Abstract
Purpose
To evaluate rest and exercise hemodynamics in patients with abdominal aortic aneurysms (AAA) and peripheral occlusive disease (claudicants) using phase-contrast MRI.
Materials and Methods
Blood velocities were acquired via cardiac-gated cine phase-contrast in a 0.5T open MRI. Volumetric flow was calculated at the supraceliac (SC), infrarenal (IR), and mid-aneurysm (MA) levels during rest and upright cycling exercise using an MR-compatible exercise cycle.
Results
Mean blood flow increased during exercise (AAA: 130%, Claudicants: 136% of resting heart rate) at the SC and IR levels for AAA participants (2.6±0.6 vs. 5.8±1.6 L/min, P<0.001 and 0.8±0.4 vs. 5.1±1.7 L/min, P<0.001) and claudicants (2.3±0.5 vs. 4.5±0.9 L/min, P<0.005 and 0.8±0.2 vs. 3.3±0.9 L/min, P<0.005). AAA participants had a significant decrease in renal and digestive blood flow from rest to exercise (1.8±0.7 to 0.7±0.6 L/min, P<0.01). The decrease in renal and digestive blood flow during exercise correlated with daily activity level for claudicants (R=0.81).
Conclusion
Abdominal aortic hemodynamic changes due to lower extremity exercise can be quantified in patients with AAA and claudication using PC-MRI. The redistribution of blood flow during exercise was significant and different between the two disease states.
Keywords: exercise imaging, blood flow, vascular disease, adominal aortic aneurysm, phase-contrast MRI
INTRODUCTION
Aneurysmal and occlusive vascular disease represents two major forms of vascular degenerative conditions. Abdominal aortic aneurysms (AAA) pose risks of rupture and death in patients (1). Intermittent claudication is a symptom that may arise in advanced stages of lower extremity peripheral vascular occlusive disease. Clinically, intermittent claudication manifests itself primarily in exercise intolerance. During increased oxygen demands, reduced blood flow to metabolically active tissues may result in ischemia-related pain, weakness and gait changes. Supervised or independent exercise therapy, most commonly consisting of recommendations to increase walking activity is the primary initial management for intermittent claudication (2, 3), and long-term exercise has been shown to reduce cardiovascular and all-cause mortality associated with peripheral vascular occlusive disease (4). Although unproven, exercise training may also modify disease progression in AAA disease (5).
Recent advances and applications in cardiovascular imaging have documented the favorable changes in flow parameters as a result of exercise in healthy subjects. Exercise eliminates retrograde flow during diastole in the infrarenal aorta, while increasing wall shear stress at both the infrarenal and supraceliac levels (6, 7). These hemodynamic changes have been documented for healthy subjects (ages 50–70) during exercise (8) and may prevent progression of vascular disease. Advanced vascular disease states affecting the abdominal aorta and downstream vasculature include AAA and intermittent claudication although these disease states have not been previously characterized using phase-contrast MRI.
In this study, we characterized in vivo abdominal aortic blood flow conditions using MRI at rest and during upright lower limb exercise in participants with AAAs and intermittent claudication. Results from this study enable hemodynamic comparisons between these disease states, and may provide evidence for the role of exercise in altering the hemodynamic conditions that contribute to pathophysiology.
MATERIALS AND METHODS
Eight male participants with small AAA disease and six male participants with peripheral arterial occlusive disease and symptoms of intermittent claudication were recruited to participate in the study. Patients with the diagnosis of intermittent claudication were recruited from an outpatient vascular clinic. All patients were capable of exercise, and patients with rest pain or limb amputation were excluded from participation. Ankle Brachial Index values were calculated for claudicants (mean ABI = 0.70 ± 0.17). Of the claudicants, three of six patients had a history of tobacco use and three patients had a history of diabetes. Patients with AAA disease were recruited from three hospital systems. Two of eight patients currently used tobacco products (four patients had a history of tobacco use) and none of these patients had a history of diabetes. The presence and severity of AAA disease was confirmed using abdominal ultrasonography (mean diameter = 3.8 ± 0.6 cm, range = 3.0 to 4.6 cm). All subjects were capable of exercising and complied with the imaging protocol. The Institutional Review Boards (IRB) from each recruitment center approved the study-related protocol.
