Abstract
Asymmetric dimethylarginine inhibits nitric oxide synthase, cationic amino acid transport and endothelial function. Patients with cardiovascular risk factors often have endothelial dysfunction associated with increased plasma asymmetric dimethylarginine and markers of reactive oxygen species. We tested the hypothesis that reactive oxygen species, generated by nicotinamide adenine dinucleotide phosphate oxidase, enhance cellular asymmetric dimethylarginine. Incubation of rat preglomerular vascular smooth muscle cells with angiotensin II doubled the activity of nicotinamide adenine dinucleotide phosphate oxidase, but decreased the activities of dimethylarginine dimethylaminohydrolase by 35% and of cationic amino acid transport by 20% and doubled cellular (but not medium) asymmetric dimethylarginine concentrations (p<0.01). This was blocked by tempol or candesartan. Cells stably transfected with p22phox had a 50% decreased protein expression and activity of dimethylarginine dimethylaminohydrolase despite increased promoter activity and mRNA. The decreased DDAH protein expression and the increased asymmetric dimethylarginine concentration in p22phox transfected cells were prevented by proteosomal inhibition. These cells had enhanced protein arginine methylation, a 2-fold increased expression of protein arginine methyltransferase-3 (p<0.05), and a 30% reduction in cationic amino acid transport activity (p<0.05). Asymmetric dimethylarginine was increased from 6±1 to 16±3μmol·l−1 (p<0.005) in p22phox transfected cells. Thus, angiotensin II increased cellular asymmetric dimethylarginine via type 1 receptors and reactive oxygen species. Nicotinamide adenine dinucleotide phosphate oxidase increased cellular asymmetric dimethylarginine by increasing enzymes that generate it, enhancing the degradation of enzymes that metabolize it, and reducing its cellular transport. This could underlie increases in cellular asymmetric dimethylarginine during oxidative stress.
Keywords: dimethylarginine dimethylaminohydrolase (DDAH), protein arginine methyltransferase (PRMT), tempol, cationic aminoacid transferase (CAT), hypertension
Introduction
Asymmetric dimethylarginine (ADMA) inhibits nitric oxide synthase (NOS) and cationic amino acid transport (CAT) 1. ADMA is generated by protein arginine methyltransferases (PRMTs) and, after proteolysis, cellular ADMA is metabolized by dimethylarginine dimethylaminohydrolases (DDAHs) or exported by CATs 2,3. Angiotensin II (Ang II) can generate reactive oxygen species (ROS) in blood vessels by activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4. Patients with early hypertension or kidney disease have elevated plasma levels of ADMA and markers of ROS 5 which may contribute to endothelial dysfunction and subsequent cardiovascular or renal events. While increased ADMA occurs in several conditions associated with ROS 6, it is unclear how ROS increase ADMA. Moreover, infusions of Ang II sufficient to increase ROS have variable effects on plasma ADMA 7–9.
The present studies were designed to test the hypothesis that NADPH oxidase enhances PRMT and/or reduces DDAH, but that a reduction in CAT activity may limit cellular ADMA export. First, we assessed the effects of Ang II on ROS and ADMA in cultured cells. Thereafter, we investigated the mechanism of NADPH oxidase-induced changes in ADMA directly in cells stably transfected with p22phox which increases NADPH oxidase activity 10. We selected preglomerular vascular smooth muscle cells (PGVSMCs) since the afferent arteriole is the main resistance vessel in the kidney and generates ROS with Ang II 11 and VSMCs produce little nitric oxide (NO), which obviates its confounding effects on DDAH activity 12.
Methods
All animal care and experimental procedures complied with National Institutes of Health guidelines and were approved by Georgetown University Animal Care and Use Committee. Details of methods appear in supplement (please see http://hyper.ahajournals.org).
Cell culture
Preglomerular vascular smooth muscle cells (PGVSMCs) were isolated from 13 to 15 week old male WKY rats purchased from Tacomic Farms (Germantown, N.Y., USA) as described in detail 11 (see supplement).
Measurement of superoxide production in PGVSMCs
Cells were seeded into a 96 well plate at densities of 1 × 105 cells per well in 200 μl of the DMEM-F12 medium. After 24 hours, the cells were incubated overnight in serum free medium, which was replaced 4 hours before incubation with added vehicle or indicated dose of Ang II. Measurement of superoxide (O2· −) was as described in detail for these cells using low concentration (5μM) lucigenin-enhanced chemiluminescence 11. NADPH oxidase activity was assessed from the increase in superoxide generated after addition of 100 μM of NADPH.
