Abstract
Our goal was to determine whether exercise training (ExT) alleviates impaired nitric oxide synthase (NOS)-dependent dilation of pial arterioles during chronic exposure to nicotine. We measured dilation of cerebral (pial) arterioles in sedentary and exercised control and nicotine-treated (2 mg·kg−1·day−1 for 4 wk via an osmotic minipump) rats to an endothelial NOS (eNOS)-dependent (ADP), a neuronal NOS (nNOS)-dependent [N-methyl-d-aspartic acid (NMDA)], and a NOS-independent (nitroglycerin) agonist. In addition, we harvested brain tissue from sedentary and exercised control and nicotine-treated rats to measure the production of superoxide anion and measured superoxide dismutase-1 (SOD-1) protein in cerebral microvessels using Western blot. We found that eNOS-and nNOS-dependent, but not NOS-independent, vasodilation was impaired in nicotine-treated compared with control rats. In addition, the production of superoxide anion (lucigenin chemiluminescence) was increased, and SOD-1 protein decreased, in rats treated with nicotine compared with control rats. Further, although ExT did not significantly affect eNOS- or nNOS-dependent vasodilation in control rats, ExT restored impaired eNOS- and nNOS-dependent responses in nicotine-treated rats. In addition, the increase in superoxide anion production observed in nicotine-treated rats was reduced by ExT, and SOD-1 protein was increased in nicotine-treated rats by ExT. We suggest that ExT restores impaired NOS-dependent dilation of pial arterioles during chronic exposure to nicotine by a mechanism related to the formation of superoxide anion.
Keywords: smoking, brain, nitric oxide, endothelial nitric oxide synthase, neuronal nitric oxide synthase
cigarette smoking and/or the use of smokeless tobacco products leads to vascular damage in several major organ systems, including the lung, heart, and brain (1, 24, 29). In addition, investigators have shown that cigarette smoking and/or the use of smokeless tobacco contributes to the pathogenesis of ischemic and hemorrhagic stroke (4, 7, 21). Although cigarette smoke contains many toxic substances that could potentially damage cells composing the vasculature and surrounding tissue, it has been suggested that nicotine may be a contributing agent to vascular dysfunction during exposure to cigarette smoke and/or smokeless tobacco. Previous studies have shown that nicotine is toxic to endothelium (27, 33) and exposure (acute and chronic) to nicotine impairs nitric oxide synthase (NOS)-dependent dilation of large (50, 52) and small (28, 39, 40) peripheral vessels and cerebral vessels (13, 14, 19, 37). The mechanism by which nicotine impairs NOS-dependent dilation of large and small peripheral and cerebral blood vessels appears to be related to an increase in oxidative stress (6).
The beneficial effects of exercise training (ExT) on the prevention of cardiovascular-related diseases have been well-documented. Although the precise mechanism accounting for the beneficial effects of ExT on the cardiovascular system remains uncertain, several studies have suggested that ExT may modulate vascular endothelial function. Investigators have shown that ExT enhances eNOS-dependent dilation of skeletal muscle and cutaneous vessels in animals and humans via a change in shear stress to increase eNOS activity and/or via an increase in activity of antioxidant enzymes (17, 32, 51, 53, 63). In addition, ExT improves responses of peripheral and cerebral vessels during chronic disease states (3, 10, 25, 38, 44, 56). However, no studies to our knowledge have examined the influence of ExT on responses of cerebral arterioles during exposure to nicotine. Thus the goal of the present study was to examine the influence of ExT on reactivity of cerebral arterioles during chronic exposure to nicotine and determine a possible mechanism for the influence of ExT on reactivity of cerebral arterioles. To accomplish this goal, we measured eNOS- and nNOS-dependent reactivity of pial arterioles, superoxide anion production by brain tissue, and superoxide dismutase-1 (SOD-1) protein in sedentary and exercised control and nicotine-treated rats.
MATERIALS AND METHODS
Preparation of animals.
