Abstract
Smokers are highly reactive to smoking-related cues that are directly linked, or most proximal, to actual smoking behavior (e.g., lit cigarettes). However, over the course of smoking, proximal cues may not be the only stimuli to become strongly associated with smoking. Distal cues, such as the environments in which smoking occurs (e.g., bar) might also gain associative properties and come to evoke robust reactivity from smokers. To test this, a pilot study was first conducted to develop standard pictorial stimuli of smoking and nonsmoking environments, all of which were completely devoid of proximal smoking cues. A comparison set of smoking and nonsmoking proximal cues was then created. Using the 12 total pictorial cues developed, 62 adult smokers participated in a cue-reactivity study during which they viewed and rated pictorial smoking and nonsmoking environment and proximal cues. Results demonstrate that, similar to proximal cues, environments associated with smoking can alone function as stimuli capable of evoking strong subjective reactivity from smokers. This work supports a broader conceptualization of drug-related cues in cue-based research and treatment development that includes proximal and distal cues as distinct categories.
Keywords: craving, cue reactivity, proximal and distal stimuli, environments, smoking
Cue-based research offers a wealth of information regarding how smokers respond to smoking-related stimuli, or cues, associated with past cigarette use. Studies aimed at eliciting responses to such cues in smokers typically employ a paradigm in which smokers are exposed to smoking- and nonsmoking-related stimuli through in vivo (e.g., watching someone smoke), imaginal (e.g., imagining smoking scenes), audio (e.g., hearing a description of smoking), and less commonly video (e.g., seeing a film of someone smoking), and/or pictorial (e.g., viewing pictures of smoking-related paraphernalia) stimulus modes. On balance this research has revealed that, regardless of presentation mode, smokers consistently show robust subjective responding and moderate physiological reactivity to smoking-related cues (Carter & Tiffany, 1999).
The cues typically targeted in cue-reactivity studies are those most proximal to drug administration (e.g., in the case of smoking, stimuli such as viewing or handling a lit cigarette, ashtrays, or lighters). Such cues are most often chosen because they are presumably ubiquitous across the smoking experience. Thus, it is advantageous to use proximal cues for investigating the impact of conditioned drug stimuli, as smokers’ reliable reactivity to such cues reduces within-group variability, making the effects of within- and between-group manipulations more readily observable. Despite this advantage, when conditioning occurs, it is not only proximal cues that may come to be associated with smoking. An array of cues more distal to actual drug administration (e.g., the environments in which smoking occurs), may also become strongly linked with smoking.
Distal cues can be conceptualized as stimuli that have been regularly present during drug use, but, unlike proximal cues, are not directly linked to actual drug administration. For example, key distal cues include the environments in which smoking typically occurs and the people around whom one most often smokes (Conklin, 2006). Whereas proximal cues, such as cigarettes and lighters, are integral to the drug use ritual, distal cues are less reliably linked to actual drug administration. Although smokers can easily identify salient distal cues, such as the environments or people that are regularly present when they smoke (Conklin & Tiffany, 2001), they also smoke in the absence of those cues. Most smokers have also been in smoking-related environments and occasionally not being able to smoke, or have chosen to wait before actually smoking. In contrast, smokers rarely hold or light cigarettes (a key proximal cue) for any length of time without smoking or preparing to smoke. In general, proximal cues reliably predict smoking, whereas distal cues are more variably predictive. Still, it may be the case that distal cues, like proximal cues, can come to be strongly associated with smoking. Using environments associated with smoking as the distal cue of interest, the present study aimed to determine the associative strength of that key distal smoking cue.
The notion that cues other than those most proximally linked to drug administration (i.e., distal cues) might come to gain associative control over conditioned responding was first introduced by Wikler (1965/1984). Wikler observed that previously drug-dependent individuals experienced “abstinence distress” when they encountered environments and/or situations similar to those experienced during their active drug use. Wikler’s patients anecdotally reported that their reactions upon returning, or even just nearing, environments in which they once used drugs, motivated their resumption of drug seeking (Wikler, 1965/1984). These early observations, although not an empirical test, suggest support for the notion that distal cues, particularly environments, might become strongly associated with drug use to the extent that, like proximal cues, they can evoke robust drug urges.
Examining the impact of distal cues, such as environments, on cue responding is an infrequent practice across cue-reactivity research. When such studies are conducted, distal cues (most often environments or other people) typically occur jointly with proximal cues. This method, while important in presenting a potentially more naturalistic exposure to proximal cues, essentially creates a compound stimulus that prevents examination of the independent contribution of the distal cues. One such combined cue study was conducted by Wall, McKee, and Hinson (2000). The authors examined the impact of environmental setting (bar vs. laboratory) on college students’ alcohol outcome expectancies. They found that students who drank in a bar setting reported greater disinhibition and stimulation/perceived dominance as a function of environmental setting (Wall et al., 2000). The authors note, however, that the bar setting also contained a number of uncontrolled stimuli (e.g., other patrons drinking and talking) and the environment was always tested with the proximal alcohol cue. Therefore, conclusions regarding the independent effects of environment alone on alcohol expectations and ingestion were not possible. Other research incorporating exposure to naturalistic environments such as drug-buying corners (Kasvikis, Bradley, Powell, Marks, & Gray, 1991; Dawe et al., 1993) have met with similar limitations in controlling stimuli and parsing out the effect of environment exposure from other stimuli present during the cue-reactivity session.