Overexpression of NADPH oxidase subunit p22phox in rat preglomerular vascular smooth muscle cells
A full-length rat p22phox cDNA fragment (709 bp) was cloned into the EcoR I/Xba I site of pcDNA4 HisMax vector which contained ampicillin and zeocin-resistant genes to allow positive clone (see supplement).
DDAH2 promoter activity assay
The mouse DDAH-2 promoter (−924 to −36 bp from transcription start site) that drives a luciferase construct was cloned into pGL3-Basic vector (Promega) as described previously 13. This was a gift from Dr. Satoshi Tanaka (The University of Tokyo, Japan) (see supplement).
Medium or cell lysate ADMA, symmetric dimethylarginine (SDMA) and L-arginine
VSMCs were grown to full confluence in 100-mm dishes and cultured for 48h in 5 ml of serum-free, phenol-red-free medium. Some dishes of cells were directly treated with indicated dose of Ang II for 48h and some of them were pretreated for 2 hours with Candesartan(10−7 M), Candesartan + PD-123,319 (3×10−6 M) or Tempol(10−4 M) and then coincubated with vehicle or Ang II (10−6 M) for 48 hours. Measurement of ADMA, SDMA, and L-arginine in the medium and cell lysate was performed using HPLC as previously described 14 (see supplement).
Medium H2O2
Measurement of H2O2 released fromintact PGVSMCs was performed using Amplex Red hydrogen peroxide/peroxidase assay kit (Molecular Probes Inc., OR) (see supplement).
DDAH activity
The conversion of [14C]-ADMA to [14C]-citrulline was used to quantify DDAH activity based on previous assays 15 (see supplement).
CAT activity assays
CAT activity was assessed in intact cells from 10mM lysine-inhibitable [14C]-ADMA uptake 2 (see supplement).
RNA isolation and real-time quantitative RT-PCR
Real-time quantitative PCR was performed in anABI Prism 7700 sequence-detection system (Applied Biosystems, Foster City, CA) as described 16 (see supplement).
Protein expression
This used Western blot as described 16 (see supplement).
Analysis of protein carbonylation
The carbonylated proteins from lysed cells were detected with an OxyBlot Oxidized Protein Detection Kit (Millipore, Billerica, MA) as previously reported 17 (see supplement).
Materials
Ang II, tempol and NADPH were obtained from Sigma-Aldrich (St. Louis, M.O., USA), DMEM-F12 from Gibco (Carlsband, C.A., USA) and FBS from American Type Culture Collection (Manassas, V.A., USA).
Statistical analysis
Results are expressed as mean +/− standard error of the mean. Analysis of variance was performed and differences between experimental groups were compared by Students t test, when appropriate. A value of p<0.05 was considered as statistically significant.
Results
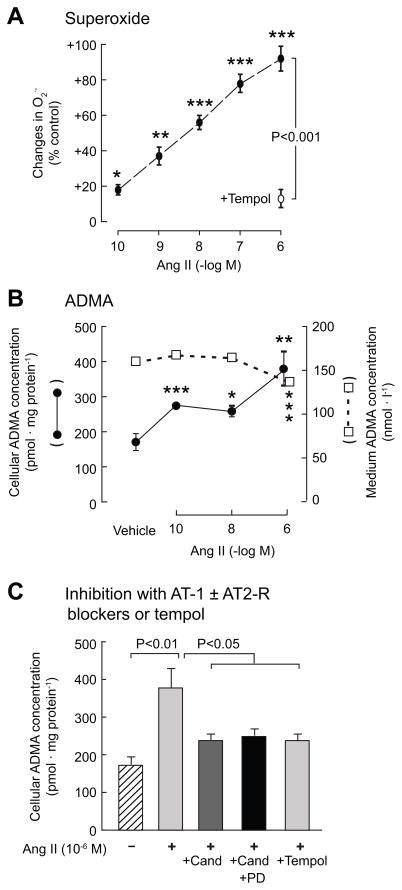
Ang II produced concentration-dependent increases in cellular O2· − from 10−10M (Fig 1A), confirming prior findings 11. Cellular ADMA doubled with 10−6M Ang II but the medium ADMA was modestly reduced (Fig 1B). Coincubation of Ang II-stimulated cells with candesartan or tempol reduced cellular ADMA (Fig 1C), which was not altered by addition of the AT2-R blocker PD-123,319. Ang II increased the activity of NADPH oxidase (2-fold; p<0.01; Fig 2A), but decreased the activities of DDAH by 35% (p<0.01) and CAT by 20% (p<0.05) (Fig 2B and 2C).
Figure 1.