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Nebraska Medical Center, and all studies conform to the American Physiological Society's “Guiding Principles in the Care and Use of Animals.” Adult male Sprague-Dawley rats (250–300 g) were divided into saline-treated (control) and nicotine-treated groups. In both groups, an osmotic minipump (model 2006; Alzet; Cupertino, CA) was implanted subcutaneously under anesthesia (pentobarbital sodium; 35–50 mg/kg ip). In the control group, the minipump contained vehicle (saline) and in the nicotine group the minipump contained nicotine at a concentration of 175 mg/ml. The minipump released saline or nicotine at a rate of 0.15 μl/h, to provide a concentration of nicotine at ∼2 mg·kg−1·day−1, similar to that described previously (15, 19, 36). Four weeks after implantation of the minipump, rats were anesthetized with thiobutabarbital sodium (Inactin; 100 mg/kg body wt ip). A tracheotomy was performed and the animals were mechanically ventilated. A catheter was placed in a femoral vein for injection of supplemental anesthetic (10–20 mg/kg), and a femoral artery was cannulated to measure arterial pressure. After these procedures, a window was prepared over the parietal cortex to expose the pial microcirculation (14). The cranial window was suffused with artificial cerebral spinal fluid bubbled with 95% nitrogen-5% carbon dioxide. Temperature of the suffusate was maintained at 37 ± 1°C. The cranial window was connected via a three-way valve to an infusion pump, which allowed for infusion of agonists into the suffusate. Arterial blood gases were monitored and maintained within normal limits.
Exercise training.
Rats were exercised using standard techniques similar to what we (38) and others have described (10, 26, 46, 53, 63). Treadmill exercise was started 3 days following implantation of minipumps and was carried out 5 days/wk until the day before the experiment (4 wk after implantation of the minipumps). The length of time on the treadmill was initially 10 min/day for the first 3 days at 0% grade and a speed of 15–20 m/min. Then, over a 7-day period, the speed was increased to 25 m/min, the grade was increased to 10%, and the duration on the treadmill was increased to 60 min. This regimen for ExT is considered moderate and results in about a 45% increase in citrate synthase activity in the soleus muscle of rats (38) and is similar to that reported (23, 59).
Pial arteriolar diameter.
Inner diameter of pial arterioles was measured using a video image-shearing device coupled to a video monitor. Diameter of arterioles was measured before, at 1-min intervals for 5 min during application of agonists, and after application of agonists was completed.
Experimental protocol.
The cranial window was suffused for 30–45 min before testing responses to the agonists. In the first group (n = 5), we examined the effects of chronic treatment with nicotine on reactivity of pial arterioles in sedentary rats to an eNOS-dependent agonist [ADP (10 and 100 μM)], to an nNOS-dependent agonist [N-methyl-d-aspartic acid (NMDA; 100 and 300 μM)], and to a NOS-independent agonist [nitroglycerin (1.0 and 10 μM)]. In a second group (n = 7), we examined responses of pial arterioles to the agonists in exercised rats chronically treated with nicotine. In a third group (n = 7), we examined responses of arterioles to the agonists in sedentary control rats. In a fourth group (n = 8), we examined responses of pial arterioles to the agonists in exercised control rats.
Measurement of superoxide.
Superoxide levels were measured by lucigenin-enhanced chemiluminescence as described previously (11, 12, 42). In separate groups of sedentary and exercised control (n = 11) and nicotine-treated (n = 8) rats, the brain was removed and placed in a Krebs/HEPES buffer (pH 7.4) with the following composition (in mmol/l): 118 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.2 MgCl2, 1.3 CaCl2, 10 HEPES, 25 NaHCO3, and 5 glucose. Samples of cortex tissue, cut from brains of sedentary and exercised control and nicotine-treated rats, were placed in polypropylene tubes containing 5 μM lucigenin. The tubes were then read in a Sirius/FB15 luminometer (Berthold Detections Systems), which reports relative light units (RLU) emitted over a 30-s interval for 5 min. Levels of superoxide reported are the value of tissue plus lucigenin-containing buffer minus background (lucigenin-containing buffer without tissue) and are normalized for tissue weight (RLU·min−1·mg tissue−1). We measured levels of superoxide in tissue obtained from sedentary and exercised control and nicotine-treated rats under basal conditions.
Western blot analysis.
In separate groups of sedentary and exercised control and nicotine-treated rats (n = 12 for all groups) cerebral microvessels were isolated from brain tissue using methods described previously (62). Samples were homogenized in 20% (wt/vol) ice-cold buffer containing 10 mM Tris·HCl (pH 7.4), 1% SDS, 1 mM sodium vanadate, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride. The homogenates were centrifuged at 12,000 g for 20 min at 4°C, and the protein concentrations in supernatant were determined by the Bradford method (Bio-Rad; Richmond, CA) with BSA as the standard. Protein was mixed and boiled in SDS-PAGE sample buffer for 5 min, then loaded and run on standard 7.5% or 12.5% gels using 20 μg of protein. After SDS-PAGE, the proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane. Immunoblotting was performed with rabbit anti-SOD-1 (Santa Cruz Biotechnology) as the primary antibody and goat anti-rabbit as the secondary antibody. The bound antibody was detected using an ECL kit and quantified by scanning densitometry.