Data was collected in a 0.5T interventional magnet (GE Signa SP, GE Medical Systems, Milwaukee, WI) at seated rest and during seated lower limb exercise performed on a custom-built MR-compatible exercise cycle (Fig. 1) (9). The exercise cycle was positioned to allow for full range of leg motion, and resistance and workload were adjusted to match patient ability level and to reach desired increase in heart rate. Participants were instructed to adjust their pedaling and workload to reach a target of mild to moderate exercise levels (up to 150% resting heart rate) and were given verbal instructions to adjust pedaling speed to maintain a constant heart rate during the exercise scans over a duration of 10–15 minutes. Each subject was positioned in the center of the magnet and securely strapped to the seat to ensure optimal image quality and minimize motion artifact.
Figure 1.
MR Compatible Exercise Cycle composed of plastic and wood was used to perform exercise for each participant in the 0.5T interventional magnet. Adjustments of resistance provided ability-appropriate exercise to reach target increase in heart rate of up to 50%.
Cine phase contrast MRI (PC-MRI) acquisitions measured axial blood velocities in the abdominal aorta at up to three anatomical positions: superior to the celiac artery (supraceliac), immediately inferior to the renal arteries (infrarenal), and mid-aneurysm (defined at level of maximal dilatation) for AAA participants (Fig. 2). Double oblique localizers were performed to ensure perpendicular prescription to the vessel lumen at each anatomical location. From these prescriptions, upright resting scans were performed, followed by exercise scans. The cine PC-MRI acquisition was cardiac-gated using a plethysmograph and respiratory-compensated using chest bellows positioned inferior to the xyphoid process. Scan parameters included: 25 ms TR, 9 ms TE, 30° flip angle, 10-mm slice thickness, appropriate field of view (26 to 32 cm square field of view) and image matrix (256 X 128 or 192), 150 cm/s through-plane velocity encoding, and reconstruction to a minimum of 16 time points per cardiac cycle.
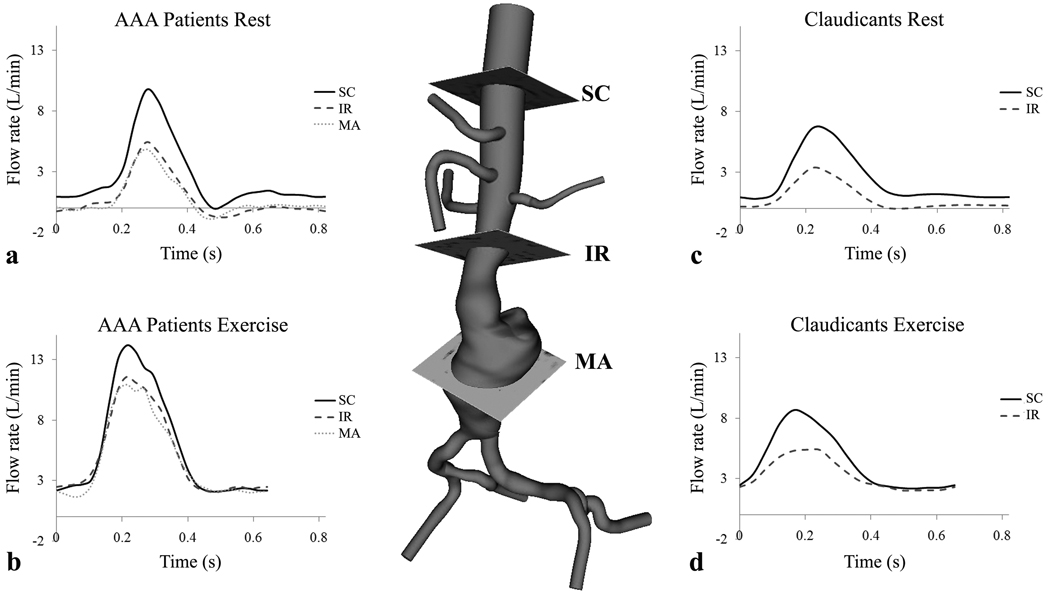
Figure 2.
