed] [Google Scholar]
- 21.Francavilla A, Starzl TE, Porter K, et al. Hepatology. 1991;14:665. doi: 10.1016/0270-9139(91)90055-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mozes MF, Moran M, Ketel B, et al. Kidney Int. 1989;35:520. [Google Scholar]
- 23.Greig PD, Woolf GM, Abecassis M, et al. Transplant Proc. 1989;21:2385. [PubMed] [Google Scholar]
- 24.Sinclair S, Levy G. Ital J Gastro. 1990;22:205. [PubMed] [Google Scholar]
- 25.Pollak R, Dumble LJ, Wiederkehr JC, et al. Transplantation. 1990;50:834. doi: 10.1097/00007890-199011000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Miura Y, Fukui N. Cell Mol Biol. 1979;25:179. [PubMed] [Google Scholar]
- 27.Andreis PG, Whitfield JF, Armato U. Exp Cell Res. 1981;134:265. doi: 10.1016/0014-4827(81)90425-0. [DOI] [PubMed] [Google Scholar]
- 28.Skouteris GG, McMenamin M. Biochem J. 1992;281:729. doi: 10.1042/bj2810729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anggard E, Samuelsson B. J Biol Chem. 1964;239:4097. [PubMed] [Google Scholar]
- 30.Francavilla A, Azzarone A, Carrieri G, et al. Hepatology. (submitted for publication) [Google Scholar]
- 31.Levy GA, Abecassis M. Rev Infect Dis. 1989;11:712. doi: 10.1093/clinids/11.Supplement_4.S712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson PD, Withrington PG. Br J Pharmacol. 1978;48:337. doi: 10.1111/j.1476-5381.1978.tb17320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]