Drugs.
ADP, lucigenin, and nicotine were purchased from Sigma Chemical (St. Louis, MO). Nitroglycerin was purchased from SoloPak Laboratories (Elk Grove Village, IL).
Statistical analysis.
ANOVA with Scheffé's test was used to compare responses of pial arterioles to the agonists and superoxide production in sedentary and exercised control and nicotine-treated rats. A P value of 0.05 or less was considered to be significant.
RESULTS
Baseline parameters.
We found that baseline diameter of pial arterioles was similar in sedentary and exercised control and in exercised nicotine-treated rats (Fig. 1). In addition, there was no significant difference in blood pressure between sedentary and exercised control and nicotine-treated rats (Fig. 1).
Fig. 1.
Baseline diameter of pial arterioles and mean arterial pressure in sedentary and exercised control and nicotine-treated rats. Values are means ± SE. ExT, exercise training.
Reactivity of pial arterioles.
First, we determined the effect of ExT on responses of pial arterioles in control rats. We found that application of ADP (Fig. 2), NMDA (Fig. 3) and nitroglycerin (Fig. 4) dilated pial arterioles in sedentary and exercised control rats, and the magnitude of this dilation was similar in sedentary and exercised control rats.
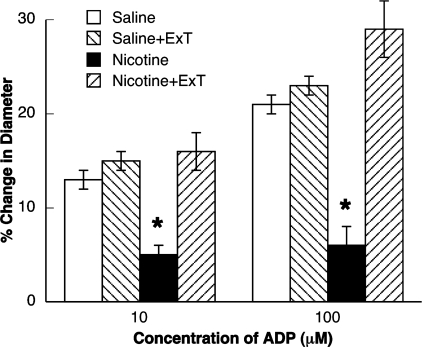
Fig. 2.
Responses of pial arterioles to ADP in sedentary and exercised saline-treated and nicotine-treated rats. Values are means ± SE. *P < 0.05 vs. response in sedentary and exercised control and exercised nicotine-treated rats.
Fig. 3.
Responses of pial arterioles to N-methyl-d-aspartic acid (NMDA) in sedentary and exercised saline-treated and nicotine-treated rats. Values are means ± SE. *P < 0.05 vs. response in sedentary and exercised control and exercised nicotine-treated rats.
Fig. 4.
Responses of pial arterioles to nitroglycerin in sedentary and exercised control and nicotine-treated rats. Values are means ± SE.
Next, we examined the influence of ExT on responses of pial arterioles in rats treated with nicotine. We found that dilation of pial arterioles to ADP (Fig. 2) and NMDA (Fig. 3) was impaired in sedentary rats treated with nicotine compared with sedentary and exercised control rats. However, dilation of pial arterioles in response to nitroglycerin was not altered in sedentary rats treated with nicotine (Fig. 4). In addition, we found that ExT restored impaired responses of pial arterioles to ADP (Fig. 2) and NMDA (Fig. 3) in rats treated with nicotine. However, ExT did not influence reactivity of pial arterioles to nitroglycerin (Fig. 4).
Superoxide production.
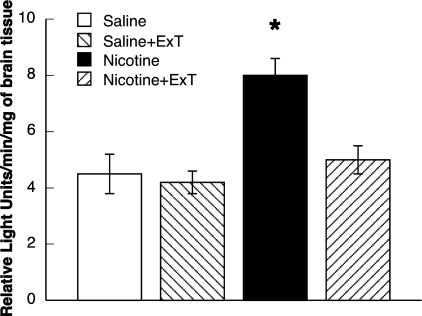
We measured superoxide production by cortex tissue in the various groups of rats. We found that the basal production of superoxide from cortex tissue was similar in sedentary and exercised control rats (Fig. 5). In addition, we found that basal production of superoxide from cortex tissue was increased in rats chronically treated with nicotine. Further, the increase in basal production of superoxide in the nicotine-treated rats was restored to that observed in control rats by ExT (Fig. 5). Thus it appears that ExT can reduce basal increases in superoxide production by cortex tissue in rats chronically treated with nicotine.
Fig. 5.
Superoxide anion production from parietal cortex tissue in sedentary and exercised control and nicotine-treated rats. Values are means ± SE. *P < 0.05 vs. response in sedentary and exercised saline-treated rats, and exercised nicotine-treated rats.
