Abstract
Criteria for diagnosing cachexia in adults include unintentional loss in body weight, decreased strength, fatigue, anorexia, and low muscle mass. Cachexia is also associated with systemic inflammation, altered metabolism, and anemia. The ApcMin/+ mouse is a model of cachexia directly related to intestinal tumor burden and subsequent chronic inflammation. These mice also demonstrate muscle weakness, fatigue, decreased volitional activity, and elevated circulating IL-6 levels. The purpose of this study was to determine the time course of changes in physical activity and their relationship to anemia, muscle apoptosis, and muscle mass and body mass loss during cachexia. A subset of male ApcMin/+ mice were given access to voluntary activity wheels from 5 to 26 wk of age, while sedentary male ApcMin/+ mice were housed in cages lacking wheels. At the study's end mice were stratified by cachectic symptoms. Severely cachectic mice had decreased wheel running performance at 15 wk of age, while anemia and body weight loss were not present until 18 wk of age. Severely cachectic mice had lower hemoglobin levels compared with mildly cachectic mice at 13, 18, and 22 wk of age. Severely cachectic mice also demonstrated threefold more BCL2-associated X protein (BAX) protein in the gastrocnemius muscle at 26 wk of age compared with mildly cachectic mice. In sedentary ApcMin/+ mice at 26 wk of age anemia was present, and markers of apoptosis were induced in severely cachectic muscle. Proapoptotic protein expression was induced in both red and white portions of gastrocnemius muscle as well as in soleus muscle of severely cachectic mice compared with mildly cachectic mice. These data demonstrate that decrements in wheel running performance precede loss of body mass and that inherent muscle oxidative capacity is not protective against muscle apoptosis.
Keywords: exercise, colorectal cancer, muscle wasting, anemia
cachexia is characterized as an overall state of ill health, accompanied by a loss of lean body mass and fat mass (4, 5). Cancer patients can lose up to 30% of their original body weight, and cachexia accounts for ∼20–33% of cancer deaths (19). Recently, the criterion for the diagnosis of cachexia in adults was defined as “a complex metabolic syndrome associated with underlying illness and characterized by loss of muscle with or without loss of fat mass” (15). To be diagnosed with cachexia, one must have at least 5% loss of body mass with three of five of the following symptoms: 1) decreased muscle strength, 2) fatigue, 3) anorexia, 4) low fat-free mass, and 5) abnormal circulating markers, such as elevated interleukin (IL)-6 or low hemoglobin levels. One interesting observation about these criteria is in their relationship to physical activity levels. Limited research exists for physical activity levels in cancer patients during cachexia. One study has found that physical activity levels did not differ between cancer and age-matched noncancer patients, but spontaneous physical activity was correlated with weight loss and blood hemoglobin concentrations (18). Conversely, others have shown that weight-losing cancer patients exhibit less physical activity than healthy control subjects (28). How physical activity levels change during cachexia and how increased muscle contractile activity alters the course of the disease remain important areas of research.
It has long been acknowledged that disuse caused by bed rest, spaceflight, limb immobilization, or hindlimb suspension in rodents all cause muscle atrophy, muscle weakness, and fatigue of slow-twitch muscles (10). This is the opposite of cancer cachexia, where fast-twitch muscles are predominantly affected, possibly because of the high glucose utilization by the tumor (32). In fact, during cachexia induced by chronic heart failure oxidative fibers are actually protected from atrophy (23). Overexpression in mice of peroxisome proliferator-activated receptor γ coactivator 1α (PPARGC1A, also known as PGC-1α), a protein important for mitochondrial biogenesis, protected skeletal muscles from undergoing atrophy during many types of atrophy, including cancer cachexia (30). These studies suggest that the stimuli for atrophy, either disease-induced cachexia or disuse, target different fiber types dependent on their oxidative capacity.