Mean ± SEM values for preglomerular vascular smooth muscle cells (n=6 per group) incubated for 4 hours with angiotensin II showing changes in superoxide and the effects of 10−4M tempol (panel A), on asymmetric dimethylarginine in the medium (open circles) and cell lysate (close circles) (panel B) and the effects of coincubation with candesartan (10−7M), PD-123,319 (3×10−6M) or tempol (10−4M). Compared to vehicle, *, p<0.05; **, p<0.01; ***, p<0.005.
Figure 2.
Mean ± SEM values (n=3 per group) for cells incubated with a vehicle (open boxes) or angiotensin II (10−6M; closed boxes) for NADPH oxidase, DDAH and CAT activities after incubation for 4 hours with vehicle or 10−6M angiotensin II. Compared to vehicle: *, p<0.05; **p<0.01.
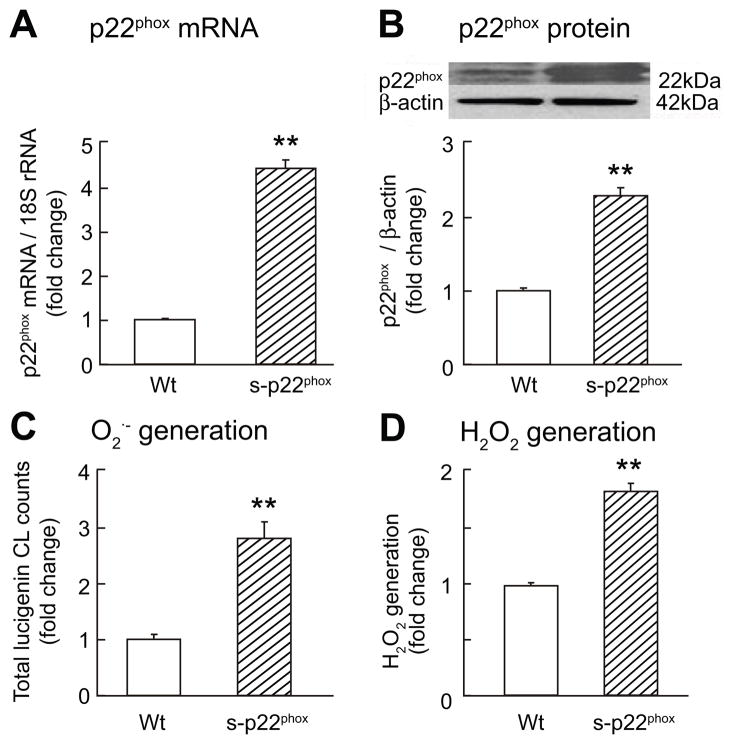
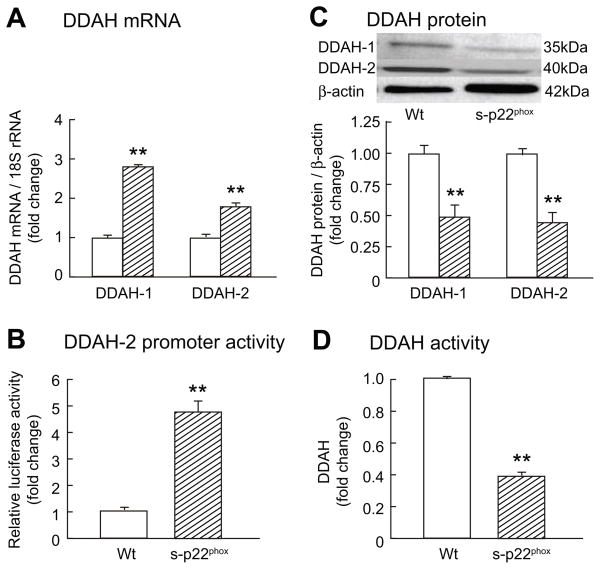
Cells stably transfected with p22phox (S- p22phox) had an increased expression of NOX-1 mRNA (2.4±0.37 fold; p<0.02) and protein (2.9±0.1 fold; p<0.003) but no significant changes for NOX-4 (Supplement Fig S1). Transcripts or protein for NOS-1, -2 or -3 were not detected in PGVSMCs (data not shown). The S- p22phox cells had a 2 -fold increased p22phox (p<0.01) and a 2½-fold increased O2· − and H2O2 (p<0.01; Fig 3). The mRNA for DDAH-1 and DDAH-2 (Fig 4A) and the DDAH-2 promoter activity (Fig 4B) were increased by 2- to 4-fold (p<0.01) in S-p22phox cells. However, the protein expression for DDAH-1 and DDAH-2 were reduced by 50% (p<0.01; Fig 4C) and the DDAH activity was reduced correspondingly (p<0.01; Fig 4D). S-p22phox cells had a marked increase in protein carbonyls that was abolished by incubation with catalase and tempol (Supplement Fig S2). After incubation of S-p22phox cells with 1 μM epoxomicin to inhibit proteosomal degradation, DDAH-1 and -2 expression increased by 50% (p<0.05; Supplement Fig S3). This was accompanied by a reduction in cellular concentrations of ADMA in S-p22phox cells to the level measured in Wt cells (Supplement Fig S4). The increased mRNA expression for DDAH-1 and -2 in S-p22phox cells persisted after epoxomicin treatment (DDAH-1 mRNA 1.6±0.1 fold increase; p<0.02 and DDAH-2 2.4±0.3 fold increase; p<0.02; data not shown).