Western blot analysis.
Chronic administration of nicotine produced a decrease in the expression of SOD-1 (Fig. 6). In addition, while ExT did not alter SOD-1 protein expression in control rats, ExT produced an increase in SOD-1 protein expression in rats treated with nicotine.
Fig. 6.
Superoxide dismutase-1 (SOD-1) protein from cerebral microvessels in sedentary and exercised control and nicotine-treated rats. Top: a representative Western immunoblot of SOD-1 protein and GAPDH protein. Bottom: quantified data from Western blots. Values are means ± SE. *P < 0.05 vs. sedentary and exercised control rats, and exercised nicotine-treated rats. **P < 0.05 vs. sedentary nicotine-treated rats.
DISCUSSION
This is the first study that we are aware of that has examined the beneficial effects of ExT on reactivity of cerebral blood vessels during chronic exposure to nicotine. There are three new findings of the present study. First, we found that ExT alleviates impaired eNOS-dependent dilation of pial arterioles in nicotine-treated rats. Second, we found that ExT alleviates impaired nNOS-dependent dilation of pial arterioles in rats treated with nicotine. This effect of ExT on eNOS- and nNOS-dependent vasodilation appears to be related to NOS activity and not altered NO reactivity since ExT did not alter dilation of pial arterioles in response to nitroglycerin. Third, it appears that ExT may restore impaired eNOS- and nNOS-dependent dilation of pial arterioles by a mechanism that involves the inhibition of superoxide anion production. We found that superoxide production by cortex tissue was increased under basal conditions in rats treated with nicotine and this basal increase in superoxide production was inhibited by ExT. Based on these findings, we suggest that ExT has beneficial effects on cerebral blood vessels and we speculate that ExT may have potential therapeutic value for the treatment of smoking-induced vascular dysfunction.
Influence of smoking/nicotine on vascular function.
Previous studies have reported that active and passive exposure to cigarette smoke/cigarette smoke extract impairs NOS-dependent reactivity of large and small peripheral (8, 41, 45, 60) and cerebral (57) vessels in animals and human subjects. Mechanisms by which cigarette smoking/cigarette smoke extract impairs NOS-dependent reactivity are not entirely clear, but it appears that an increase in the local/systemic formation of reactive oxygen species may play a role (16, 18, 22, 41, 45, 49). Over the past several years, it has become apparent that nicotine may be a candidate contributing to vascular dysfunction in smokers and users of tobacco products. Investigators have reported that treatment of human subjects (9, 55) and animals (43) with nicotine impaired NOS-dependent reactivity of peripheral vessels. In addition, we (13, 14, 37) and others (19) have shown that acute and chronic treatment with nicotine produced selective impairment in NOS-dependent reactivity of cerebral arteries and arterioles that was attributed to the formation of oxygen radicals, presumably superoxide. The results of the present study complement and extend previous findings. In the present study, we found that chronic exposure to nicotine impaired NOS-dependent responses of cerebral resistance arterioles. In addition, we found that ExT could restore this alteration in cerebrovascular function produced by chronic exposure to nicotine, and this was correlated with decreased SOD-1 content and increased brain superoxide levels.
One might suggest that it is not entirely clear whether changes in protein levels of SOD-1 and production of superoxide are mechanistically linked in improving/inhibiting dilation of cerebral arterioles, respectively. To address this concern, studies could be conducted using SOD knockout mice to determine whether nicotine and/or ExT restores impaired responses of arterioles when changes of SOD-1 protein are prevented. However, comparisons between rats and knockout mice may be problematic. Another approach could be to inhibit superoxide production and determine whether nicotine and/or ExT alter dilation of cerebral arterioles. In a previous study (13) we found that chronic treatment of rats with apocynin, to inhibit superoxide production via activation of NADPH oxidase, could prevent nicotine-induced impairment in dilation of cerebral arterioles. Thus, although we cannot exclude the possible involvement of other cellular networks in impairment of vascular function during exposure to nicotine, we suggest that are findings regarding SOD-1 and superoxide are significant and appear to be mechanistically linked to dilation of cerebral arterioles.
