Abstract
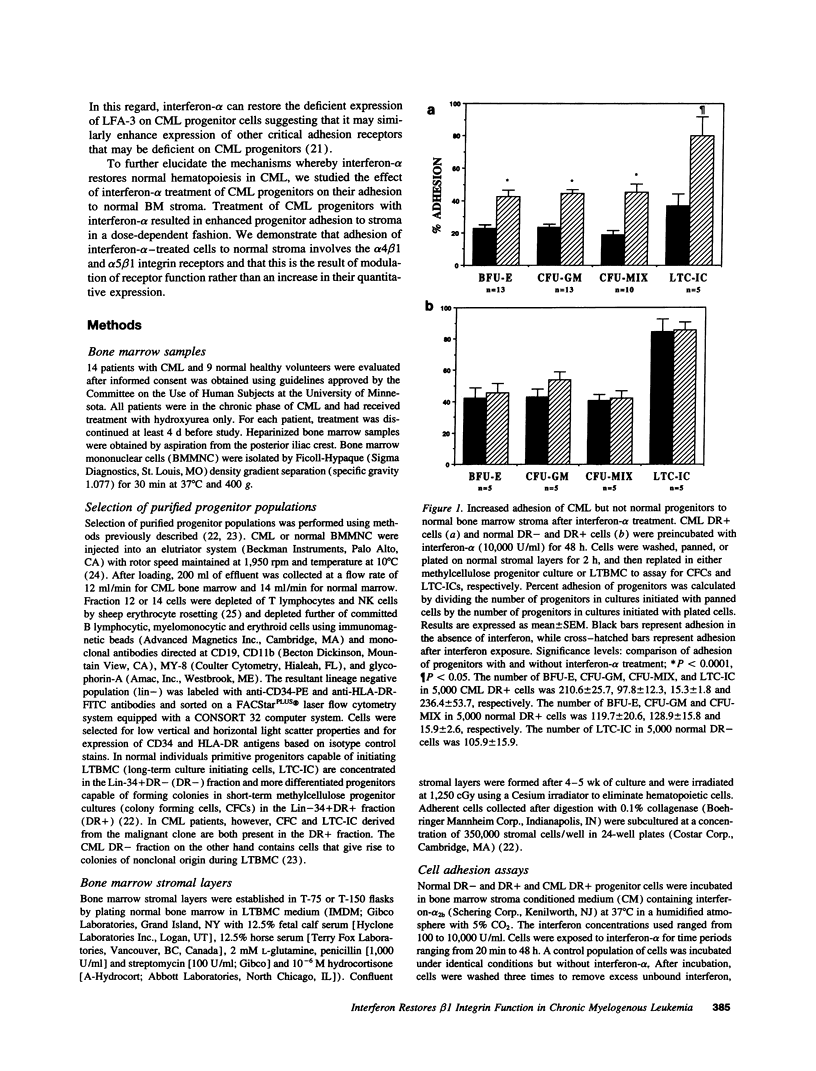
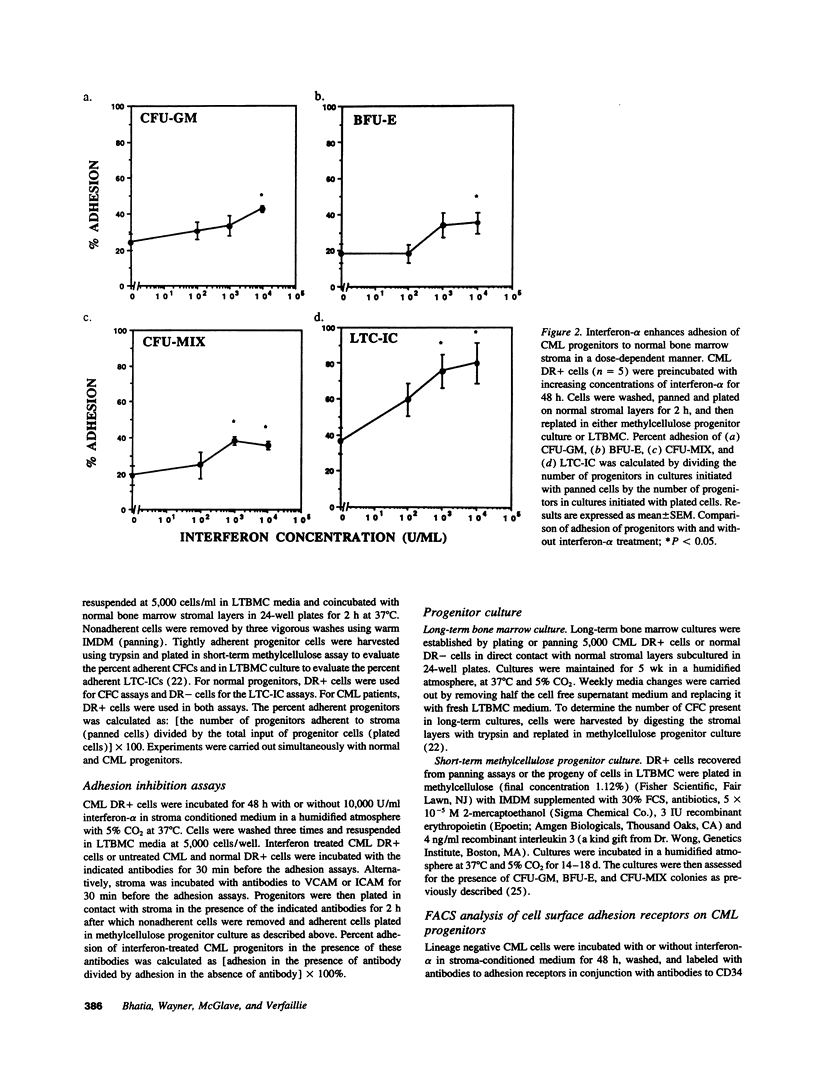
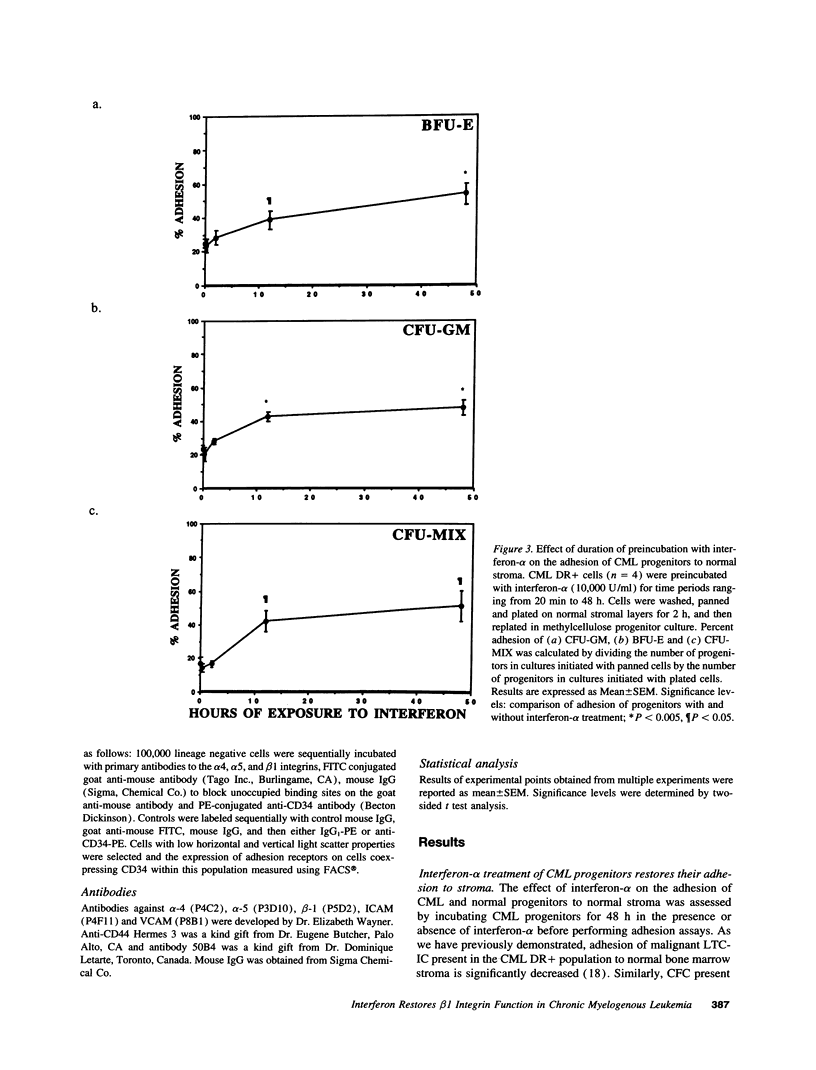
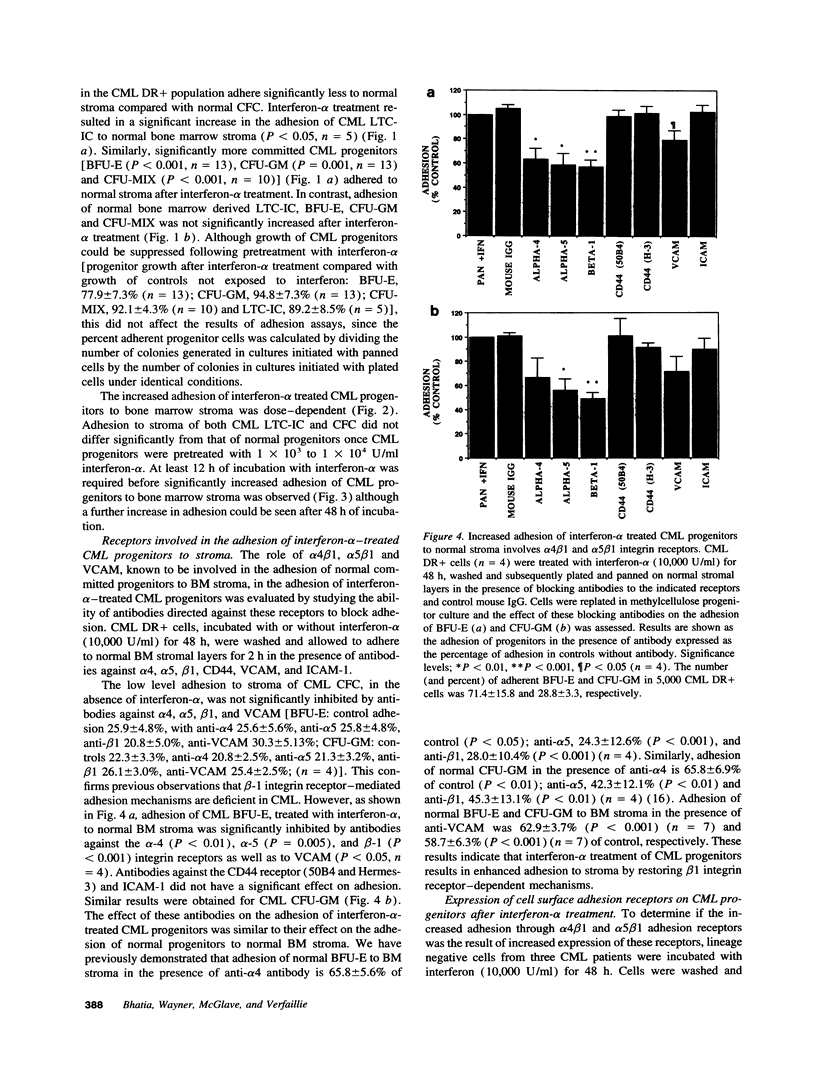
Treatment of chronic myelogenous leukemia (CML) with interferon-alpha frequently results in normalization of peripheral blood counts and, in up to 20% of patients, reestablishment of normal hematopoiesis. We hypothesize that interferon-alpha may restore normal adhesive interactions between CML progenitors and the bone marrow microenvironment and restore normal growth regulatory effects resulting from these progenitor-stroma interactions. We demonstrate that treatment with interferon-alpha induces a significant, dose-dependent increase in the adhesion of primitive long-term culture initiating cells and committed colony-forming cells (CFC) from CML bone marrow to normal stroma. Adhesion of CFC seen after interferon-alpha treatment could be inhibited by blocking antibodies directed at the alpha 4, alpha 5, and beta 1 integrins and vascular cell adhesion molecule, but not CD44 or intracellular adhesion molecule, suggesting that interferon-alpha induces normalization of progenitor-stroma interactions in CML. Because FACS analysis showed that the level of alpha 4, alpha 5, and beta 1 integrin expression after interferon-alpha treatment is unchanged, this suggests that interferon-alpha may restore normal beta 1 integrin function. Normalization of interactions between CML progenitors and the bone marrow microenvironment may then result in the restoration of normal regulation of CML progenitor proliferation, and explain, at least in part, the therapeutic efficacy of interferon-alpha in CML.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Watt F. M. Fibronectin inhibits the terminal differentiation of human keratinocytes. Nature. 1989 Jul 27;340(6231):307–309. doi: 10.1038/340307a0. [DOI] [PubMed] [Google Scholar]
