Cyclosporine (CyA) monitoring of blood or plasma drug concentrations remains an important part of the use of this agent following organ transplantation. When CyA is used as the primary immunosuppressant agent following liver transplantation (LT), monitoring becomes particularly important because of the altered pharmacokinetics of CyA in this patient population. Previous studies have demonstrated that wide variability exists in the fraction of the dose absorbed and in the clearance of Cy A in L T patients.1 Clinical situations that exacerbate the poor absorption of Cy A are frequently present in LT patients and include cholestasis and biliary diversion through a T-tube. Monitoring CyA concentrations in blood or plasma is also critically important when making the transition from intravenous to oral therapy in LT patients.
Patient monitoring using a polyclonal RIA (PC-RIA) kit for Cy A has been extensively evaluated in organ transplant patients, and its use in comparison to high-performance liquid chromatography (HPLC) in LT patients has been assessed.2 Since the PC-RIA cross-reacts with metabolites of Cy A that are present in plasma or blood, its results are affected by a number of factors. Hepatic function in a LT patient will determine CyA metabolism and clearance, and biliary function affects the appearance of metabolites in blood and plasma. Early studies indicated that the initial two postoperative weeks following LT were the period of greatest discrepancy between the PC-RIA and HPLC assay results, with the PC-RIA occasionally being 10 times the HPLC concentration.3 Later studies demonstrated that whole blood (WB) samples from LT patients contain amounts of M 17 and other CyA metabolites that are in excess of concentrations observed in other transplant populations.4 Other factors which may affect the ratio of PC-RIA results to HPLC CyA results include: the liming of the blood sample during the dosing interval since the PC-RIA/HPLC ratio is constantly changing, the absolute CyA concentration since higher levels result in better agreement between the PC-RIA and HPLC assays, and drug interactions.
More recently another immunoassay technique has been developed for CyA called fluorescence polarization immunoassay (FPIA). This technique is rapid to perform, but also uses a polyclonal antibody which is distinct from the PC-RIA. The objective of this study was to assess the factors affecting the FPIA technique in LT patients using comparisons to the PC-RIA and HPLC assays in blood and plasma.
MATERIALS AND METHODS
The FPIA assay was assessed in three separate ways. The first method involved making a comparison of the FPIA, PC-RIA, and HPLC assays on blood and plasma trough samples obtained on consecutive days from LT patients. Patients were included if samples could be obtained for the first 14 postoperative days following transplantation. The results of these assays on blood and plasma were compared to the other assays and to the biochemical profile which included blood urea nitrogen, serum creatinine, alkaline phosphatase, GGTP, ALT, AST, total and direct bilirubin, hematocrit, and albumin and total protein.
The second method of assessment included the review of the clinical course of 50 LT patients who were monitored by daily assessment of WB FPL CyA concentration. Changes in blood concentration in relation to major clinical events and in relation to other drug therapy were noted. The third method of assessment included the FPIA analysis of plasma samples from LT patients not receiving CyA in an effort to investigate the cross-reactivity of the assay with any endogenous or exogenous substrate present in the plasma of an LT patient.
The HPLC assays utilized cyclosporin D as an internal standard and followed the procedure previously described.5 The PC-RIA assay (Sandoz, East Hanover, NJ) and the FPIA assay (Abbott Laboratories, Diagnostics Division, Irving, TX) were utilized as per manufacturers’ directions.
Stepwise multiple linear regression analysis (SAS Institute Inc. Cary. NC) was utilized to identify the biochemical factors that primarily influenced CyA concentrations measured by the FPIA assay.
RESULTS
Eleven LT patients were followed for the first 14 postoperative days using FPIA, PC-RIA, and HPLC measurements on blood and plasma. Serum creatinine did not exceed 2.8 mg/dL in any of these patients during the two-week study period. Five of the 11 patients received additional corticosteroids during the study period. The highest quantitative values for CyA concentrations were recorded for the FPIA assay on WB, followed by either the PC-RIA result in whole blood or the TDX assay result from plasma.