Figure 3.
Mean ± SEM values (n=3 per group) for cells stably transfected with an empty vector (Wt, open boxes) or p22phox (S-p22phox, cross-hatched boxes). Compared to Wt: **, p<0.01.
Figure 4.
Mean ± SEM values (n=3 per group). See legend to Figure 3.
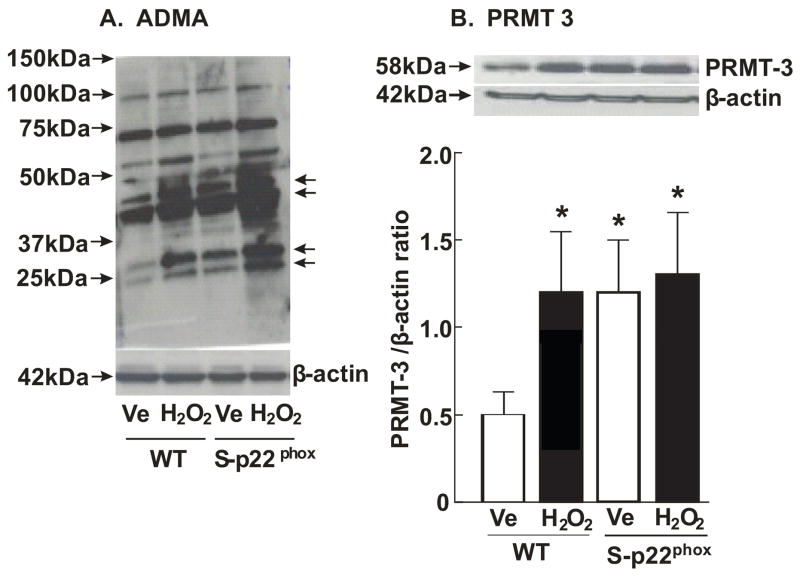
There was a marked increase in asymmetric dimethylation of proteins in S-p22phox cells and in cells incubated for 30 minutes with 100 μM H2O2 (Fig 5A) accompanied by a doubling of PRMT-3 expression (p<0.05; Fig 5B).
Figure 5.
Panel A, Western blots for asymmetrically demethylated arginine moieties on proteins (Panel A) and PRMT-3 expression in these cells (n=3 per group; Panel B).
CAT activity, assessed as lysine-inhibitable [14C]-ADMA cellular uptake (Fig 6A) was reduced by 30% in S-p22phox cells (p<0.05; Fig 6B), accompanied by a 70% reduction in CAT-1 mRNA expression (p<0.01) that was mimicked by incubation of Wt cells with H2O2 (Fig 6C).
Figure 6.
Panel A, cell diagram of method to measure CAT activity. Panel B, CAT activity showing [14C]-ADMA uptake (open bars) and the effects of inhibition with 10mM lysine (hatched bars). Panel C, CAT-1 mRNA expression in Wt cells treated with vehicle (open bars) or H2O2 (cross-hatched bars) and in S-p22phox cells (filled bars) (n=3 per group).
S-p22phox cells grown in culture medium containing L-arginine had a 10% increase in medium L-arginine concentration (p<0.005), a 60% increase in medium ADMA (p<0.005) and a 5% reduction in medium SDMA (p<0.05; Table 1a). The concentrations in cell water were calculated from the relationship between cell water and cell protein content in rat cultured VSMCs 18. S-p22phox cells had a doubling of cellular L-arginine (p<0.005), a 2.6-fold increase in cellular ADMA (p<0.005) and a 3-fold increase in cellular SDMA (p<0.05; Table 1b).
Table 1.