Apoptosis of myonuclei is a process that has been found to be important for regulating skeletal muscle catabolism induced by a variety of stimuli and has emerged as a potential control point for muscle mass loss (5, 6). Apoptosis is a specific form of cell death that occurs when the cells are not needed or become damaged or to counterbalance the effect of cellular proliferation (29). Multinucleated muscle fibers can have individual myonuclei undergo apoptosis without initiating necrosis or myofiber death (2, 29). Myofiber area is theorized to be regulated by myonuclear abundance as stated by the myonuclear domain concept, where a finite amount of cell volume can be supported by a single myonucleus (12). Thus a decrease in myonuclear number would be associated with a decrease in cell volume. Apoptotic pathways are activated in the skeletal muscle of cachectic humans (11), in tumor-bearing animals (9, 17, 20, 33), and in cell culture models of cachexia (14, 31). In fact, B cell leukemia/lymphoma 2 (BCL2)-associated X protein (BAX) protein expression appears to be a key regulator during cachexia (20, 33), and BCL2 is either not detected or weakly expressed (20). Conversely, aerobic exercise training is a potent stimulus that protects mitochondria from undergoing apoptosis in skeletal muscle (1). However, the relationship of muscle apoptosis to activity level and overall amount of muscle wasting during cancer cachexia has not been elucidated.
Our lab utilizes the ApcMin/+ mouse to study mechanisms responsible for the initiation and progression of cancer cachexia. These mice are heterozygotes for a mutation in the adenomatous polyposis coli (Apc) gene and spontaneously develop intestinal and colon adenomas. Since the APC gene is mutated in a large percentage of human colon cancer cases, this is a common model for studying environmental factors that influence a genetic predisposition for colorectal cancer occurrence (27). The advantages of using the ApcMin/+ mouse as a model of cachexia are the ratio of tumor mass to body mass, lack of anorexia, chronic inflammation, and slow rate of wasting, which are important characteristics of cachectic humans. These mice also demonstrate muscle weakness, fatigue, decreased volitional activity, and elevated circulating IL-6 levels (7, 8, 25). We have also shown that ApcMin/+ mice exhibit normal exercise adaptations to treadmill exercise and voluntary wheel running (26) and accumulate less running activity during the progression of the disease (7). The purpose of this study was to determine the time course of changes in physical activity and their relationship to anemia, apoptosis, and overall muscle mass and body mass loss during cachexia.
MATERIALS AND METHODS
Animals.
Male ApcMin/+ mice bred on a C57BL/6 background and wild-type littermates were bred and genotyped at the University of South Carolina's animal resource facility as previously described (7, 8, 25). Three separate studies were performed. Study 1 provided voluntary activity wheel access to wild-type (n = 5) and ApcMin/+ (n = 12) mice, which were then stratified by their severity of cachexia at 26 wk of age: mild (n = 4), moderate (n = 3), and severe (n = 5). The gastrocnemius muscle masses, body masses, plasma IL-6 levels, and voluntary wheel running distances presented in 4-wk intervals have been previously published for the mice in study 1 (8). All other data related to study 1 in the present report have not been previously published. Study 2 used sedentary ApcMin/+ mice housed without running wheels for the histological evaluation of muscle apoptosis in mice stratified by their severity of cachexia at 26 wk of age: mild (n = 3), moderate (n = 7), severe (n = 12). Study 3 examined 20-wk-old wild-type (n = 6) and ApcMin/+ (n = 14) mice stratified by their severity of cachexia and examined for apoptosis by muscle phenotype: mild (n = 6) and severe (n = 8). The room was maintained at 20–25°C and on a 12:12-h light-dark cycle with the light period starting at 0700. Mice were provided standard rodent chow (Harlan Teklad Rodent Diet, no. 8604, Madison, WI) and water ad libitum. All animal experimentation was approved by the University of South Carolina's Institutional Animal Care and Use Committee.
Determination of cachexia symptom severity.