A three-dimensional model of an AAA and imaging slices for supraceliac (SC), infrarenal (IR), and mid-aneurysm (MA) positions. During rest, AAA participants had retrograde flow at each anatomic location (a), and claudicants had retrograde flow at the infrarenal level only during diastole (c). During exercise, the peak systolic blood flow rate increases, diastole shortens, and retrograde flow is eliminated for AAA participants (b) and claudicants (d). Note that at rest, AAA participants exhibited a triphasic waveform (a) compared to a biphasic waveform for claudicants (c).
Using manual segmentation on the magnitude images, vessel lumen boundaries were defined. Using these lumen boundaries, integration of blood velocities for each time step was used to calculate instantaneous blood flow rates and cross-sectional areas at each anatomical landmark. To minimize phase errors that result from gradient eddy currents and concomitant magnetic fields, second-order baseline corrections were applied to each time frame (10). From these time-resolved flow data, blood flow rate (11) and oscillatory flow index (OFI) (12) for each vessel position at rest and exercise were calculated. OFI was defined by:
where T is the period of the cardiac cycle and Q is the blood flow rate. An OFI value of zero denotes positive flow throughout the cardiac cycle whereas a value of 0.5 correspond to equal amounts of antegrade and retrograde flow.
Statistical Analysis
Average population data are reported as means ± SD. Paired two-tailed t-tests were performed within subject groups with Bonferroni-Holm adjustment for multiple comparisons for hemodynamic values (mean flow rate and OFI) between aortic locations and between rest and exercise conditions (13). Comparisons between populations were performed by unpaired two-tailed t-tests. Statistical significance was set at a threshold value of P < 0.05.
RESULTS
Demographic data for each group is listed in Table 1. Rest and exercise heart rates were 73 ± 13 and 94 ± 14 bpm for AAA participants and 68 ± 11 and 92 ± 16 bpm for claudicants, representing heart rate increases during exercise of 30 ± 12% and 36 ± 18%, respectively.
Table 1.
Demographic Data for Subject Populations
| AAA Patients (N=8) | Claudicants (N=6) | Statistical Significance (P-value) a | |
|---|---|---|---|
| Age | 72.5 ± 9.3 | 62.3 ± 10.3 | ns |
| Height (cm) | 180.0 ± 9.0 | 174.0 ± 4.0 | ns |
| Weight (kg) | 77.0 ± 15 | 83.0 ± 11 | ns |
| BMI | 27.3 ± 2.4 | 27.4 ± 2.5 | ns |
| Resting HR | 73.0 ± 13 | 68.0 ± 11 | ns |
| Exercise HR | 94.0 ± 14 | 92.0 ± 16 | ns |
| % Change | 30.0 ± 12 | 36.0 ± 18 | ns |
P-value < 0.05
Values are means ± SD. A P-value of < 0.05 indicates a statistically significant difference between groups. Both groups had similar age, body composition, and heart rate values during rest and exercise and were able to increase their heart rates similarly during exercise.
Lumen cross-sectional area was greater at the supraceliac level for AAA participants (5.4 ± 1.0 cm2) than claudicants (3.6 ± 0.4 cm2, P < 0.005) as measured during rest. Lumen cross-sectional area was also greater at the infrarenal level for AAA participants (4.2 ± 0.9 cm2) than claudicants (2.0 ± 0.5 cm2, P < 0.001).