Understanding how distal cues function independent of proximal cues has important implications for smoking cessation and relapse avoidance. If distal cues are capable of alone evoking excitatory responses from smokers, treatments that instruct smokers to eliminate exposure to key proximal drug cues, or that provide extinction training only for proximal cues, are likely inadequate, as distal cues might motivate smoking when proximal cues are absent. Therefore, the present study was aimed at examining if distal cues, specifically environments associated with smoking but completely devoid of proximal cues, can alone function as salient stimuli in a cue reactivity paradigm; and, to compare distal cue reactivity with smokers’ responses to standard proximal cues. To accomplish this, we first conducted a pilot stimulus development study to create a set of pictorial smoking and nonsmoking environment cues, as well as comparable pictorial smoking and nonsmoking proximal cues. We then used those stimulus sets in a larger study to compare smokers’ subjective and physiological reactivity to proximal versus distal smoking and nonsmoking cues in a 2 cue (smoking and nonsmoking) × 2 type (distal and proximal) cue-reactivity study. A main effect of the cue variable was hypothesized such that smoking cues, regardless of type, would evoke stronger reactivity than nonsmoking cues. This effect should be robust for distal as well as proximal cues, demonstrating the potential influence of distal cues on smoking motivation.
Method
Pilot Study: Stimuli Development
Extensive stimulus development work was conducted to create a set of environment cues. In past work (Conklin & Tiffany, 2001), smokers were interviewed regarding the most common places in which they do and do not smoke. For the present study, we compiled a list from that earlier data of the eight most commonly reported smoking and eight nonsmoking environments. (See Table 1 for a list of the 16 environments tested.) Worth noting, at the time of the present study, no public smoking bans were in place in the city of Pittsburgh where the study was conducted. Thus, smokers were still free to smoke in all of the places identified by smokers in 2001 as being smoking-related (e.g., bars and restaurants.) In order to potentially enhance the realness of environment cues, a pictorial mode of cue presentation was used. Photographs of the 16 environments were taken from four angles each (two approaching the environment; two from within the environment). Importantly, we excluded any proximal smoking cues in the pictures (e.g., cigarettes, lighters, ashtrays) or other possible distal cues, for example, other people. The goal of this work was to determine if there are ubiquitous smoking and non-smoking-related environments. Moreover, if there are, we aimed to discover which environments are strongly associated with smoking (smoking environments) and which are not (nonsmoking environments), as indexed by smokers’ self-reported craving upon exposure to the pictorial cues. All research presented here was approved by the Institutional Review Board of the University of Pittsburgh.
Table 1.
Average Craving Rating for Each Pictorial Context
| Smoking | Craving mean (SD) | Neutral | Craving mean (SD) |
|---|---|---|---|
| Coffee shop | 60 (28) | Bookstore | 39 (31) |
| Bar | 59 (28) | Classroom | 39 (25) |
| Restaurant | 55 (29) | Office | 38 (26) |
| Bus stop | 54 (30) | Post Office | 37 (29) |
| Bench | 48 (29) | Store | 36 (27) |
| Ballpark | 48 (34) | Church | 35 (26) |
| Car | 47 (29) | Shower | 29 (26) |
| Gas station | 36 (27) | Gym | 27 (30) |
Sixteen smokers came into the laboratory for one cue-reactivity session during which they viewed and rated each of the 16 environment cues. (More specific details of the cue-reactivity procedure are given in the Procedure section for the larger study below.) These participants were on average 30.1 years of age, smoked 21.1 cigarettes per day, had been smoking 14.1 years, and had an average Fagerström Test of Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) score of 4.6 (range 2–9). Prior to the session, participants abstained from smoking for 6 hrs. During the session, participants viewed all 16 environment cues (four angles of each environment per cue trial) and completed self-report craving ratings following each trial. Two counterbalanced cue-orders were developed such that no more than two smoking or nonsmoking cues occurred consecutively and an equal number of smoking and nonsmoking cues occurred in the first and second half of the trials. In this initial pilot work, we were interested in examining the strength of smokers’ craving ratings for each environment and determining if there was a significant difference between ratings for smoking-related and nonsmoking-related environments.
The results showed an overall significant effect of craving on the cue manipulation (smoking vs. nonsmoking environment cues), t(15) = 4.1, p < .001, such that smoking-related environments evoked greater craving to smoke (M = 51) than nonsmoking environments (M = 35). This effect was quite robust regardless of several environments not functioning as initially anticipated. For example, a gas station led to very low craving ratings even though it was anecdotally identified in a previous study as being related to smoking. The opposite was true of a bookstore and classroom, which were both thought to be nonsmoking environments but led to higher craving than many of the smoking-related places. (See Table 1 for the mean craving rating and SD for each environment tested.)
In general, the results of the initial pilot study suggest that environments do vary in the strength of their association with smoking and their subsequent impact on craving. Furthermore, these early results demonstrated that smoking-related environments alone, completely devoid of proximal smoking cues, can elicit strong craving to smoke, while nonsmoking-related environments (e.g., shower) do not.