Concentrations of L-arginine, ADMA and SDMA in the medium and cell lysate or cell water of preglomerular vascular smooth muscle cells transfected with p22phox or wild-type cells transfected with an empty vector
| Cell type | Arginine | ADMA | SDMA |
|---|---|---|---|
| (a) Medium | |||
| μmol·l−1 |
nmol·l−1 |
||
| Wt. | 529 ± 5 | 220 ± 7 | 143 ± 6 |
| S-p22phox | 588 ± 2 | 357 ± 4 | 135 ± 4 |
| p value: | <0.005 | < 0.005 | < 0.05 |
| (b) Calculated concentrations in cell water* | |||
| μmol·l−1 |
μmol·l−1 |
||
| Wt. | 1,645 ± 63 | 5.9 ± 1.2 | 1.6 ± 0.7 |
| S-p22phox | 3,222 ± 340 | 15.7 ± 3.4 | 4.9 ± 2.7 |
| p value: | <0.005 | < 0.005 | < 0.05 |
| (c) Cell: Medium concentration ratio* | |||
| Wt. | 3.1 ± 0.06 | 26.9 ± 3.5 | 11.5 ± 2.8 |
| S-p22phox | 5.5 ± 0.33 | 51.9 ± 13.9 | 36.1 ± 12.0 |
| p value: | <0.005 | < 0.05 | < 0.05 |
Mean ± SEM (n=6 per group);
based on a cell water content of cultured vascular smooth muscle cells of 2.53 μl·mg protein−1.18
The cell:medium concentration ratio for L-arginine, ADMA and SDMA were all increased significantly (p<0.05) in S-p22phox cells (Table 1c).
Discussion
The main new findings are: Incubation of VSMCs with Ang II for 4 hours increased O2· − and cellular, but not medium, ADMA. Inhibition of AT1-receptors or ROS blocked the increase in cellular ADMA. Ang II stimulated NADPH oxidase, but inhibited DDAH and CAT activities. Stable transfection of cells with p22phox increased mRNA and protein expression for NOX-1 and increased the ROS generation, protein arginine methylation and PRMT-3 expression, but decreased DDAH protein expression and activity, CAT activity and CAT-1 expression. The decrease in DDAH protein and activity occurred despite an increase in DDAH mRNA expression and was prevented by blockade of proteosomal degradation which also reduced cellular ADMA of S-p22phox cells to the level of Wt cells. These changes in S-p22phox cells were accompanied by an increase in medium, and a greater increase in cellular levels of ADMA.
The function of ADMA in VSMCs is not clear. We could not detect expression of mRNA or protein for any NOS isoform in our isolated PGVSMCs. However, ADMA can have adverse vascular effects in eNOS knockout mice 19. ADMA can stimulate ROS production from some NOS isoforms if arginine is limited or tetrahydrobiopterin is oxidized 20. Moreover, Vallance and Leiper 1 have shown that ADMA released from one cell can inhibit NOS in an adjacent cell and that this could be a mechanism for VSMCs to signal to endothelial cells. Additionally, Ang II doubled the release of ADMA from cultured endothelial cells 21. Thus, a similar mechanism for angiotensin-induced ADMA generation may occur in VSMCs and endothelial cells, but this remains to be explored.
We found an increase in cellular ADMA with Ang II that was related to AT1 receptor activation and to ROS. Thus, cellular O2· − and ADMA increased in parallel from 10−10M Ang II and the increase in ADMA was prevented by candesartan, but not PD-123,319 and by tempol, which prevented Ang II-stimulated increases in O2· − in these cells 11. A decreased ADMA metabolism by DDAH during Ang II could contribute to increased cellular ADMA concentrations while a decreased CAT activity could reduce cellular export of ADMA into the medium. Further studies focused on the direct effects of prolonged NADPH oxidase activity on ADMA.
The membrane protein p22phox is a critical component of NADPH oxidase 10. Smooth muscle specific overexpression of p22phox in mice increased aortic p22phox and NOX-1 proteins and increased O2· − and H2O2 generation 22, whereas knockdown of p22phox in vivo reduced the protein expression for NOX-1, -2 and -4 23. Stable transfection of cells with p22phox provided a robust model of cellular oxidative stress with increased O2· − and H2O2 generation and increased NOX-1 expression. In apparent contrast to the finding that Ang II decreased DDAH activity in PGVSMCs, we detected an increase in the mRNA for DDAH-1 and -2 in S-p22phox cells accompanied by an increase in the promoter activity for DDAH-2. However, this was accompanied by a reduction in protein expression for DDAH-1 and -2 and cellular DDAH activity. This discrepancy was related to enhanced proteosomal degradation since the reduced protein expression for DDAH-1 and -2 in S-p22phox cells was mitigated (for DDAH-1) or prevented (for DDAH-2) by inhibition of proteosomal degradation by epoxomicin which also normalized the increased cellular ADMA concentrations. This extended prior studies where H2O2 enhanced proteosomal degradation of the inositol 1,4,5-triphosphate receptor in VSMCs 24. Since restoration of DDAH-1 or -2 protein expression with epoxomicin did not correct the increased mRNA in S-p22phox cells, the increased mRNA was not likely a compensation for reduced DDAH expression.