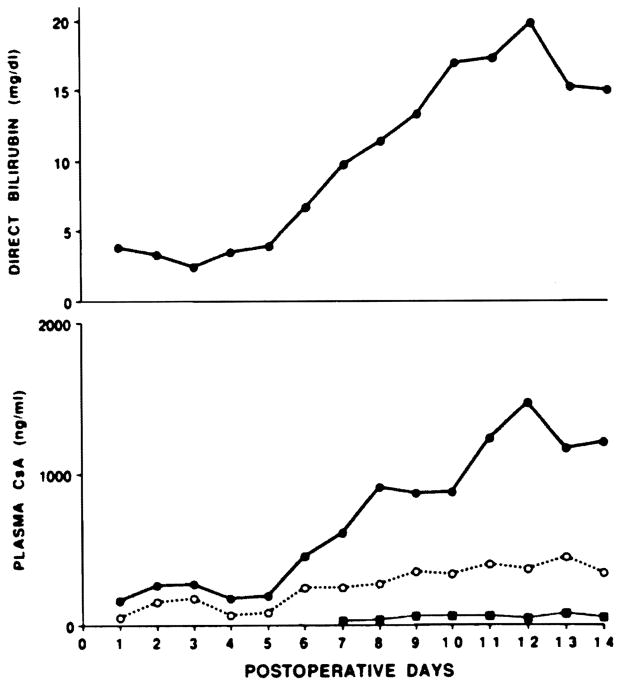
In the regression analysis, 20% of the variability in FPIA plasma CyA concentrations was related to the direct serum bilirubin concentration. Forty-eight percent of the variability in the FPIA/HPLC ratio was also related to serum bilirubin. As suggested by the regression analysis, the FPIA results were most disparate with the PC-RIA and the HPLC results when the serum bilirubin was elevated. Fig 1 demonstrates the manner in which the FPIA-measured CyA plasma concentration appears to follow the serum bilirubin concentration while consistent results were obtained using the PC-RIA and HPLC assays. During these periods of hyperbilirubinemia, the peak FPIA/HPLC ratio in plasma exceeded 40: 1 in one patient, was 30–40: 1 in two patients, and was between 20–30: 1 in an additional two patients.
Fig 1.
Serum bilirubin and plasma CyA concentrations over the 14-day postoperative period in patient 1. For the lower figure: ● = FPIA, ○ = PC-RIA, and ■ = HPLC.
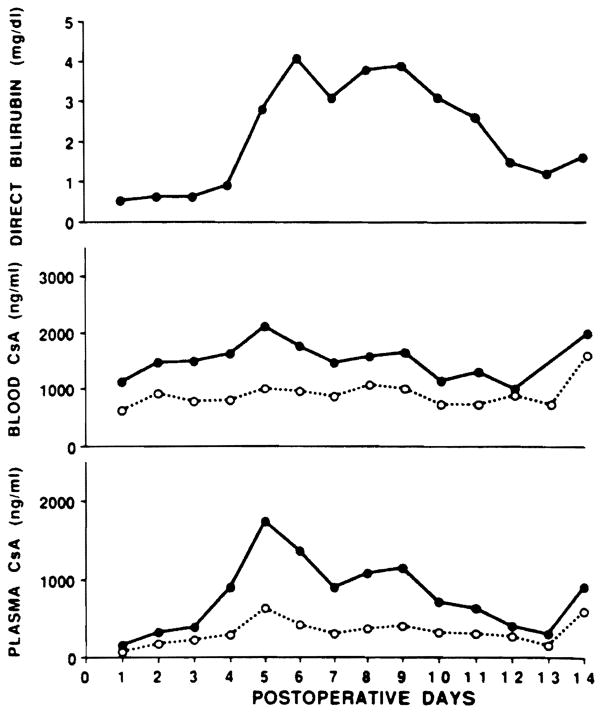
Figure 2 also includes the WB PC-RIA and FPIA, and demonstrates that assay differences in CyA measurement are not as striking with WB. Table 1 lists the percentage of samples analyzed that had varying ratios of FPIA to PC-RIA measured CyA concentration. While the majority of WB samples had reasonable agreement between the PC-RIA and FPIA assays, over 75% of plasma samples had an FPIA to PC-RIA ratio of 1.5 to 3.5 times.
Fig 2.
Serum bilirubin, WB, and plasma CyA concentrations by FPIA and PC-RIA over the 14-day postoperative period in patient 11. For the CyA concentration figures: ● = FPIA and ○ = PC-RIA.