- Brandt J., Srour E. F., van Besien K., Briddell R. A., Hoffman R. Cytokine-dependent long-term culture of highly enriched precursors of hematopoietic progenitor cells from human bone marrow. J Clin Invest. 1990 Sep;86(3):932–941. doi: 10.1172/JCI114795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H. E., Sherry B., Lu L., Cooper S., Oh K. O., Tekamp-Olson P., Kwon B. S., Cerami A. Enhancing and suppressing effects of recombinant murine macrophage inflammatory proteins on colony formation in vitro by bone marrow myeloid progenitor cells. Blood. 1990 Sep 15;76(6):1110–1116. [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Cashman J. D., Eaves A. C., Raines E. W., Ross R., Eaves C. J. Mechanisms that regulate the cell cycle status of very primitive hematopoietic cells in long-term human marrow cultures. I. Stimulatory role of a variety of mesenchymal cell activators and inhibitory role of TGF-beta. Blood. 1990 Jan 1;75(1):96–101. [PubMed] [Google Scholar]
- Cashman J., Eaves A. C., Eaves C. J. Regulated proliferation of primitive hematopoietic progenitor cells in long-term human marrow cultures. Blood. 1985 Oct;66(4):1002–1005. [PubMed] [Google Scholar]
- Davis L. S., Oppenheimer-Marks N., Bednarczyk J. L., McIntyre B. W., Lipsky P. E. Fibronectin promotes proliferation of naive and memory T cells by signaling through both the VLA-4 and VLA-5 integrin molecules. J Immunol. 1990 Aug 1;145(3):785–793. [PubMed] [Google Scholar]
- Dowding C. R., Gordon M. Y., Goldman J. M. Primitive progenitor cells in the blood of patients with chronic granulocytic leukemia. Int J Cell Cloning. 1986 Sep;4(5):331–340. doi: 10.1002/stem.5530040505. [DOI] [PubMed] [Google Scholar]
