In previous reports we identified several risk factors that significantly affected patient and graft survival.1,2 Of these, four were major immunologic risk factors: the number of intestinal allograft rejections, high blood FK 506 levels, high dose steroid requirements, and the need for adjunct OKT3 therapy. The remaining significant risk factors were the operative and cold ischemia times, number of previous abdominal surgeries, cytomegaloviral (CMV) and post-transplant lymphoproliferative diseases. To decrease the immunosuppressive requirements and improve survival outcome, we declared a 1 year moratorium in 1994, pending the results of extensive clarifying investigations by Murase et al in rats.3 When the program reopened, two major changes in management strategy were instituted. One was an attempt to avoid, when possible, the transplantation of organs from CMV-positive donors to CMV-negative recipients. The second change was to give perioperative adjunct donor bone marrow when this was available, in order to take advantage of the more tolerogenic profile of bone marrow cells compared to that of the intestinal passenger leukocytes.3–6 Other adopted strategies were careful patient selection, exclusion of the colon from the intestinal allograft, and monitoring of Epstein-Barr virus (EBV) infection by serial quantitative EBV polymerase chain reaction (PCR) measurements.
MATERIALS AND METHODS
We report here our overall long-term results with 115 intestinal transplantation in 109 consecutive patients that were transplanted over 8 years. Of these 64 (59%) were children and 45 (41%) were adults. The causes of intestinal failure were short gut syndrome (SGS) in 89 (81%), dysmotility syndrome in 11 (10%), intestinal neoplasm in 6 (6%), and enterocyte dysfunction in 3 (3%). The leading causes of SGS were vascular occlusion. Crohn’s disease, and trauma in adults and volvulus, gastroschisis, atresia and necrotizing enterocolitis in children. The intestine was engrafted alone (n = 43), or as part of a composite graft (n = 72), before and after the moratorium. All of the isolated intestinal recipients were suffering from frequent central line sepsis, vanishing central venous access, and reversible total parenteral nutrition (TPN) induced hepatic dysfunction. The donors were all ABO identical and HLA histoincompatible. The lymphocytotoxic crossmatch was positive in 14 patients. No attempts were made to alter the graft lymphoreticular tissue with antilymphocyte preparations or other modalities. Of the last 55 recipients, 23 were infused with bone marrow, the distinction from the other 32 being the willingness of the donor family to permit the extra procurement procedure. The surgical techniques of the donor and recipient operation and details of bone marrow augmentation are fully described elsewhere.7–10
The management of the intestinal allograft recipients is fully described elsewhere.11 The immunosuppressive therapy was based on FK 506 and prednisone. Cyclophosphamide was given to 23 patients after the moratorium at a dose of 2 to 3 mg/kg/d for 4 weeks and then switched to mycophenolate mofetil (15 to 30 mg/kg/d) or azathioprine. In a few cases, azathioprine was given as a third drug from the outset. The level of maintenance immunosuppression was individualized and adjusted based on the clinical course of each patient with the intention to reduce the drug dosage and levels whenever possible. Episodes of rejection were treated with adjustments of FK 506 dose and/or supplemental prednisone. OKT3 was given only as a rescue therapy. Upward dose adjustments of mycophenolate mofetil, azathioprine, or steroids, were frequently needed to compensate for FK-506 dose reductions mandated by FK 506–related adverse effects. Patient and graft survival curves were generated using the Kaplan-Meier method and group comparisons were performed using the log-rank test.
RESULTS
Survival
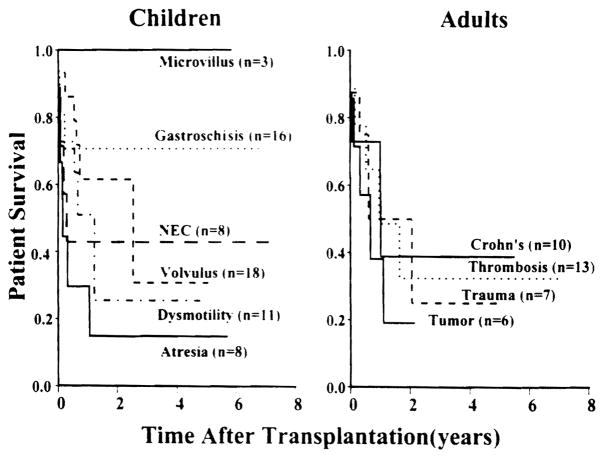
The overall cumulative patient survival is 72% at 1 year and 48% at 5 years with a graft survival rate of 64% and 40%, respectively. With a mean follow-up of 40 ± 29 months (range, 1 to 94 months), 31 patients are alive with good nutrition beyond the third postoperative year and 18 are well beyond the 5-year mile stone. The survival benefits of intestinal transplantation has been better (P = .57) achieved among children compared to adults with the best outcome among patients between 2 and 17 years of age, in whom the 5-year cumulative survival rate was 68%. Although both the isolated intestinal and composite visceral grafts had similar (P = .72) survival rates, the cumulative risk of graft loss due to rejection was significantly (P = .045) higher among the isolated intestine. The Kaplan-Meier graft survival curves stratified according to the cause of intestinal failure are depicted in Figure 1. The best survival rates have been achieved among children with microvillus disease and gastroschisis and in adults with Crohn’s disease and vascular thrombosis. Figure 2 shows the significant (P = .04) improvement in intestinal allograft survival during the last four years (after the moratorium) with a cumulative rate of 65% at 4 years. Such an achievement reflects further refinement in operative techniques, immunomodulation, and postoperative management as described above.
Fig 1.
Graft survival according to the native intestinal disease.
Fig 2.