Table 1.
Ratio of FPIA to PC-RIA CyA Analysis in Plasma and WB
| FPIA/PC-RIA Ratio | Percentage of Results Within Ratio Range |
|||
|---|---|---|---|---|
| <1.5 | 1.5–2.5 | 2.5–3.5 | >3.5 | |
| Whole blood | 53.4 % | 45.4 % | 1.2 % | – |
| Plasma | 11.5% | 50.7% | 28.4% | 9.2% |
Considerable variability in measured CyA concentrations were observed for the 50 LT patients followed with daily FPIA WB concentrations. Day-to-day variations in measured trough concentrations were frequently 300 to 600 ng/mL, necessitating daily patient sampling for CyA blood concentrations to determine a trend in concentration changes. As previously documented with other assay techniques, dramatic changes in FPIA-assayed CyA blood concentrations frequently accompanied clamping the T - tube with return of bile to the small bowel. No clear association between FPIA-measured CyA levels and biopsy-proven rejection could be ascertained, with the occasional occurrence of mild to moderate rejection in patients with blood concentrations in the accepted 800 to 1200 ng/mL therapeutic blood concentration range. The FPIA assay did clearly demonstrate the effect of diltiazem to increase CyA concentrations, occasionally to twice the pre-diltiazem level.
Another drug interaction which has been reported using the PC-RIA to measure CyA was also detectable using the FPIA assay. A bolus of methylprednisolone, which was usually 1 g intravenously, frequently increased the FPIA blood concentration of Cy A. Previous report of this interaction has theorized that methylprednisolone is inhibiting the metabolism of CyA.6 but HPLC studies have not demonstrated that change.7 Intravenous corticosteroids may produce a redistribution or compete for biliary elimination of CyA metabolites, thereby increasing metabolites in blood that are detected by the immunoassays.
The studies of serum concentrations of CyA by FPIA in patients not receiving CyA provided additional information on the high levels observed in patients with hyperbilirubinemia. Twenty-six plasma samples were analyzed from 13 patients who underwent orthotopic liver transplantation and who were not receiving CyA at the time of the study. The highest concentration measured by FPIA in plasma was 148 ng/mL in a patient with a direct bilirubin of 18.3 mg/dL and in whom CyA had been discontinued 8 days earlier. Only 8 of the 26 samples had concentrations above 50 ng/mL in plasma; in each case, the patient had received CyA 4 to 15 days prior to obtaining the plasma sample. One patient had a serum direct bilirubin of 34.6 mg/dL and yet did not have any CyA detected by FPIA. Six plasma samples from 6 patients with cirrhosis who were being evaluated for LT were also analyzed; all 6 samples were assayed by FPIA as having less than 30 ng/mL CyA.
DISCUSSION
CyA measurement on the blood or plasma of LT patients is complicated by the presence of high concentrations of a large number of metabolites. Our studies of CyA metabolites in WB samples from various transplant populations have concentrated on the most lipid-soluble and pharmacologically active8 compounds such as M17, M1, M18, and M21. These metabolites are found primarily within red blood cells. Little quantitative information on the more polar di- and trihydroxylated CyA metabolites has been performed. The present study suggests that these polar metabolites may influence assay results for CyA using polyclonal antibody immunoassay techniques.
The FPIA assay for CyA produces results that are approximately double that of the PC-RIA in half of the samples analyzed from LT patients. Parallel changes in the FPIA results in relation to the PC-RIA assay would allow the use of this assay in the LT population since experience with CyA monitoring with the PC-RIA is extensive. We have observed that increases in the FPIA assay are however disproportionate with other assays when hepatic function is compromised through immunologic rejection or through technical complications. The results of the plasma studies in patients not receiving CyA confirmed two points: (1) that bilirubin alone has little influence on the FPIA assay as stated by the manufacturer and (2) assay variability results in the occasional detection of amounts of CyA in plasma by FPIA when the patient is not receiving the drug. Therefore, the most reasonable explanation for the increased FPIA concentrations during hyperbilirubinemia is that CyA metabolites accumulate in plasma and blood and cross-react with the FPIA to a greater extent than with the PC-RIA assay. The observation that plasma FPIA concentrations appear to be disproponionately influenced by hepatic dysfunction in relation to blood CyA concentrations suggests that the FPIA assay is more cross-reactive with the polar CyA metabolites than is the PC-RIA.