- Dowding C., Guo A. P., Osterholz J., Siczkowski M., Goldman J., Gordon M. Interferon-alpha overrides the deficient adhesion of chronic myeloid leukemia primitive progenitor cells to bone marrow stromal cells. Blood. 1991 Jul 15;78(2):499–505. [PubMed] [Google Scholar]
- Du X. P., Plow E. F., Frelinger A. L., 3rd, O'Toole T. E., Loftus J. C., Ginsberg M. H. Ligands "activate" integrin alpha IIb beta 3 (platelet GPIIb-IIIa). Cell. 1991 May 3;65(3):409–416. doi: 10.1016/0092-8674(91)90458-b. [DOI] [PubMed] [Google Scholar]
- Eaves A. C., Cashman J. D., Gaboury L. A., Kalousek D. K., Eaves C. J. Unregulated proliferation of primitive chronic myeloid leukemia progenitors in the presence of normal marrow adherent cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5306–5310. doi: 10.1073/pnas.83.14.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S. S., Collea R. P., Appenheimer M. M., Gollnick S. O. Interferon-alpha induces the expression of the L-selectin homing receptor in human B lymphoid cells. J Cell Biol. 1993 Dec;123(6 Pt 2):1889–1898. doi: 10.1083/jcb.123.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon M. Y., Dowding C. R., Riley G. P., Goldman J. M., Greaves M. F. Altered adhesive interactions with marrow stroma of haematopoietic progenitor cells in chronic myeloid leukaemia. Nature. 1987 Jul 23;328(6128):342–344. doi: 10.1038/328342a0. [DOI] [PubMed] [Google Scholar]
- Horvath A. R., Elmore M. A., Kellie S. Differential tyrosine-specific phosphorylation of integrin in Rous sarcoma virus transformed cells with differing transformed phenotypes. Oncogene. 1990 Sep;5(9):1349–1357. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Kornberg L., Earp H. S., Parsons J. T., Schaller M., Juliano R. L. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992 Nov 25;267(33):23439–23442. [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Boyd F. T., Andres J. L. TGF-beta receptors and TGF-beta binding proteoglycans: recent progress in identifying their functional properties. Ann N Y Acad Sci. 1990;593:59–72. doi: 10.1111/j.1749-6632.1990.tb16100.x. [DOI] [PubMed] [Google Scholar]