We did not observe an effect of nicotine treatment on resting diameter of cerebral arterioles. Given the dramatic effect of nicotine treatment on cerebrovascular function, it was somewhat surprising that chronic treatment with nicotine did not influence basal diameter of cerebral arterioles. One might have predicted that since nicotine treatment influenced NOS-dependent responses of cerebral arterioles that this treatment may have led to a decrease in baseline diameter of cerebral arterioles. Although we are not certain as to why treatment with nicotine did not influence baseline diameter, we suggest that since baseline tone of cerebral arterioles may be related to a complex interplay between dilator/constrictor pathways, it is possible that compensatory mechanisms contribute to maintenance of vascular tone during treatment with nicotine.
Effects of ExT on vascular function.
Many studies have examined the effects of ExT on NOS-dependent reactivity (31, 32, 61, 63). Sun et al. (63) found that short-term (2–4 wk) daily ExT increased NOS-dependent, but not -independent, dilation of skeletal muscle arterioles in rats. In addition, Kvernmo et al. (32) found that NOS-dependent dilation of cutaneous vessels from human subjects was increased by physical conditioning. Further, Koller et al. (31) found that ExT increased flow-dependent dilation of skeletal muscle arterioles from rats. The precise mechanisms by which ExT enhances NOS-dependent relaxation of blood vessels are not entirely clear but have been reported to be related to an increase in shear forces to increase the release of nitric oxide from the endothelium (53, 63), an increase in the release of prostanoids (31), an increase in the activity of superoxide dismutase (35, 54) and/or an increase in the activity of other antioxidant enzymes (glutathione peroxidase and catalase) (51). However, not all studies have shown an affect of ExT on NOS-dependent reactivity of blood vessels. Franke et al. (17) found that while ExT enhanced forearm vascular conductance in humans, it did not increase vascular responsiveness to NOS-dependent agonists. Oltman et al. (48) found that ExT did not influence NOS-dependent responses of porcine coronary arteries. Further, we (38) have shown that ExT does not alter responses of the basilar artery in rats to an eNOS-dependent agonist (acetylcholine). In the present study, we did not find a difference in reactivity of pial arterioles to ADP or NMDA between sedentary and ExT control rats.
While many studies have examined the influence of ExT on reactivity of blood vessels during physiological states, others have examined the effects of ExT during pathophysiological states. Although no studies to our knowledge have examined the effects of ExT on responses of cerebral arterioles during chronic exposure to nicotine, others have reported beneficial effects of ExT on responses of peripheral blood vessels during chronic hypertension (3, 25), heart failure (20, 30), and Type 2 diabetes (38, 44). In addition, we (38) have shown that ExT restores impaired responses of the basilar artery during Type 1 diabetes. The mechanism for the effects of ExT on improving NOS-dependent responses of blood vessels during disease states is not entirely clear but may be related to a decrease in oxidative stress and/or an increase in nitric oxide synthesis/release (3, 25, 34, 38, 47). In the present study, we found that ExT restored impaired eNOS- and nNOS-dependent dilation of pial arterioles in rats chronically treated with nicotine. This restoration in NOS-dependent reactivity appeared to be related to an influence of ExT on oxidative stress since ExT reduced basal production of superoxide.
Implications of ExT during smoking/exposure to nicotine.
Some may suggest that ExT may not be appropriate and/or feasible in smokers or users of tobacco products. However, there are many examples in the literature that suggest that ExT is tolerated by smokers and users of tobacco products and can reduce the risk of many diseases by a mechanism that may be related to a reduction in oxidative stress. A recent study by Anton et al. (2) found that ExT (8 h/wk) in individuals that smoked between 8–10 cigarettes/day for at least 2 years could be easily tolerated and that ExT reduced the risk for cardiovascular-related diseases in smokers. In addition, a study by Sinner et al. (58) found that the risk of lung cancer was decreased by physical activity in women that smoke. Further, it has been reported that ExT in smokers can lower lipid peroxidation, suggesting that the beneficial effects of ExT during smoking may be related to a reduction in oxidative stress (5). Thus we suggest that ExT is an appropriate and feasible methodology for the treatment of vascular dysfunction in smokers or users of nicotine-containing tobacco products.
In summary, this is the first study that we are aware of to examine the effects of ExT on eNOS- and nNOS-dependent reactivity of cerebral resistance arterioles. We found that ExT alleviated impaired eNOS- and nNOS-dependent dilation of pial arterioles in rats treated with nicotine but did not alter NOS-independent vasodilation. In addition, we found that nicotine produced an increase in superoxide formation from brain tissue and that ExT alleviated nicotine-induced superoxide formation. On the basis of our findings, we suggest ExT improves NOS-dependent responses of pial arterioles during chronic exposure to nicotine by a mechanism that appears to suppress the formation of superoxide. We speculate that our findings may have important implications for the pathogenesis of cerebrovascular abnormalities, including stroke, observed in smokers and users of tobacco products.