The clinical observations with the use of the FPIA CyA assay in monitoring LT patients demonstrate the usefulness of this analytical technique for patient monitoring when a kinetic assessment of CyA therapy is not required. Changes in FPIA whole blood concentrations were observed following T-tube clamping, during transition from intravenous to oral therapy, and in relation to other drug administration, such as methyl prednisolone1 and diltiazem.9 as would be expected using other assay methods.10 The day-to-day variability in trough WB CyA level measured by FPIA must be considered when monitoring an LT patient so that abrupt changes in therapy are not based upon single trough CyA observations. The disproportionate change in the FPIA assay in relation to the PC-RIA during hyperbilirubinemia also demands flexibility in what is accepted as the therapeutic range of CyA concentrations in WB. For example, trough CyA concentrations in LT patients were commonly observed in excess of the 800 to 1200 ng/mL therapeutic range, and yet were not associated with CyA-induced renal dysfunction in the population reviewed.
SUMMARY
The factors affecting CyA dosing and kinetics in LT patients are complex, and have been thoroughly investigated and reviewed.11 Plasma or WB CyA concentration monitoring remains the best method presently available for adjusting CyA dosage in LT patients in a timely manner. The availability of an FPIA assay for CyA has produced rapid drug analysis for transplant patient monitoring, but adds additional factors that must be considered in interpreting CyA concentrations. Liver dysfunction may disproportionately elevate CyA plasma or blood levels when analyzed by FPIA in relation to PC-RIA or HPLC, and adjustment of the therapeutic range or analysis by a more specific assay method may be necessary for dosage adjustment in these patients. The availability of a more specific antibody in an FPIA assay may avert these problems, as would the development of immunologic monitoring techniques that provide a global assessment of immune suppression produced by increasingly complex immunosuppressive regimens in LT patients.
Acknowledgments
Supported in part by Grant DK-34475. National Institutes of Health. Bethesda. Maryland.
References
- 1.Burckart GJ, Venkataramanan R, Ptachcinski RJ, et al. J Clin Pharmacol. 1986;26:647. doi: 10.1002/j.1552-4604.1986.tb03538.x. [DOI] [PubMed] [Google Scholar]
- 2.Burckart GJ, Ptachcinski RJ, Venkataramanan R, et al. Transplant Proc. 1986;18:188. [PMC free article] [PubMed] [Google Scholar]
- 3.Burckart GJ, Starzl TE, Williams L, et al. Transplant Proc. 1985;17:1172. [PMC free article] [PubMed] [Google Scholar]
- 4.Burckart GJ, Venkataramanan R, Ptachcinski RJ, et al. Transplant Proc. 1986;18:129. [PMC free article] [PubMed] [Google Scholar]
- 5.Sawchuk RJ, Cartier LL. Clin Chem. 1981;27:1368. [PubMed] [Google Scholar]
- 6.Klintmalm G, Sawe J. Lancet. 1984;1:731. doi: 10.1016/s0140-6736(84)92239-6. [DOI] [PubMed] [Google Scholar]
- 7.Ptachcinski RJ, Venkataramanan R, Burckart GJ, et al. Transplant Proc. 1987;19:1728. [PubMed] [Google Scholar]
- 8.Zeevi A, Venkataramanan R, Burckart GJ, et al. Human Immunol. 1988;21:143. doi: 10.1016/0198-8859(88)90089-4. [DOI] [PubMed] [Google Scholar]
- 9.McCauley J, Ptachcinski RJ, Shapiro R. Transplant Proc. (in press) [PubMed] [Google Scholar]
- 10.Venkataramanan R, Burckart GJ, Ptachcinski RJ. Seminars in Liver Dis. 1985;5:357. doi: 10.1055/s-2008-1040633. [DOI] [PubMed] [Google Scholar]
- 11.Venkataramanan R, Habucky K, Burckart GJ, et al. Clin Pharmacokinet. 1989;16:134. doi: 10.2165/00003088-198916030-00002. [DOI] [PubMed] [Google Scholar]