- Matulonis U., Salgia R., Okuda K., Druker B., Griffin J. D. Interleukin-3 and p210 BCR/ABL activate both unique and overlapping pathways of signal transduction in a factor-dependent myeloid cell line. Exp Hematol. 1993 Oct;21(11):1460–1466. [PubMed] [Google Scholar]
- Rohrschneider L. R. Adhesion plaques of Rous sarcoma virus-transformed cells contain the src gene product. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3514–3518. doi: 10.1073/pnas.77.6.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons P. J., Masinovsky B., Longenecker B. M., Berenson R., Torok-Storb B., Gallatin W. M. Vascular cell adhesion molecule-1 expressed by bone marrow stromal cells mediates the binding of hematopoietic progenitor cells. Blood. 1992 Jul 15;80(2):388–395. [PubMed] [Google Scholar]
- Talpaz M., Kantarjian H., Kurzrock R., Trujillo J. M., Gutterman J. U. Interferon-alpha produces sustained cytogenetic responses in chronic myelogenous leukemia. Philadelphia chromosome-positive patients. Ann Intern Med. 1991 Apr 1;114(7):532–538. doi: 10.7326/0003-4819-114-7-532. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Adams D. H., Hubscher S., Hirano H., Siebenlist U., Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature. 1993 Jan 7;361(6407):79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Albelda S. M., Horgan K. J., van Seventer G. A., Shimizu Y., Newman W., Hallam J., Newman P. J., Buck C. A., Shaw S. CD31 expressed on distinctive T cell subsets is a preferential amplifier of beta 1 integrin-mediated adhesion. J Exp Med. 1992 Jul 1;176(1):245–253. doi: 10.1084/jem.176.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixidó J., Hemler M. E., Greenberger J. S., Anklesaria P. Role of beta 1 and beta 2 integrins in the adhesion of human CD34hi stem cells to bone marrow stroma. J Clin Invest. 