After developing effective pictorial cues of environments during this initial pilot work, we next created proximal stimuli to be used in a larger study of smokers’ reactivity to both cue types. Although past cue-reactivity work has identified specific proximal cues to which smokers are reliably reactive, we knew of no set of pictorial proximal stimuli that depicted cues taken from four angles, to match our environmental contextual cues, or on a neutral background in order to be completely devoid of distal cues. Therefore, we created a set of proximal cues including three smoking cues (i.e., cigarette in ashtray, lighter, and pack of cigarettes); all of which are common and reliable cues used in smoking cue-reactivity studies [See Brandon, Piasecki, Quinn, & Baker, 1995, for discussion of cues incorporated in past studies]) and three nonsmoking cues (i.e., pen & pad, lip balm, and bar of soap; chosen for their neutral properties, as well as their physical similarity to the smoking cues) each taken from four angles and shown on a cream colored background. This allowed for a more direct comparison of these two cue types, independent of one another, within our larger cue-reactivity study. We did not test the evocativeness of the proximal stimuli during piloting as we created pictorial representations of basic proximal cues that have been shown to work effectively across many past smoking cue studies (e.g., Carter & Tiffany, 1999; Field & Duka, 2004).
Study 2: Proximal Versus Distal Cue-Reactivity
After developing pictorial cues of salient smoking and nonsmoking environments and proximal cues, we next conducted a full cue-reactivity study aimed at examining smokers’ subjective and physiological reactivity to both types of cues, and potential differences in responding between the two (proximal vs. distal).
Stimuli Selection
Using the three smoking contexts that generated the strongest craving (i.e., bar, restaurant, and bus stop) and the three nonsmoking contexts that generated the least (i.e., church, shower, and gym), we compiled the set of standard smoking and nonsmoking environment cues to use as the distal stimuli in this larger cue-reactivity study. Worth noting is the decision not to use the coffee shop. Past research has shown that other psychoactive substances such as alcohol (Burton & Tiffany, 1997) and caffeine (e.g., Lane, 1996) can increase smokers’ cigarette cravings. Unlike the bar pictures in which all specific alcohol cues were eliminated, the coffee shop pictures included two cups of coffee that we did not edit out prior to the pilot study. For this reason, we decided not to use the coffee shop pictures in this main study.
The six environment cues were combined with the six proximal cues in a 12 trial cue-reactivity paradigm. To control for order effects, four counterbalanced orders were developed for cue presentation using the following rules: (1) three smoking and three nonsmoking cues had to occur in the first six and the last six trials, (2) three proximal and three distal cues had to occur in the first six and the last six trials, (3) a smoking or nonsmoking cue could not occur more than twice in a row, (4) a proximal or distal cue could not occur more than twice in a row, (5) each of the four cue types had to occur in each of the 12 positions an equal number of times across the four randomizations.
Participants
Sixty-two smokers (31 men and 31 women) were recruited for this study through local flyers and newspaper advertisements, which invited “healthy male and female smokers between the ages of 20–65 to participate in research study on smoking cues.” Participants were on average 34.4 (range 20–57) years of age, smoked 20. (range 15–40) cigarettes per day, had been smoking regularly for 19.5 (range 3–44) years, and scored 6.1 (range 3–9) on the FTND (Heatherton et al., 1991). Participants were told to remain abstinent for 6 hrs prior to the session, and a carbon monoxide (CO) air sample less than 13ppm was required for participation. The 13ppm CO level was based on a 50% reduction from the average baseline CO level of nondeprived participants who come into our laboratory for various studies and is similar to cutoffs used verify comparable durations of abstinence (e.g., Madden Bickel, 1999; Perkins et al., 2006). Three participants did not meet this criterion and were paid for their time and excused from the study. Participants’ average CO measurement was 8.7 ppm. Each participant was paid $40 for completing the study.
Apparatus
The presentation of the pictorial stimuli was controlled by Microsoft PowerPoint, 2000 (Microsoft Corporation; Red wood, WA) software on a Compaq Evo computer (Hewlett Packard Company; Palo Alto, CA) and displayed on a 22″ monitor (ViewSonic Corporation; Walnut, CA). Heart rate (HR) and skin conductance (SC) were the physiological data obtained throughout the session and were collected using BIOPAC physiological recording equipment (BioPac Systems; Goleta, CA). Pulse was recorded from a BIOPAC photoelectric pulse plethysmograph transducer attached the nondominant index finger. SC was recorded from two BIOPAC Ag/AgCl electrodes filled with isotonic gel and attached to the ring and middle fingers of the nondominant hand.