Time scaled blood flow waveforms of rest and exercise conditions for AAA (Fig. 2a, 2b) and intermittent claudication participants (Fig. 2c, 2d) are depicted in Fig. 2. Two data sets acquired during exercise (one of mid-aneurysm flow values for an AAA participant and one of infrarenal flow values for a claudicant) were excluded from analysis due to poor image quality. Note that during exercise, peak systolic blood flow rate increased, diastole shortened, and retrograde flow was eliminated in both groups. Average peak systolic blood flow values were lower for claudicants than AAA participants during rest at the supraceliac (6.8 ± 0.8 to 9.7 ± 2.3 L/min, P < 0.01) and infrarenal positions (3.3 ± 0.7 to 5.4 ± 1.1 L/min, P < 0.01). Differences in peak systolic flow were also recorded during exercise at the supraceliac (8.6 ± 1.9 to 14.1 ± 4.0 L/min, P < 0.01) and infrarenal position (5.6 ± 1.0 to 11.6 ± 3.7 L/min, P < 0.01) for claudicants compared to AAA participants.
Group averages for mean blood flow and oscillatory flow index for both groups during rest and exercise are listed in Table 2. Mean blood flow for AAA participants increased from 2.6 ± 0.6 to 5.8 ± 1.6 L/min at the supraceliac level (P < 0.001), 0.8 ± 0.4 to 5.1 ± 1.7 L/min at the infrarenal level (P < 0.001), and 0.8 ± 0.4 to 4.8 ± 1.7 L/min at the mid-aneurysm level (P < 0.001) from rest to exercise. Claudicants recorded gains in blood flow from 2.3 ± 0.5 to 4.5 ± 0.9 L/min at the supraceliac level (P < 0.005) and 0.8 ± 0.2 to 3.3 ± 0.9 L/min at the infrarenal level (P < 0.005) with exercise. Infrarenal flow was subtracted from supraceliac flow to compute blood flow distributed to the renal and digestive (celiac trunk, superior mesentery artery) circulations. The AAA participants experienced reduced renal and digestive blood flow from rest (1.8 ± 0.7 L/min) to exercise (0.7 ± 0.6 L/min, P < 0.005), a finding not seen in claudicants (1.5 ± 0.5 to 1.5 ± 0.8 L/min, ns).
Table 2.
Rest and Exercise Values for subject populations
| AAA Patients (N=8) |
Claudicants (N=6) |
Between Group P-Value |
||||||
|---|---|---|---|---|---|---|---|---|
| Rest | Exercise | P-value | Rest | Exercise | P-value | Rest | Exercise | |
| Blood flow rate in 1/min | ||||||||
| Supraceliac | 2.6 ±0.6 | 5.8 ±1.6 | <0.001 | 2.3 ±0.5 | 4.5 ±0.9 | <0.005 | ns | ns |
| Infrarenal | 0.8 ± 0.4 | 5.1 ± 1.7 | <0.001 | 0.8 ± 0.2 | 3.3 ± 0.9 | <0.001 | ns | ns |
| Mid-Aneurysm | 0.8 ± 0.4 | 4.8 ± 1.7 | <0.001 | -- | -- | -- | -- | -- |
| SC-IR | 1.8 ± 0.7 | 0.7 ± 0.6 | <0.005 | 1.5 ± 0.5 | 1.5 ± 0.8 | ns | ns | ns |
| OFI | ||||||||
| Supraceliac | 0.02 ± 0.01 | 0.00 ± 0.00 | <0.025 | 0.00 ± 0.00 | 0.00 ± 0.00 | ns | <0.05 | ns |
| Infrarenal | 0.18 ± 0.14 | 0.00 ± 0.01 | <0.010 | 0.03 ± 0.04 | 0.00 ± 0.00 | ns | <0.05 | ns |
| Mid-Aneurysm | 0.19 ± 0.12 | 0.02 ± 0.04 | <0.010 | -- | -- | -- | -- | -- |
Values are means ± SD. Both groups had significant increases in blood flow at each anatomical position from rest to exercise. All groups had non-zero OFI values at the infrarenal position that were nearly eliminated during exercise. AAA participants had non-zero OFI at SC that was significantly reduced during exercise. AAA participants had a reduction in SC to IR flow (renal and digestive blood flow) during exercise, a finding not seen in claudicants. P-values are listed by threshold of significance accounting for Bonferroni-Holm adjustments for multiple comparisons, and ns indicates not significant.