1992 Aug;90(2):358–367. doi: 10.1172/JCI115870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya G., Guba S. C., Sih S. A., Feinberg A. P., Talpaz M., Kantarjian H. M., Deisseroth A. B., Emerson S. G. Interferon-alpha restores the deficient expression of the cytoadhesion molecule lymphocyte function antigen-3 by chronic myelogenous leukemia progenitor cells. J Clin Invest. 1991 Dec;88(6):2131–2136. doi: 10.1172/JCI115543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten R. A., Jackson P., Baltimore D. The mouse type IV c-abl gene product is a nuclear protein, and activation of transforming ability is associated with cytoplasmic localization. Cell. 1989 Aug 25;58(4):669–678. doi: 10.1016/0092-8674(89)90102-5. [DOI] [PubMed] [Google Scholar]
- Verfaillie C. M. Direct contact between human primitive hematopoietic progenitors and bone marrow stroma is not required for long-term in vitro hematopoiesis. Blood. 1992 Jun 1;79(11):2821–2826. [PubMed] [Google Scholar]
- Verfaillie C. M., McCarthy J. B., McGlave P. B. Differentiation of primitive human multipotent hematopoietic progenitors into single lineage clonogenic progenitors is accompanied by alterations in their interaction with fibronectin. J Exp Med. 1991 Sep 1;174(3):693–703. doi: 10.1084/jem.174.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaillie C. M., McCarthy J. B., McGlave P. B. Mechanisms underlying abnormal trafficking of malignant progenitors in chronic myelogenous leukemia. Decreased adhesion to stroma and fibronectin but increased adhesion to the basement membrane components laminin and collagen type IV. J Clin Invest. 1992 Oct;90(4):1232–1241. doi: 10.1172/JCI115985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaillie C. M., Miller W. J., Boylan K., McGlave P. B. Selection of benign primitive hematopoietic progenitors in chronic myelogenous leukemia on the basis of HLA-DR antigen expression. Blood. 1992 Feb 15;79(4):1003–1010. [PubMed] [Google Scholar]