GRANTS
This study was supported by National Institutes of Health Grants DA-14258, HL-090657, and AA 11288 and funds from the University of Nebraska Medical Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Abbott RD, Yin Y, Reed DM, Yano K. Risk of stroke in male cigarette smokers. N Engl J Med 315: 717–720, 1986 [DOI] [PubMed] [Google Scholar]
- 2. Anton MM, Cortez-Cooper MY, DeVan AE, Neidre DB, Cook JN, Tanaka H. Cigarette smoking, regular exercise, and peripheral blood flow. Atherosclerosis 185: 201–205, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Arvola P, Wu X, Kahonen M, Makynen H, Riutta A, Mucha I, Solakivi T, Kainulainen H, Porsti I. Exercise enhances vasorelaxation in experimental obesity associated hypertension. Cardiovasc Res 43: 992–1002, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Asplund K, Nasic S, Janlert U, Stegmayr B. Smokeless tobacco as a possible risk factor for stroke in men: a nested case-control study. Stroke 34: 1754–1759, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Bloomer RJ, Fisher-Wellman KH. Postprandial oxidative stress in exercise trained and sedentary cigarette smokers. Int J Environ Res Public Health 6: 579–591, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burke A, Fitzgerald GA. Oxidative stress and smoking-induced vascular injury. Prog Cardiovasc Dis 46: 79–90, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Burns DM. Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis 46: 11–29, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield JE. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med 334: 150–154, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Chalon S, Moreno H, Benowitz NL, Hoffman BB, Blaschke TF. Nicotine impairs endothelium-dependent dilatation in human veins in vivo. Clin Pharmacol Ther 67: 391–397, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Chen Y, Collins HL, DiCarlo SE. Daily exercise enhances acetylcholine-induced dilation in mesenteric and hindlimb vasculature of hypertensive rats. Clin Exper Hypertens 21: 353–376, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Didion SP, Faraci FM. Effects of NADH and NADPH on superoxide levels and cerebral vascular tone. Am J Physiol Heart Circ Physiol 282: H688–H695, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Didion SP, Hathaway CA, Faraci FM. Superoxide levels and function of cerebral blood vessels after inhibition of CuZn-SOD. Am J Physiol Heart Circ Physiol 281: H1697–H1703, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Fang Q, Sun H, Arrick DM, Mayhan WG. Inhibition of NADPH oxidase improves impaired reactivity of pial arterioles during chronic exposure to nicotine. J Appl Physiol 100: 631–636, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Fang Q, Sun H, Mayhan WG. Impairment of nitric oxide synthase-dependent dilatation of cerebral arterioles during infusion of nicotine. Am J Physiol Heart Circ Physiol 284: H528–H534, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Feitelson JBA, Rowell PP, Roberts CS, Fleming JT. Two week nicotine treatment selectively increases bone vascular constriction in response to norepinephrine. J Orthop Res 21: 497–502, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Fennessy FM, Moneley DS, Wang JH, Kelly CJ, Bouchier-Hayes DJ. Taurine and vitamin C modify monocyte and endothelial dysfunction in young smokers. Circulation 107: 410–415, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Franke WD, Stephens GM, Schmid PG. Effects of intense exercise training on endothelium-dependent exercise-induced vasodilatation. Clin Physiol 18: 521–528, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Gamble J, Grewal PS, Gartside IB. Vitamin C modifies the cardiovascular and microvascular responses to cigarette smoke inhalation in man. Clin Sci 98: 455–460, 2000 [PubMed] [Google Scholar]
- 19. Gerzanich V, Zhang F, West GA, Simard JM. Chronic nicotine alters NO signaling of Ca2+ channels in cerebral arterioles. Circ Res 88: 359–365, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 107: 3152–3158, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Hawkins BT, Brown RC, Davis TP. Smoking and ischemic stroke: a role for nicotine. Trends Pharmacol Sci 23: 78–82, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Heitzer T, Just H, Munzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation 94: 6–9, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Helwig B, Schreurs KM, Hansen J, Hageman KS, Zbreski MG, McAllister RM, Mitchell KE, Musch TI. Training-induced changes in skeletal muscle Na+-K+ pump number and isoform expression in rats with chronic heart failure. J Appl Physiol 94: 2225–2236, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Higa M, Davanipour Z. Smoking and stroke. Neuroepidemiology 10: 211–222, 1991 [DOI] [PubMed] [Google Scholar]