Oscillatory flow index was decreased for AAA participants at the SC, IR, and MA locations due to exercise (Table 2). In contrast, claudicants had little or no retrograde flow at the SC and IR locations at rest or during exercise. At rest, claudicants had lower OFI at the infrarenal level (0.03 ± 0.04) compared to AAA participants (0.18 ± 0.14, P < 0.05).
DISCUSSION
The supraceliac and infrarenal cross-sectional areas were greater in the AAA participants than claudicants, a result consistent with the presence of aneurysmal disease. Mean blood flow values were similar between AAA participants and claudicants at the supraceliac and infrarenal levels during rest. With exercise, both groups significantly increased their mean blood flow values at each location with similar increases in heart rate.
Significant decreases in renal and digestive blood flow were recorded for AAA participants from rest to exercise. Dilatation of peripheral downstream arterioles in metabolically active tissues provides a path of lower resistance and may explain the preferential shunt in blood flow to the lower extremities. Accordingly, OFI at the supraceliac and infrarenal positions is largely eliminated for AAA participants during exercise. The large difference between supraceliac and infrarenal flow during rest may contribute to the pathophysiology of infrarenal AAA disease, and exercise appears to significantly reduce this difference in blood flow.
In contrast, claudicants did not exhibit a decrease in renal and digestive blood flow during exercise compared to rest. Advanced occlusive vascular disease may alter physiological mechanisms that normally lower resistance to flow in distal vascular beds during exercise. In addition, there was a strong linear correlation (R = 0.81) between subjectively reported daily activity level (rated on a scale of 1 = sedentary, 2 = somewhat active, 3 = active, 4 = extremely active) and the amount of flow decrease to the renal and digestive circulation. The variance for individual response to exercise likely represents severity in peripheral vascular disease process, including the degree and extent of vascular involvement. For subjects with ineffective redistribution of blood flow to the lower extremities during exercise, metabolically active tissues may develop unmet oxygen demands that result in ischemia, exercise intolerance, and pain. Interestingly, this described method of measuring changes in renal and digestive blood flow may provide a tool for monitoring downstream adaptations in blood vessels related to exercise.
At rest, the claudicants exhibited a biphasic flow (constant flow during diastole) waveform at the SC and IR position (Fig. 2c) compared to the triphasic wave forms (a decrease followed by an increase in flow during diastole) as seen in AAA participants (Fig. 2a) and healthy subjects (8). Compared to AAA participants, claudicants have lower OFI values at the supraceliac and infrarenal level during rest and have lower recorded peak systolic values at rest and exercise. This can be explained by occlusive vascular disease in claudicants decreasing compliance and elastic recoil, thereby dampening peak-to-peak flow pulsatility. Cheng, et al. 2003 reported on a healthy population 50–70 years old with resting OFI values of 0.14 ± 0.09 at the infrarenal position during rest that was reduced to zero during exercise (8). The AAA participants had similar recorded resting OFI values at this location and responded with near elimination of OFI flow during exercise, despite having advanced vascular disease. Exercise increases the proportion of the cardiac cycle spent in systole, which helps provide greater flow rate and reduces stagnation in aneurysms. Reduction of OFI for AAA participants during exercise may provide hemodynamic conditions that slow the course of disease progression (6, 12, 14).