- Verfaillie C., Blakolmer K., McGlave P. Purified primitive human hematopoietic progenitor cells with long-term in vitro repopulating capacity adhere selectively to irradiated bone marrow stroma. J Exp Med. 1990 Aug 1;172(2):509–502. doi: 10.1084/jem.172.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaillie C., McGlave P. Leukemia inhibitory factor/human interleukin for DA cells: a growth factor that stimulates the in vitro development of multipotential human hematopoietic progenitors. Blood. 1991 Jan 15;77(2):263–270. [PubMed] [Google Scholar]
- Wetzler M., Talpaz M., Van Etten R. A., Hirsh-Ginsberg C., Beran M., Kurzrock R. Subcellular localization of Bcr, Abl, and Bcr-Abl proteins in normal and leukemic cells and correlation of expression with myeloid differentiation. J Clin Invest. 1993 Oct;92(4):1925–1939. doi: 10.1172/JCI116786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Rios M., Stephens C., Patel V. P. Fibronectin and VLA-4 in haematopoietic stem cell-microenvironment interactions. Nature. 1991 Aug 1;352(6334):438–441. doi: 10.1038/352438a0. [DOI] [PubMed] [Google Scholar]
- Yamada A., Nikaido T., Nojima Y., Schlossman S. F., Morimoto C. Activation of human CD4 T lymphocytes. Interaction of fibronectin with VLA-5 receptor on CD4 cells induces the AP-1 transcription factor. J Immunol. 1991 Jan 1;146(1):53–56. [PubMed] [Google Scholar]
- de Klein A., van Kessel A. G., Grosveld G., Bartram C. R., Hagemeijer A., Bootsma D., Spurr N. K., Heisterkamp N., Groffen J., Stephenson J. R. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982 Dec 23;300(5894):765–767. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]