Self-Report Measures
Participants initially completed a series of self-report measures including a Smoking History Form that investigated past and current smoking patterns; the FTND; the Balanced Inventory of Desired Responding-Impression Management section (BIDR-IM; Paulhus, 1991), which al lows for investigation of possible associations between impression management and self-report measures; the four item Questionnaire of Smoking Urges (QSU-4; Carter Tiffany, 2001); the Diener and Emmons Mood Form (1984); and a visual analog scale of nicotine withdrawal (VAS; Hughes & Hatsukami, 1986). Furthermore, participants completed subjective ratings after each cue-exposure trial including: a four-item version of the QSU craving measure, a four-item relevance measure, as well as single item measures of vividness, positive and negative mood, and excitement and calmness. Each response form in structed the participant to answer the ratings based on how he or she felt while viewing the picture in that trial. The four QSU items included: (1) Nothing would have been better than smoking a cigarette, (2) I had an urge for a cigarette, (3) All I wanted right then was a cigarette, and (4) I craved a cigarette (Carter & Tiffany, 2001). The four relevance questions generally probed how relevant the stimulus (environment or object) was for the participant, and the extent to which he or she could imagine ever encountering it in real life. All ratings were done on a 100-point scale.
Procedure
After providing written informed consent, participants were asked how long it had been since their last cigarette and a CO measure was taken using a Vitalograph CO monitor (Vitalograph; Lenexa, KS). If participants met the <13ppm cutoff, they then completed the series of initial self-report forms (listed above under Self-report Measures). The experimenter then attached the two SC electrodes and the photoplethysmograph transducer to fingers on the participant’s nondominant hand.
Participants were then given an overview of the remainder of the session. After the instructions, participants underwent a neutral practice trial to ensure that they understood the format of the procedure. Following the practice trial, the experimenter dimmed the lights and left the room. Participants then completed 12 automated cue-exposure trials, which followed a standard format: 20-s relaxation, during which they were told to relax and silently repeat the word “one” to themselves, 20-s baseline, during which they sat quietly while baseline physiological measures were collected, 40-s picture viewing (10 s per photo angle) during which they had been instructed to focus on each picture and imagine that they were actually in the place pictured or came across the item they saw, and post-trial subjective ratings (described under Self-report Measures above). After filing out ratings forms, participants clicked a button that began the next trial, starting again with the 20-s relaxation period. After the final cue-exposure trial, the experimenter returned to the room, removed the monitors, and gave a debriefing before paying the participant.
Data Reduction and Analyses
An interactive editing program was used to eliminate artifacts from the pulse data and convert it to beat-per-minute HR. The SC signal was amplified at 10 Amho/V (0–100 Amho range) and bandpass-filtered online (1.0 to 0.05 Hz). All signals were digitized at 250 Hz, passed to a PC-based BIOPAC MP100 data acquisition workstation, and saved to disk. Mean HR and mean, area, and maximum SC, during each picture trial were quantified for two discrete periods: (1) baseline—the average of seconds 4–16 of the baseline period, (2) picture exposure—the average of seconds 3–36 of the picture viewing period. For each measure, deviation scores were computed by subtracting the baseline average from the picture exposure average for each trial.
To first verify the hypothesis that an excitatory response to the cue manipulation would be present for both distal and proximal stimuli, t tests were conducted for smoking versus nonsmoking craving report for the proximal and distal conditions separately. Next, the overall data analytic strategy focused on the impact of cue (Smoking, Nonsmoking) and type (Proximal, Distal) on self-report and physiological indices of reactivity using a 2 (type) × 2 (cue) within-subject repeated measures analysis of variance (ANOVA). To control experimenter-wise error, a modified Bonferroni correction (Simes, 1986) was applied to analyses of the self-report data. Follow-up evaluation of significant interactions was conducted using pairwise comparisons, which allows for comparisons of each cue type. Correlational analyses were conducted to determine possible relationships between measures collected upon arrival at the session, (i.e., QSU, VAS withdrawal scale, and Mood) and post-trial self-report ratings. Correlational analyses were also conducted to examine possible associations between self-report measures and trait scales of impression management (BIDR-IM score) and nicotine dependence (FTND).
Results
Average post-trial ratings for subjective measures are presented in Table 2.
Table 2.
Dependent Measure Means (SD) as a Function of Type and Cue
| Variable | Proximal |
Distal |
||
|---|---|---|---|---|
| Smoking | Nonsmoking | Smoking | Nonsmoking | |
| Craving | 76.2 (24.1) | 39.4 (27.6) | 64.1 (23.8) | 35.8 (28.9) |
| Vividness | 92.8 (9.3) | 81.8 (16.4) | 78.3 (18.6) | 78.5 (14.0) |
| Relevance | 90.4 (13.2) | 82.2 (15.2) | 77.1 (19.0) | 74.8 (15.7) |
| Negative affect | 32.6 (27.3) | 19.0 (19.6) | 23.5 (20.5) | 18.8 (16.7) |
| Positive affect | 32.6 (19.9) | 34.6 (21.9) | 36.8 (19.4) | 40.4 (20.9) |
| Excited | 47.2 (21.0) | 28.7 (21.3) | 36.5 (18.7) | 36.4 (20.6) |
| Calm | 38.0 (23.7) | 49.6 (23.1) | 43.7 (22.0) | 55.4 (20.5) |
| Heart rate | −0.52 (4.0) | −0.26 (3.7) | 0.35 (3.1) | −0.18 (1.9) |
| Skin conductance | 0.0098 (0.032) | 0.0048 (0.033) | 0.50 (3.6) | 0.019 (0.048) |
Craving
The individual t tests for proximal and distal smoking versus nonsmoking stimuli revealed a significant cue effect for each, t(29) = 7.5, p < .001, and t(29) = 6.3, p < .001, respectively. As anticipated, for both cue types, proximal and distal, smoking cues led to greater craving compared to nonsmoking cues. This supports the initial assumption that smoking stimuli of both types can elicit a significant craving response from smokers.