Inhibition of DDAH by oxidative stress has been ascribed to oxidation of a cystein residue in the catalytic site of the enzyme 1,12 or to down-regulation of protein expression 25. A novel finding was that this also can involve proteosomal degradation of the DDAH protein. We detected increased asymmetric dimethylarginine in proteins, and increased PRMT-3 protein expression in S-p22phox cells, which suggests that increased PRMT activity contributed to increased cellular ADMA. Recently, Chen et al 26 reported that bovine retinal capillary endothelial cells cultured in high glucose solution that elevated ROS production had increased PRMT-1 expression, and decreased DDAH activity and DDAH-2 expression. These were corrected by antioxidants suggesting that ROS increased PRMT-1 and decreased DDAH, as in our study.
The finding that Ang II or activation of NADPH oxidase reduced cellular CAT activity and that the latter reduced CAT-1 mRNA expression is compatible with prior studies that have reported diminished CAT activity or expression under conditions that induce oxidative stress, for example exposure to cigarette smoke 27 or homocysteine 28.
ADMA concentrations in cultured endothelial cells are reported to be about 10-fold above that in the medium 29. We measured ADMA concentrations in PGVSMCs to be 27-fold above that in the medium. The calculated intracellular concentrations of ADMA of 6 μmol·l−1 in Wt cells and 16μmol·l−1 in S-p22phox cells are expected to inhibit NOS in other cell types that express NOS isoforms but the higher calculated intracellular arginine concentrations would offset this effect. The doubling of intracellular L-arginine in p22phox cells may be secondary to increased cellular proliferation which enhances arginine turnover 30 since p22phox overexpression induces proliferation in VSMCs 10.
Perspectives
Since Ang II upregulated ROS in many tissues 4,11 and ROS increased cellular ADMA, Ang II should increase ADMA. Indeed, Ang II doubled ADMA release from cultured endothelial cells. However, we found that relatively short-term incubation of VSMCs with Ang II for 4 hours did not change, or even reduced, medium ADMA, despite a rise in cellular ADMA. This may be a consequence of a reduced CAT activity which could slow the cellular export of ADMA. Infusions of Ang II were reported to increase 7 or maintain 8 plasma ADMA, or to increase ADMA only at high rates of Ang II infusion 9. Our findings in isolated cells suggest that there could be a substantial increase in cellular ADMA in conditions that enhance ROS that may not be reflected reliably in plasma levels because of decreased CAT activity. The finding that Ang II and NADPH oxidase expression both reduced CAT activity supports the possibility that in the short term, ADMA export may be diminished. However, in the long term, the increased intracellular ADMA will stress the CAT system and export will increase until a new steady state has been reached where the rate of ADMA production will equal the sum of degradation by DDAH and export by CAT 2. The main effect of CAT inhibition is that this steady state should be reached at higher intracellular levels of ADMA. ADMA production in S-p22phox cells was increased, as evidenced by increased PRMT expression and increased levels of ADMA moieties on proteins. From Table 1, it is apparent that ADMA in the medium increased 1.6-fold compared to wild-type cells. This is less than the 2.7-fold increase in intracellular ADMA, compatible with reduced CAT activity, but clearly shows an increased net export of ADMA. Thus, under steady-state conditions, export of ADMA by VSMCs may be increased by ROS although this may not hold for other cell types. Uptake of ADMA by neighboring endothelial cells with NOS inhibition is plausible, but remains to be demonstrated experimentally.
Supplementary Material
Acknowledgments
We thank Ms Sigrid de Jong for expert technical assistance, Ms Emily Wing Kam Chan for preparing and editing the manuscript and Dr. Satoshi Tanaka (University of Tokyo, Japan) for a gift of a plasmid containing a DDAH-2 promoter/luciferase construct.
Sources of funding
The work described in this review was supported by research grants to Christopher S. Wilcox from the NIDDK (DK-049870 and DK-036079) and from the NHLBI (HL-68686) and by funds from the George E. Schreiner Chair of Nephrology and by grant to William J. Welch from NHLBI (HL-89583). Zaiming Luo was supported by a Nephrology Research Training Grant (T32-DK-059274).