- 25. Higashi Y, Sasaki S, Kurisu S, Yoshimizu A, Sasaki N, Matsuura H, Kajiyama G, Oshima T. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects. Role of endothelium-derived nitric oxide. Circulation 100: 1194–1202, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Hirai T, Zelis R, Musch TI. Effects of nitric oxide synthase inhibition on the muscle blood flow response to exercise in rats with heart failure. Cardiovasc Res 30: 469–476, 1995 [PubMed] [Google Scholar]
- 27. Hladovec J. Endothelial injury by nicotine and its prevention. Experientia 34: 1585–1586, 1978 [DOI] [PubMed] [Google Scholar]
- 28. Ijzerman RG, Serne EH, van Weissenbruch MM, de Jongh RT, Stehouwer CD. Cigarette smoking is associated with an acute impairment of microvascular function in humans. Clin Sci (Lond) 104: 247–252, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Kannel WB. Update on the role of cigarette smoking in coronary artery disease. Am Heart J 101: 319–328, 1981 [DOI] [PubMed] [Google Scholar]
- 30. Katz SD, Yuen J, Bijou R, LeJemtel TH. Training improves endothelium-dependent vasodilation in resistance vessels of patients with heart failure. J Appl Physiol 82: 1488–1492, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Koller A, Huang A, Sun D, Kaley G. Exercise training augments flow-dependent dilation in rat skeletal muscle arterioles. Circ Res 76: 544–550, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Kvernmo HD, Stefanovska A, Kirkeboen KA, Osterud B, Kvernebo K. Enhanced endothelium-dependent vasodilatation in human skin vasculature induced by physical conditioning. Eur J Appl Physiol 79: 30–36, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Lakier JB. Smoking and cardiovascular disease. Am J Med 93, Suppl 1A: 8S–12S, 1992 [DOI] [PubMed] [Google Scholar]
- 34. Lawler JM, Kwak HB, Kim JH, Suk MH. Exercise training inducibility of MnSOD protein expression and activity is retained while reducing prooxidant signaling in the heart of senescent rats. Am J Physiol Regul Integr Comp Physiol 296: R1496–R1502, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lawson DL, Chen L, Mehta JL. Effects of exercise-induced oxidative stress on nitric oxide release and antioxidant activity. Am J Cardiol 80: 1640–1642, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Li Z, Barrios V, Buchholz JN, Glenn TC, Duckles SP. Chronic nicotine administration does not affect peripheral vascular reactivity in the rat. J Pharmacol Exper Ther 271: 1135–1142, 1994 [PubMed] [Google Scholar]
- 37. Mayhan WG, Arrick DM, Sharpe GM, Sun H. Nitric oxide synthase-dependent responses of the basilar artery during acute infusion of nicotine. Nicotine Tob Res 11: 270–277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mayhan WG, Sun H, Mayhan JF, Patel KP. Influence of exercise on dilatation of the basilar artery during diabetes mellitus. J Appl Physiol 96: 1730–1737, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Mayhan WG, Patel KP. Effect of nicotine on endothelium-dependent arteriolar dilatation in vivo. Am J Physiol Heart Circ Physiol 272: H2337–H2342, 1997 [DOI] [PubMed] [Google Scholar]
- 40. Mayhan WG, Sharpe GM. Chronic exposure to nicotine alters endothelium-dependent arteriolar dilatation: effect of superoxide dismutase. J Appl Physiol 86: 1126–1134, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Mays BW, Freischlag JA, Eginton MT, Cambria RA, Seabrook GR, Towne JB. Ascorbic acid prevents cigarette smoke injury to endothelium-dependent arterial relaxation. J Surg Res 84: 35–39, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Miller FJ, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res 82: 1298–1305, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Miller VM, Clouse WD, Tonnessen BH, Boston US, Severson SR, Bonde S, Rud KS, Hurt RD. Time and dose effect of transdermal nicotine on endothelial function. Am J Physiol Heart Circ Physiol 279: H1913–H1921, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Miorana A, O'Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, Taylor R, Green D. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetics. J Am Coll Cardiol 38: 860–866, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Murohara T, Kugiyama K, Ohgushi M, Sugiyama S, Yasue H. Cigarette smoke extract contracts isolated porcine coronary arteries by superoxide anion-mediated degredation of EDRF. Am J Physiol Heart Circ Physiol 266: H874–H880, 1994 [DOI] [PubMed] [Google Scholar]