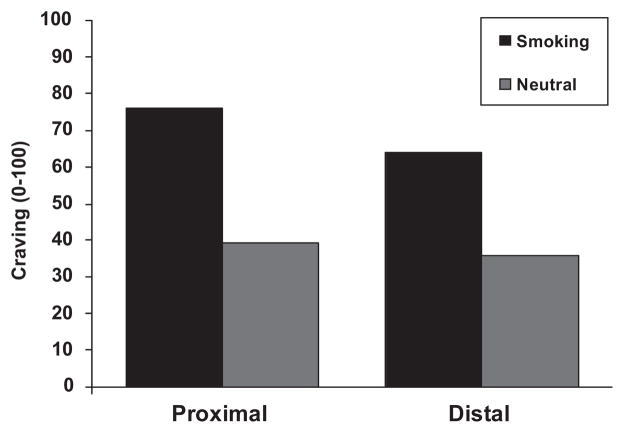
The overall 2 × 2 ANOVA on craving revealed a main effect of cue (Smoking, Nonsmoking) such that exposure to smoking cues clearly led to stronger craving ratings (M = 70.2) compared to nonsmoking cues (M = 37.6); F(1, 61) = 121.2, p < .001. A main effect of type (Proximal, Distal) was also found, F(1, 61) = 33.0, p < .001, such that proximal cues elicited higher craving ratings from smokers than distal cues. The interaction of Cue × Type was also significant, F(1, 61) = 5.4, p < .05. Post hoc evaluation, via pairwise comparisons, revealed that the magnitude of the smoking-nonsmoking difference was greater for proximal cues (mean difference = 36.8, 95% CI 29.6–44.0) than distal cues (mean difference = 28.28, 95% CI 21.5–35.0). However, for both types these smoking-nonsmoking differences were significant. Additionally, both led to effect sizes for the smoking-nonsmoking difference that would be considered large (Cohen, 1988): proximal d = 1.41, distal d = 1.07 (See Figure 1 for craving graph.)
Figure 1.
Craving as a function of stimulus cue (smoking, non-smoking) and type (proximal, distal) depicting significant main effects of cue and type.
Vividness
The ANOVA on the vividness data revealed a significant main effect for cue, F(1, 61) = 17.3, p < .001, as well as an effect of type, F(1, 61) = 42.4, p < .001. The Cue × Type interaction was also significant, F(1, 61) = 11.41, p < .001. Smokers rated smoking cues as more vivid than nonsmoking cues, and proximal cues as more vivid than distal cues. Pairwise comparisons revealed a significant effect on vividness of cue type for proximal (mean difference = 11.02, 95% CI 6.913 – 15.130) but not distal (mean difference = .16, 95% CI −4.482 – 4.159). Therefore, the significant interaction appears to reflect the stronger vividness of smoking compared to nonsmoking cues under the proximal cue condition compared to the difference reported for distal cues.
Relevance
Smoking stimuli were rated as more relevant than nonsmoking stimuli, as evidenced by a significant cue effect, F(1, 61) = 9.8, p < .01. A significant type effect was also found, F(1, 61) = 37.9, p < .001. Proximal cues were rated as more relevant than distal cues. No significant Cue × Type interaction was revealed.
Negative Affect (NA)
A significant main effect of cue on NA was found, F(1, 61) = 31.6, p < .001. Smoking stimuli elicited greater NA report than nonsmoking stimuli. A significant main effect of type was also found, F(1, 61) = 11.8, p < .001, such that proximal cues were rated as significantly higher on NA than distal cues. A significant Cue × Type interaction was also found, F(1, 61) = 7.3, p < .01. Pairwise comparisons revealed that, although both proximal and distal cues led to significant smoking-non-smoking differences, the mean difference was greater for proximal cues (mean difference = 13.55, 95% CI 8.5–18.6) than distal cues (mean difference = 4.67, 95% CI .5 – 8.8).
Positive Affect (PA)
A significant main effect of type on PA was found, F(1, 61) = 11.8, p < .001, such that distal cues were rated as significantly higher on PA than proximal cues.
Excitement
A main effect of cue on arousal was found, F(1, 61) = 13.9, p < .001. As found in past studies, exposure to smoking-related stimuli increased excitement as compared with nonsmoking stimuli. No main effect of the type factor was found; however a significant Cue × Type interaction was revealed, F(1, 61) = 37.8, p < .001. Pairwise comparisons revealed that the smoking-nonsmoking difference was significant only under proximal cues, for which there was a much greater mean difference (mean difference = 18.4, 95% CI 12.6–24.3) compared to distal cues (mean difference = .1, 95% CI −5.6–5.8).