Footnotes
Conflict of Interest
None.
Reference List
- 1.Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb Vasc Biol. 2004;24:1023–1030. doi: 10.1161/01.ATV.0000128897.54893.26. [DOI] [PubMed] [Google Scholar]
- 2.Teerlink T, Luo Z, Palm F, Wilcox CS. Cellular ADMA: regulation and action. Pharmacol Res. 2009;60:448–460. doi: 10.1016/j.phrs.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol. 2007;293:H3227–H3245. doi: 10.1152/ajpheart.00998.2007. [DOI] [PubMed] [Google Scholar]
- 4.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertens. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Strandgaard S, Iversen J, Wilcox CS. Asymmetric dimethylarginine, oxidative stress, and vascular nitric oxide synthase in essential hypertension. Am J Physiol Regul Integr Comp Physiol. 2009;296:R195–R200. doi: 10.1152/ajpregu.90506.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stuhlinger MC, Oka RK, Graf EE, Schmolzer I, Upson BM, Kapoor O, Szuba A, Malinow MR, Wascher TC, Pachinger O, Cooke JP. Endothelial dysfunction induced by hyperhomocyst(e)inemia: role of asymmetric dimethylarginine. Circ. 2003;108:933–938. doi: 10.1161/01.CIR.0000085067.55901.89. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa K, Wakino S, Tatematsu S, Yoshioka K, Homma K, Sugano N, Kimoto M, Hayashi K, Itoh H. Role of asymmetric dimethylarginine in vascular injury in transgenic mice overexpressing dimethylarginie dimethylaminohydrolase 2. Circ Res. 2007;101:e2–e10. doi: 10.1161/CIRCRESAHA.107.156901. [DOI] [PubMed] [Google Scholar]
- 8.Jacobi J, Maas R, Cordasic N, Koch K, Schmieder RE, Boger RH, Hilgers KF. Role of asymmetric dimethylarginine for angiotensin II-induced target organ damage in mice. Am J Physiol Heart Circ Physiol. 2008;294:H1058–H1066. doi: 10.1152/ajpheart.01103.2007. [DOI] [PubMed] [Google Scholar]
- 9.Sasser JM, Moningka NC, Cunningham MW, Jr, Croker B, Baylis C. Asymmetric dimethylarginine in angiotensin II-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;298:R740–R746. doi: 10.1152/ajpregu.90875.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 11.Luo Z, Chen Y, Chen S, Welch WJ, Andresen BT, Jose PA, Wilcox CS. Comparison of inhibitors of superoxide generation in vascular smooth muscle cells. Br J Pharmacol. 2009;157:935–943. doi: 10.1111/j.1476-5381.2009.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leiper J, Murray-Rust J, McDonald N, Vallance P. S-nitrosylation of dimethylarginine dimethylaminohydrolase regulates enzyme activity: Further interactions between nitric oxide synthase and dimethylarginine dimethylaminohydrolase. Proc Nat Acad Sci USA. 2002;99:13527–13532. doi: 10.1073/pnas.212269799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomikawa J, Fukatsu K, Tanaka S, Shiota K. DNA methylation-dependent epigenetic regulation of dimethylarginine dimethylaminohydrolase 2 gene in trophoblast cell lineage. J Biol Chem. 2006;281:12163–12169. doi: 10.1074/jbc.M513782200. [DOI] [PubMed] [Google Scholar]
- 14.de Jong S, Teerlink T. Analysis of asymmetric dimethylarginine in plasma by HPLC using a monolithic column. Anal Biochem. 2006;353:287–289. doi: 10.1016/j.ab.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Ayling LJ, Whitley GS, Aplin JD, Cartwright JE. Dimethylarginine dimethylaminohydrolase (DDAH) regulates trophoblast invasion and motility through effects on nitric oxide. Hum Reprod. 2006;21:2530–2537. doi: 10.1093/humrep/del111. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Gill P, Chabrashvili T, Onozato ML, Raggio J, Mendonca M, Dennehy K, Li M, Modlinger P, Leiper J, Vallance P, Adler O, Leone A, Tojo A, Welch WJ, Wilcox CS. Isoform-specific regulation by N(G), N(G)-dimethylarginine dimethylaminohydrolase of rat serum asymmetric dimethylarginine and vascular endothelium-derived relaxing factor/NO. Circ Res. 2007;101:627–635. doi: 10.1161/CIRCRESAHA.107.158915. [DOI] [PubMed] [Google Scholar]