In a murine model, exercise has been shown to alter the expression of several genes in the aorta including nitric oxide synthase and prostaglandins that may be cardioprotective (15). The periodic flushing effect of blood flow through the aneurysm during exercise may also disrupt the natural course of disease and provide a therapeutic tool to slow the progression of aneurysm dilatation and increased thrombus burden(16, 17). Long-term low-grade exercise has been shown to decrease platelet aggregation (18), which is a key component in the inflammatory process and thrombus formation. Although clinical bias may exist to avoid exercise at risk of dissection or rupture, AAA participants with small aneurysms demonstrated significant hemodynamic changes that were similar to previous values reported in healthy controls and hypothesized to promote vascular health (8).
Future research must answer additional questions related to exercise therapy for patients with AAA and claudication disease. The findings reported here only reflect blood flow conditions during rest and exercise for male participants at one time point. Females develop AAA disease at older ages and have significant differences in therapeutic options and clinical outcomes(19). Further work is warranted to investigate optimal activity levels for patients with each form of vascular disease. Follow-up imaging at multiple time points could eludicate long-term adaptations to exercise. Additionally, findings from this study provide in vivo baseline and exercise blood flow conditions that can be applied to computerized modeling of each disease state and used to calculate additional elements of hemodynamic flow including wall shear stress.
In conclusion, this study presents in vivo abdominal aortic hemodynamic conditions at rest and during lower limb exercise in participants with advanced aneurysmal and occlusive vascular disease. Both patient populations saw significant increases in mean blood flow and near elimination of OFI at each anatomical position during exercise. AAA participants had a significant reduction in renal and digestive blood flow in response to exercise that is not observed in claudicants. Claudicants were observed to have individual differences in exercise responses characterized by decreasing renal and digestive blood flow that correlate with subjectively reported activity levels, which may provide a novel, patient-specific imaging diagnostic tool to assess the severity of occlusive vascular disease. This research demonstrates that mild exercise provides changes in hemodynamic conditions that may provide benefits in cardiovascular health for individuals with advanced vascular disease.
ACKNOWLEDGMENTS
Grant Support: National Institutes of Health (P50 HL083800, P41 RR09784, 2R01 HL064338).
Authors acknowledge the assistance of Simron Gill, Julie White, and Mary McElrath. Authors would also like to thank study volunteers for their participation.
REFERENCES
- 1.McPhee JT, Hill JS, Eslami MH. To what extent does patient gender impact presentation, therapy and mortality for abdominal aortic aneurysm (AAA) in the United States, 2001–2004? J Vasc Surg. 2007;45:891–899. doi: 10.1016/j.jvs.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 2.Kruidenier LM, Bendermacher BL, Willigendael EM, Teijink JA, Prins MH. From the Cochrane Library: increased walking distance through supervised exercise therapy in patients with intermittent claudication. Ned Tijdschr Geneeskd. 2008;152:321–323. [PubMed] [Google Scholar]
- 3.Hankey GJ, Norman PE, Eikelboom JW. Medical treatment of peripheral arterial disease. Jama. 2006;295:547–553. doi: 10.1001/jama.295.5.547. [DOI] [PubMed] [Google Scholar]