Calmness
A main effect of cue on calmness was found, F(1, 61) = 30.0, p < .001. Also consistent with past studies, subjects reported greater calmness to nonsmoking-related stimuli compared to smoking stimuli. A significant main effect of type was also revealed, F(1, 61) = 12.5, p < .001. Distal cues led to greater self-reported calmness as compared with proximal cues.
Physiological Measures
No significant effects were found for the HR or SC measures.
Correlational Analyses
Correlations between self-report measures and the BIDR-IM (impression management scale) were conducted as a means of examining if a tendency to engage in impression management, which could bias subjective reporting, was associated with self-report responding. Contrary to this notion, a significant negative correlation was found between BIDR-IM and craving during exposure to smoking-related pictures, r = −.368, p < .01. High score on the impression management scale was associated with lower levels of craving report, suggesting that individuals with a penchant for engaging in impression management were likely to under-report craving to smoking stimuli. (However, including BIDR-IM as a covariate in the craving ANOVA did not alter the analyses.) No other significant correlations between any self-report measures and BIDR-IM were found. Correlational analyses examining potential associations between subjects’ ratings on the Mood form, QSU, and VAS nicotine withdrawal, assessed upon arrival at the session, and subsequent post-trial self-report measures yielded no significant findings. Likewise, post-trial self-report measures were not associated with level of nicotine dependence (i.e., FTND score.)
Discussion
The goal of this research was to create a set of smoking and nonsmoking environment cues, as well as comparable proximal cues, and to use them in a larger cue-reactivity study with smokers. The focus of that study was to examine how smokers’ responses to environments, a key distal cue, compare to their cue-reactivity to common proximal smoking and nonsmoking cues. We developed a set of pictorial environmental context stimuli that were completely devoid of traditionally used proximal smoking cues (e.g., cigarettes, lighters, ashtrays) and found that smokers respond with robust self-reported craving upon exposure to smoking-related compared to nonsmoking-related environments. We then created a comparable set of proximal cues and tested responding to both types of cues within a larger cue-reactivity study. The results showed that smokers’ reactivity to environments was robust, although significantly less than that elicited by a set of proximal pictorial stimuli.
Although there has been considerable speculation regarding an associative link between drug use and the environments in which it occurs, we know of no other research to date systematically examining the independent impact of environments on smokers’ craving to smoke, and other indices of cue responding. Some research has investigated how contextual cues might alter responding to proximal cues (Brandon, Juliano, & Copeland, 1999), and many studies have examined proximal cues in the presence of various environmental stimuli (e.g., Wigmore & Hinson, 1991). However, past cue-reactivity research with humans has typically focused on the impact of the cues most proximal to drug administration (See Carter & Tiffany, 1999), and has not parsed out the independent effects of environments. The present study provides perhaps the first evidence that environments can themselves gain associative control over drug-cue responding and lead to robust craving to smoke, even when cues more closely tied to smoking behavior (i.e., proximal cues) are absent.
It is important to note that although environmental contexts evoked craving to smoke that was significantly lesser in magnitude than that elicited by proximal stimuli, the craving response to distal cues was still very robust. Environment cues led to a smoking-nonsmoking craving effect size of d = 1.07, while the craving effect size for proximal stimuli was d = 1.4. As noted by Cohen, an effect size of .8 or greater can be described as large (Cohen, 1988). Therefore, both of these stimuli manipulations led to very large craving effect sizes.
The somewhat weaker craving response to distal cues might be explained in part by the fact that any one distal cue occurs with smoking only some of the time, whereas proximal cues are necessary for smoking to occur and so coincide with smoking virtually all the time. For example, even if a smoker always smokes in bars, that smoker also likely smokes in many environments other than bars, but never smokes without the proximal cue of a cigarette. The association between smoking and environments might be somewhat muted by the relative number of different places encountered over the course of smoking. As evidenced in the animal laboratory, when conditioning occurs across multiple contexts, conditioned responding can be augmented or attenuating, depending on both training and the circumstances of retesting (Grahame, Barnet, & Miller, 1992). Moreover, smokers do not consistently chain smoke the entire time they are in an environment. Therefore, even in their most salient smoking-related environments smokers will have spent some time not smoking, thus potentially slowing acquisition or even attenuating the association between the environment and smoking.
Examining differences between proximal and distal cues across other dependent variables might offer additional insight into how these cues function with smokers. As compared with proximal cues, distal cues were rated as less relevant and less vivid than proximal cues. A decrease in these measures might reflect an important distinction between distal and proximal stimuli. Although generic proximal cues work well to evoke responses from most smokers, distal cues might require more personalization. That is, a generic cigarette burning in an ashtray, one of the proximal cues to which subject were exposed, might be a very salient cue for most smokers regardless of it not being an individual’s specific brand. In fact, past research has found that personalizing proximal smoking cues does not enhance smokers’ reactivity (Conklin & Tiffany, 2001). The same might not be true for distal cues of environmental contextual stimuli. The actual bar or couch in a living room where a smoker typically smokes might function as a stronger cue to smoke than the generic bar or living room presented in our standard set of environmental stimuli. The results of the present study suggest that vividness and relevance might be important characteristics affecting the evocativeness of drug-related stimuli. Future research should determine if personalizing environmental contextual stimuli enhances smokers’ reactivity to the level achieved with proximal cues (see Conklin, 2006). Still, it is important note that even generic distal cues do elicit robust increases in craving for most smokers; none of the cues used in the present study had ever actually been paired with smoking in the real lives of the smokers who participated. This suggests, as the pilot study highlighted, that there do seem to be ubiquitous smoking and nonsmoking places, and that their urge-evoking strength generalizes well across smokers.