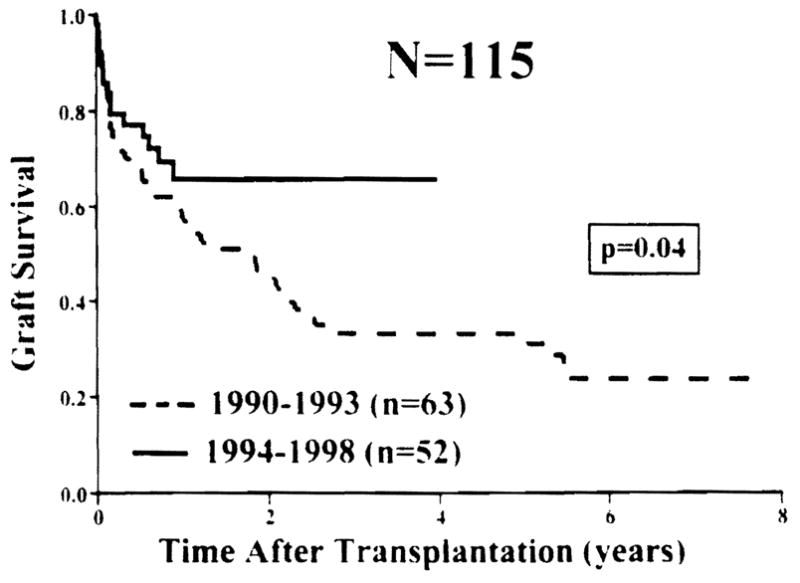
Graft survival before and after the 1994 moratorium.
Long-Term Rehabilitation
Fifty-one (93%) of 55 current survivors are home, fully active, and completely off TPN with full nutritional autonomy. These individuals have reported improved quality of life measures in comparison to TPN dependency. Two (3.5%) of the remaining four recipients require home parenteral nutrition because of chronic graft dysmotility. The other two (3.5%) patients are receiving partial intravenous support while recovering from a recent episode of moderate to severe rejection. No clinical or histopathologic evidences of disease recurrence has been documented during the 8 years of follow-up. To date, no attempts have been made to electively wean any of the intestinal recipients, including the bone marrow augmented cases, off immunosuppression.
Cost Analysis
The average cost of intestinal transplantation between 1990 and 1994 was $203,111 for the isolated intestine, $252,453 for the combined liver-intestine transplantation, and $284,452 for the multivisceral procedure. This has been significantly reduced during the last 4 years to an average of $132,285, $214,716 and $219,098, respectively. The use of intestinal transplant can be examined on a cost-effectiveness basis, due to the availability of chronic TPN as an alternative therapy for patients with irreversible gut failure. Based on Medicare data, the average yearly cost of TPN in 1992 was more than $150,000 per patient not including the cost of frequent hospitalization, medical equipment, and nursing care.12 The total dollars spent on TPN are increasing every year because of the yearly increase in TPN cost and the cumulative increase of the home and hospital bound TPN population. Based on these data, intestinal transplantation becomes cost-effective by the second year after transplantation.
CONCLUSIONS
Intestinal transplantation has become a life-saving treatment for patients with irreversible intestinal failure who cannot be maintained on TPN, and a cost-effective therapy for patients who still have the option of TPN. The long-term rehabilitation with all three kinds of intestinal transplant procedures is similar to that achieved with lung transplantation13 (currently eligible for reimbursement by Medicare) and the other kinds of organ allografts. Therefore, it is justifiable, to consider intestinal transplantation as nonexperimental procedure that is eligible for reimbursement under Medicare, Medicaid, and other third party payers in the United States. Based on the results of our most recent preclinical trials (unpublished data), further improvement in patient and graft survival is greatly anticipated with further immune modulation strategies (graft cytoreduction) that can be added to the bone marrow augmentation protocol.
References
- 1.Todo S, Reyes J, Furukawa H, et al. Ann Surg. 1995;3:270. doi: 10.1097/00000658-199509000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu-Elmagd K, Reyes J, Todo S, et al. J Am Coll Surg. 1998;186:512. doi: 10.1016/s1072-7515(98)00083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murase N, Starzl TE, Tanabe M, et al. Transplantation. 1995;60:158. doi: 10.1097/00007890-199507000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murase N, Demetris AJ, Woo J, et al. Transplant Proc. 1991;23:3246. [PMC free article] [PubMed] [Google Scholar]
- 5.Murase N, Demetris AJ, Woo J, et al. Transplantation. 1993;55:1. doi: 10.1097/00007890-199301000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanabe M, Muase N, Demetris AJ, et al. Transplant Proc. 1994;26:4325. [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Todo S, Tzakis A, et al. Ann Surg. 1992;216:605. doi: 10.1097/00000658-199211000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casavilla A, Selby R, Abu-Elmagd K, et al. Ann Surg. 1992;216:605. doi: 10.1097/00000658-199211000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furukawa F, Abu-Elmagd K, Reyes J, et al. Surgical Technology International. Vol. 2. San Francisco: Universal Medical Press; 1994. pp. 165–170. [Google Scholar]
- 10.Fontes P, Rao A, Demetris AJ, et al. Lancet. 1994;344:151. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu-Elmagd K, Fung JJ, Reyes J, et al. Transplant Proc. 1992;24:1243. [PMC free article] [PubMed] [Google Scholar]
- 12.Howard L, Ament M, Fleming R, et al. Gastroenterology. 1995;109:355. doi: 10.1016/0016-5085(95)90321-6. [DOI] [PubMed] [Google Scholar]
- 13.Hosenpud JD, Novick RJ, Bennett LE, et al. J Heart Lung Transplantation. 1996;15:655. [PubMed] [Google Scholar]