- 4.Gardner AW, Montgomery PS, Parker DE. Physical activity is a predictor of all-cause mortality in patients with intermittent claudication. J Vasc Surg. 2008;47:117–122. doi: 10.1016/j.jvs.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalman RL, Tedesco MM, Myers J, Taylor CA. AAA disease: mechanism, stratification, and treatment. Ann N Y Acad Sci. 2006;1085:92–109. doi: 10.1196/annals.1383.008. [DOI] [PubMed] [Google Scholar]
- 6.Taylor CA, Hughes TJ, Zarins CK. Effect of exercise on hemodynamic conditions in the abdominal aorta. J Vasc Surg. 1999;29:1077–1089. doi: 10.1016/s0741-5214(99)70249-1. [DOI] [PubMed] [Google Scholar]
- 7.Tang BT, Cheng CP, Draney MT, et al. Abdominal aortic hemodynamics in young healthy adults at rest and during lower limb exercise: quantification using image-based computer modeling. Am J Physiol Heart Circ Physiol. 2006;29:H668–H676. doi: 10.1152/ajpheart.01301.2005. [DOI] [PubMed] [Google Scholar]
- 8.Cheng CP, Herfkens RJ, Taylor CA. Abdominal aortic hemodynamic conditions in healthy subjects aged 50–70 at rest and during lower limb exercise: in vivo quantification using MRI. Atherosclerosis. 2003;168:323–331. doi: 10.1016/s0021-9150(03)00099-6. [DOI] [PubMed] [Google Scholar]
- 9.Cheng CP, Herfkens RJ, Taylor CA. Dynamic Exercise Imaging with an MR-Compatible Bicycle within the GE Open-Magnet. Magnetic Resonance in Medicine. 2003;49:581–585. doi: 10.1002/mrm.10364. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein MA, Zhou XJ, Polzin JA, et al. Concomitant gradient terms in phase contrast MR: analysis and correction. Magn Reson Med. 1998;39:300–308. doi: 10.1002/mrm.1910390218. [DOI] [PubMed] [Google Scholar]
- 11.Pelc NJ, Sommer FG, Li KC, Brosnan TJ, Herfkens RJ, Enzmann DR. Quantitative magnetic resonance flow imaging. Magn Reson Q. 1994;10:125–147. [PubMed] [Google Scholar]
- 12.Taylor CA, Cheng CP, Espinosa LA, Tang BT, Parker D, Herfkens RJ. In vivo quantification of blood flow and wall shear stress in the human abdominal aorta during lower limb exercise. Ann Biomed Eng. 2002;30:402–408. doi: 10.1114/1.1476016. [DOI] [PubMed] [Google Scholar]
- 13.Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- 14.Moore JE, Jr, Ku DN. Pulsatile velocity measurements in a model of the human abdominal aorta under simulated exercise and postprandial conditions. J Biomech Eng. 1994;116:107–111. doi: 10.1115/1.2895692. [DOI] [PubMed] [Google Scholar]
- 15.Maeda S, Iemitsu M, Miyauchi T, Kuno S, Matsuda M, Tanaka H. Aortic stiffness and aerobic exercise: mechanistic insight from microarray analyses. Med Sci Sports Exerc. 2005;37:1710–1716. doi: 10.1249/01.mss.0000175052.37087.f8. [DOI] [PubMed] [Google Scholar]
- 16.Hoshina K, Sho E, Sho M, Nakahashi TK, Dalman RL. Wall shear stress and strain modulate experimental aneurysm cellularity. J Vasc Surg. 2003;37:1067–1074. doi: 10.1016/s0741-5214(03)70052-4. [DOI] [PubMed] [Google Scholar]
- 17.Nakahashi TK, Hoshina K, Tsao PS, et al. Flow loading induces macrophage antioxidative gene expression in experimental aneurysms. Arterioscler Thromb Vasc Biol. 2002;22:2017–2022. doi: 10.1161/01.atv.0000042082.38014.ea. [DOI] [PubMed] [Google Scholar]
- 18.de Meirelles LR, Mendes-Ribeiro AC, Mendes MA, et al. Chronic exercise reduces platelet activation in hypertension: upregulation of the l-arginine-nitric oxide pathway. Scand J Med Sci Sports. 2009;19:67–74. doi: 10.1111/j.1600-0838.2007.00755.x. [DOI] [PubMed] [Google Scholar]
- 19.Dillavou ED, Muluk SC, Makaroun MS. A decade of change in abdominal aortic aneurysm repair in the United States: Have we improved outcomes equally between men and women? J Vasc Surg. 2006;43:230–238. doi: 10.1016/j.jvs.2005.09.043. discussion 238. [DOI] [PubMed] [Google Scholar]