That there are smoking and nonsmoking environments capable of alone evoking strong craving to smoke may have important implications for the design and conduct of behavioral treatments for smoking, specifically those that focus on coping with cues and/or reducing or extinguishing cue reactivity as a component of treatment. Human laboratory research on drug cues, as well as cue-related treatments, that focus only on proximal cues may be inadequate. The present study shows that such a narrow focus is likely insufficient, as in the absence of proximal cues, environments alone might comparably augment craving to smoke. Thus, treatments that instruct smokers to avoid or eliminate exposure to key proximal drug cues (e.g., cigarettes, lighters, ashtrays), or that provide extinction training only for proximal cues, as is most often the case in cue-exposure treatment (Conklin & Tiffany, 2002), are likely inadequate in guarding abstaining smokers against some of the most salient cues to which they might be highly reactive, that is, the places they will return to during and/or after they quit. Whereas proximal cues might be avoided in the interest of promoting abstinence, distal cues could easily remain as common after one quits as they were during active drug use.
The methods used in the present study could also be applied on a broader scale to address a variety of questions regarding how environments influence smoking and craving. For example, although not true in Pittsburgh at the time the study was conducted, smoking indoors is becoming less common with the adoption of legislation to restrict smoking in public places. Consequently, smokers’ responses to environments could be assessed longitudinally to determine how quickly and in what ways their reactivity changes as a function of environmental smoking restrictions. Additionally, of considerable recent interest is the goal of assessing the emergence of nicotine dependence (Brandon, Herzog, Irvin, & Gwaltney, 2004; Conklin, Clayton, Tiffany, & Shiffman, 2004). Determining how quickly environments, or other distal cues, come to evoke robust craving responses from young smokers is highly relevant to understanding the onset of dependence. The present procedures could be used to longitudinally track adolescents’ responses to environments as they become regular smokers.
The physiological data collected in the larger study presented here failed to show significant differences in HR or SC as a function of the study manipulations. This is not uncommon as past research has found that physiological measures are, in general, less sensitive to cue manipulations than self-report, (Carter & Tiffany, 1999; Drobes & Tiffany, 1997), and in some cases fail to emerge altogether (Tiffany, 1990). Therefore, in the present study conclusions about proximal and distal cue reactivity are drawn solely from self-report measures, which some researchers have suggested might be subject to experimental demand or report bias (Orne, 1962). Analysis of correlations between impression management (BIDR-IM) and self-report measures revealed that BIDR-IM score was negatively correlated with craving report for smoking stimuli, suggesting that individuals who might be responding with a proclivity toward creating a favorable impression did so by underreporting the amount of craving they experienced upon exposure to smoking-related stimuli. Therefore, the craving effect might actually have been somewhat muted by some subjects’ desire to appear less affected by the manipulation than they possibly were. This, in turn, argues for the importance of examining smokers’ reactivity across multiple domains of functioning. Future cue-reactivity studies examining these manipulations should include not only subjective and physiological response domains, but behavioral and cognitive as well, each of which might be differentially affected not only by study manipulations but also by experimental demand and/or response biases.
In summary, this study demonstrated that environments generally differ in their association with smoking and can independently function as stimuli capable of evoking strong subjective responses from smokers. Whereas past cue-reactivity work has largely focused on proximal cues alone or presenting proximal cues in the presence of distal cues, it is clear that distal cues, less proximal to the actual act of smoking, can alone also come to be strongly associated with smoking. Therefore, treatments focused on eliminating reactivity to proximal cues, while offering an important component of cue-based treatments, may be less thorough than they need to be in order to effectively guard smokers against cue-driven urges to smoke. The present work suggests that a conceptualization of smoking cues that better captures the full array of stimuli capable of becoming associated with this behavior, both proximal and distal, should offer a more complete picture of the salient stimuli that might function to motivate smoking maintenance and/or relapse. Consequently, applying a more inclusive perspective to future cue-based research and treatment development might prove to enhance the efficacy of cue-extinction treatment techniques for drug dependence.
Acknowledgments
This work was supported by National Institute on Drug Abuse Grants DA017582 & DA019269 awarded to Cynthia A. Conklin, and by The Pittsburgh Mind-Body Center’s National Institute of Health Grants HL076852/076858 awarded to Karen A. Matthews and Michael Scheier.
Contributor Information
Cynthia A. Conklin, University of Pittsburgh
Nathalie Robin, University of Pittsburgh.