- 17.Wong CM, Cheema AK, Zhang L, Suzuki YJ. Protein carbonylation as a novel mechanism in redox signaling. Circ Res. 2008;102:310–318. doi: 10.1161/CIRCRESAHA.107.159814. [DOI] [PubMed] [Google Scholar]
- 18.Orlov SN, Tremblay J, Hamet P. Cell volume in vascular smooth muscle is regulated by bumetanide-sensitive ion transport. Am J Physiol. 1996;270:C1388–C1397. doi: 10.1152/ajpcell.1996.270.5.C1388. [DOI] [PubMed] [Google Scholar]
- 19.Suda O, Tsutsui M, Morishita T, Tasaki H, Ueno S, Nakata S, Tsujimoto T, Toyohira Y, Hayashida Y, Sasaguri Y, Ueta Y, Nakashima Y, Yanagihara N. Asymmetric dimethylarginine produces vascular lesions in endothelial nitric oxide synthase-deficient mice: involvement of renin-angiotensin system and oxidative stress. Arterioscler Thromb Vasc Biol. 2004;24:1682–1688. doi: 10.1161/01.ATV.0000136656.26019.6e. [DOI] [PubMed] [Google Scholar]
- 20.Cardounel AJ, Xia Y, Zweier JL. Endogenous methylarginines modulate superoxide as well as nitric oxide generation from neuronal nitric-oxide synthase: differences in the effects of monomethyl- and dimethylarginines in the presence and absence of tetrahydrobiopterin. J Biol Chem. 2005;280:7540–7549. doi: 10.1074/jbc.M410241200. [DOI] [PubMed] [Google Scholar]
- 21.Chen MF, Xie XM, Yang TL, Wang YJ, Zhang XH, Luo BL, Li YJ. Role of asymmetric dimethylarginine in inflammatory reactions by angiotensin II. J Vasc Res. 2007;44:391–402. doi: 10.1159/000103284. [DOI] [PubMed] [Google Scholar]
- 22.Laude K, Cai H, Fink B, Hoch N, Weber DS, McCann L, Kojda G, Fukai T, Schmidt HH, Dikalov S, Ramasamy S, Gamez G, Griendling KK, Harrison DG. Hemodynamic and biochemical adaptations to vascular smooth muscle overexpression of p22phox in mice. Am J Physiol Heart Circ Physiol. 2005;288:H7–H12. doi: 10.1152/ajpheart.00637.2004. [DOI] [PubMed] [Google Scholar]
- 23.Modlinger P, Chabrashvili T, Gill PS, Mendonca M, Harrison DG, Griendling KK, Li M, Raggio J, Wellstein A, Chen Y, Welch WJ, Wilcox CS. RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertens. 2006;47:238–244. doi: 10.1161/01.HYP.0000200023.02195.73. [DOI] [PubMed] [Google Scholar]
- 24.Martin-Garrido A, Boyano-Adanez MC, Alique M, Calleros L, Serrano I, Griera M, Rodriguez-Puyol D, Griendling KK, Rodriguez-Puyol M. Hydrogen peroxide down-regulates inositol 1,4,5-trisphosphate receptor content through proteasome activation. Free Radic Biol Med. 2009;47:1362–1370. doi: 10.1016/j.freeradbiomed.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Tain YL, Baylis C. Determination of dimethylarginine dimethylaminohydrolase activity in the kidney. Kidney Int. 2007;72:886–889. doi: 10.1038/sj.ki.5002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Xu X, Sheng M, Zhang X, Gu Q, Zheng Z. PRMT-1 and DDAHs-induced ADMA upregulation is involved in ROS- and RAS-mediated diabetic retinopathy. Exp Eye Res. 2009;89:1028–1034. doi: 10.1016/j.exer.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Zhang WZ, Venardos K, Chin-Dusting J, Kaye DM. Adverse effects of cigarette smoke on NO bioavailability: role of arginine metabolism and oxidative stress. Hypertens. 2006;48:278–285. doi: 10.1161/01.HYP.0000231509.27406.42. [DOI] [PubMed] [Google Scholar]
- 28.Jin L, Caldwell RB, Li-Masters T, Caldwell RW. Homocysteine induces endothelial dysfunction via inhibition of arginine transport. J Physiol Pharmacol. 2007;58:191–206. [PubMed] [Google Scholar]
- 29.Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, Tsai AL, Berka V, Zweier JL. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J Biol Chem. 2007;282:879–887. doi: 10.1074/jbc.M603606200. [DOI] [PubMed] [Google Scholar]
- 30.Morris SM., Jr Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharmacol. 2009;157:922–930. doi: 10.1111/j.1476-5381.2009.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.