- 46. Musch TI, Terrell JA. Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise. Am J Physiol Heart Circ Physiol 262: H411–H419, 1992 [DOI] [PubMed] [Google Scholar]
- 47. Navarro A, Gomez C, Lopez-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol 286: R505–R511, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Oltman CL, Parker JL, Laughlin MH. Endothelium-dependent vasodilation of proximal coronary arteries from exercise-trained pigs. J Appl Physiol 79: 33–40, 1995 [DOI] [PubMed] [Google Scholar]
- 49. Ota Y, Kugiyama K, Sugiyama S, Ohgushi M, Matsumura T, Doi H, Ogata N, Oka H, Yasue H. Impairment of endothelium-dependent relaxation of rabbit aortas by cigarette smoke extract-role of free radicals and attenuation by captopril. Atherosclerosis 131: 195–202, 1997 [DOI] [PubMed] [Google Scholar]
- 50. Pellaton C, Kubli S, Feihl F, Waeber B. Blunted vasodilatory responses in the cutaneous microcirculation of cigarette smokers. Am Heart J 144: 269–274, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Powers SK, Ji LL, Leeuwenburgh C. Exercise training-induced alterations in skeletal muscle antioxidant capacity: a brief review. Med Sci Sports Exerc 107: 987–997, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Puranik R, Celermajer DS. Smoking and endothelial function. Prog Cardiovasc Dis 45: 443–458, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Roberts CK, Barnard RJ, Jasman A, Balon TW. Acute exercise increases nitric oxide synthase activity in skeletal muscle. Am J Physiol Endocrinol Metab 277: E390–E394, 1999 [DOI] [PubMed] [Google Scholar]
- 54. Rush JWE, Laughlin MH, Woodman CR, Price EM. SOD-1 expression in pig coronary arterioles is increased by exercise training. Am J Physiol Heart Circ Physiol 279: H2068–H2076, 2000 [DOI] [PubMed] [Google Scholar]
- 55. Sabha M, Tanus-Santos JE, Toledo JCY, Cittadino M, Rocha JC, Moreno H. Transdermal nicotine mimics the smoking-induced endothelial dysfunction. Clin Pharmacol Ther 68: 167–174, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Sakamoto S, Minami K, Niwa Y, Ohnaka M, Nakaya Y, Mizuno A, Kuwajima M, Shima K. Effect of exercise training and food restriction on endothelium-dependent relaxation in the Otsuka Long-Evans Tokushima fatty rat, a model of spontaneous NIDDM. Diabetes 47: 82–86, 1998 [DOI] [PubMed] [Google Scholar]
- 57. Silvestrini M, Troisi E, Matteis M, Cupini LM, Bernardi G. Effect of smoking on cerebrovascular reactivity. J Cereb Blood Flow Metab 16: 746–749, 1996 [DOI] [PubMed] [Google Scholar]
- 58. Sinner P, Folsom AR, Harnack L, Eberly LE, Schmitz KH. The association of physical activity with lung cancer incidence in a cohort of older women: the Iowa Women's Health Study. Cancer Epidemiol Biomarkers Prev 15: 2359–2363, 2006 [DOI] [PubMed] [Google Scholar]
- 59. Siu PM, Donley DA, Bryner RW, Alway SE. Citrate synthase expression and enzyme activity after endurance training in cardiac and skeletal muscles. J Appl Physiol 94: 555–560, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Sumida H, Watanabe H, Kugiyama K, Ohgushi M, Matsumura T, Yasue H. Does passive smoking impair endothelium-dependent coronary artery dilation in women? J Am Coll Cardiol 31: 811–815, 1998 [DOI] [PubMed] [Google Scholar]
- 61. Sun D, Huang A, Koller A, Kaley G. Enhanced NO-mediated dilations in skeletal muscle arterioles of chronically exercised rats. Microvasc Res 64: 491–496, 2002 [DOI] [PubMed] [Google Scholar]
- 62. Sun H, Zheng H, Molacek E, Fang Q, Patel KP, Mayhan WG. Role of NAD(P)H oxidase in alcohol-induced impairment of endothelial nitric oxide synthase-dependent dilation of cerebral arterioles. Stroke 37: 495–500, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Sun D, Huang A, Koller A, Kaley G. Short-term daily exercise activity enhances endothelial NO synthesis in skeletal muscle arterioles of rats. J Appl Physiol 76: 2241–2247, 1994 [DOI] [PubMed] [Google Scholar]