Kenneth A. Perkins, University of Pittsburgh
Ronald P. Salkeld, University of Pittsburgh
F. Joseph McClernon, Duke University Medical Center.
References
- Brandon TH, Herzog TA, Irvin JE, Gwaltney CJ. Cognitive and social learning models of drug dependence: Implications for the assessment of tobacco dependence in adolescents. Addiction. 2004;99:51–77. doi: 10.1111/j.1360-0443.2004.00737.x. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Juliano LM, Copeland AL. Expectancies for tobacco smoking. In: Kirsh I, editor. How expectancies shape experience. Washington, DC: American Psychological Association; 1999. pp. 263–299. [Google Scholar]
- Brandon TH, Piasecki TM, Quinn EP, Baker TB. Cue exposure treatment in nicotine dependence. In: Drummond DC, Tiffany ST, Glautier S, Remington B, editors. Addictive behaviour: Cue exposure theory and practice. New York: Wiley; 1995. pp. 211–227. [Google Scholar]
- Burton SM, Tiffany ST. The effect of alcohol consumption on imaginal and in vivo manipulations of smoking urges. Addiction. 1997;92:15–26. [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta analysis of cue reactivity in addiction research. Addiction. 1999;92:15–26. [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: The effects of cigarette availability on cue reactivity in smokers. Experimental and Clinical Psychopharmacology. 2001;9:183–190. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Conklin CA. Environments as cues to smoke: Implication for human extinction-based research and treatment. Experimental and Clinical Psychopharmacology. 2006;14:12–19. doi: 10.1037/1064-1297.14.1.12. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Clayton RR, Tiffany ST, Shiffman S. Introduction to concepts and measurement of the emergence of tobacco dependence: The Tobacco Etiology Research Network. Addiction. 2004;99:1–4. doi: 10.1111/j.1360-0443.2004.00749.x. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. The impact of imagining personalized versus standardized urge scenarios on cigarette craving and autonomic reactivity. Experimental and Clinical Psychopharmacology. 2001;9:399–408. doi: 10.1037//1064-1297.9.4.399. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Dawe S, Powell JH, Richards D, Gossop M, Marks I, Strang J, et al. Does post-withdrawal cue exposure improve outcome in opiate addiction? A controlled trial. Addiction. 1993;88:1233–1245. doi: 10.1111/j.1360-0443.1993.tb02146.x. [DOI] [PubMed] [Google Scholar]
- Diener E, Emmons RA. The independence of positive and negative affect. Journal of Personality and Social Psychology. 1984;47:1105–1117. doi: 10.1037//0022-3514.47.5.1105. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Tiffany ST. Induction of smoking urges through imaginal and in vivo procedure: Physiological and self-report manifestations. Journal of Abnormal Psychology. 1997;106:15–25. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- Field M, Duka T. Cue reactivity in smokers: The effects of perceived cigarette availability and gender. Pharmacology, Biochemistry, and Behavior. 2004;78:647–652. doi: 10.1016/j.pbb.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Barnet RC, Miller RR. Pavlovian conditioning in multiple contexts: Competition between contexts for comparator status. Animal Learning and Behavior. 1992;20:329–338. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom tolerance questionnaire. Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Kasvikis Y, Bradley B, Powell J, Marks I, Gray JA. Postwithdrawal exposure treatment to prevent relapse in opiate addicts: A pilot study. International Journal of Addictions. 1991;26:1187–1195. doi: 10.3109/10826089109062154. [DOI] [PubMed] [Google Scholar]
- Lane J. Association of coffee drinking with cigarette smoking in the natural environment. Experimental and Clinical Psychopharmacology. 1996;4:409–412. [Google Scholar]
- Madden GJ, Bickel WK. Abstinence and price effects on demand for cigarettes: A behavioral-economic analysis. Addiction. 1999;94:577–588. doi: 10.1046/j.1360-0443.1999.94457712.x. [DOI] [PubMed] [Google Scholar]
- Orne MT. On the social psychology of the psychological experiment: With particular reference to demand characteristics and their implication. American Psychologist. 1962;17:776–783. [Google Scholar]
- Paulhus DL. Measurement and control of response bias. In: Robinson JP, Shaver PR, editors. Measures of personality and social psychological attitudes. Vol. 1. San Diego, CA: Academic Press, Inc; 1991. pp. 17–59. [Google Scholar]
- Perkins KA, Doyle T, Ciccocioppo M, Conklin CA, Sayette M, Caggiula A. Sex differences in the influence of nicotine dose instructions on the reinforcing and self-reported rewarding effects of smoking. Psychopharmacology. 2006;184:600–607. doi: 10.1007/s00213-005-0103-7. [DOI] [PubMed] [Google Scholar]
- Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73:751–754. [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: The role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Wall AM, McKee SA, Hinson RA. Assessing variation in alcohol outcome expectancies across environmental context: An examination of the situational-specificity hypothesis. Psychology of Addictive Behaviors. 2000;14:367–375. [PubMed] [Google Scholar]
- Wigmore SW, Hinson RE. The influence of setting on consumption in the balanced placebo design. Addiction. 1991;86:205–215. doi: 10.1111/j.1360-0443.1991.tb01770.x. [DOI] [PubMed] [Google Scholar]
- Wikler A. Conditioning factors in opiate addiction and relapse. Journal of Substance Abuse Treatment. 1984;1:279–285. (Reprinted from Narcotics, pp. 85–100, by D. I. Wilner & G. G. Kassebaum, Eds., 1965, New York: McGraw-Hill.) [PubMed] [Google Scholar]