A cachexia symptom severity score was assigned to age-matched ApcMin/+ mice as previously described (7). Mice were classified as having mild, moderate, or severe cachexia based on their body mass, gastrocnemius muscle mass, and epididymal fat pad mass. Mice were categorized as having mild cachexia if all of these variables were within 1 standard deviation of the mean of age-matched wild-type mice. Mice with moderate cachexia were 1–2 standard deviations and mice with severe cachexia were >2 standard deviations away from the means of age-matched wild-type mice.
Voluntary wheel running.
Voluntary wheel running was used as a marker of volitional physical activity and was performed as previously described (26). At 5 wk of age, wild-type and ApcMin/+ mice were housed individually in cages with 9.5-in.-diameter stainless steel activity wheels (MiniMitter, Bend, OR). Running activity was monitored daily from 5 to 26 wk of age. Bicycle computers (Specialized, Morgan Hill, CA) with magnetic sensors measured average speed, distance, time, and maximum speed, and the data were recorded daily. The maximum speed was retained in the bicycle computer's memory until a higher speed was maintained for 3 s. Voluntary wheels were only provided to mice in study 1. Mice from the other studies were housed in regular cages, and spontaneous activity levels were not monitored.
Blood variables.
Anemia was measured by taking ∼75 μl of whole blood from the retroorbital sinus under brief isoflurane anesthesia. Blood was collected in EDTA-coated tubes (BD Microtainer) and analyzed with the VetScan HMT Hematology Analyzer (Abaxis, Union City, CA). Blood measurements were taken at 13, 18, 22, and 26 wk of age for study 1 and at 26 wk of age for study 2.
Tissue collection.
Mice were given a subcutaneous injection of ketamine-xylazine-acepromazine cocktail (1.4 ml/kg body mass). Gastrocnemius muscles and tibias were excised. For study 1, gastrocnemius muscles were rinsed in PBS, snap frozen in liquid nitrogen, weighed, and stored at −80°C until further analysis. For study 2, gastrocnemius muscles were fixed in formalin overnight. After fixation, the muscles were placed in 70% ethanol until histological analysis. For study 3, gastrocnemius muscles were rinsed in PBS, weighed, dissected into their red and white portions, snap frozen in liquid nitrogen, and stored at −80°C until further analysis.
Gastrocnemius morphology.
Sectioning of muscle and staining was performed in the same way as described previously (25). Briefly, transverse sections (10 μm) were cut from the midbelly of the medial gastrocnemius on a cryostat at −20°C. Myosin ATPase staining was performed on the sections to delineate the type IIa and type IIb fibers to measure fiber cross-sectional area (CSA). Digital images were taken from each section with a Nikon spot camera, and fibers were traced with imaging software (Scion Image, Frederick, MD). Approximately 200 fibers per mouse were traced at ×200 magnification in a blinded fashion.
Western blotting.
Western blotting was performed as previously described (7, 8, 25). Primary antibodies for BAX, BCL2 (Calbiochem, San Diego, CA), and apoptotic peptidase activating factor 1 (APAF1), (Cell Signaling, Danvers, MA) were added to each membrane at a dilution of 1:500–1:1,000 in 5% BSA in Tris-buffered saline + Tween 20 (TBS-T) and incubated overnight at 4°C. Anti-rabbit IgG horseradish-peroxidase-conjugated secondary antibodies (GE Healthcare Life Sciences, Piscataway, NJ) were incubated with the membranes at 1:2,000–1:5,000 dilutions for 2 h in 5% TBS-T milk. Enhanced chemiluminescence (GE Healthcare Life Sciences) was used to visualize the antibody-antigen interactions and developed by autoradiography. Film was digitally scanned, and blots were quantified by densitometry using scientific imaging software (Scion Image). The Ponceau-stained membranes were also digitally scanned, and the 43-kDa actin bands were quantified by densitometry. All integrated optical densities (IODs) for BAX and APAF1 were normalized by the IODs for actin.
TUNEL assay and apoptotic nuclei.
Apoptotic cells were detected with a kit purchased from Chemicon International (Billerica, MA). Formalin-fixed, paraffin-embedded medial gastrocnemius sections (10 μm) taken from the midbelly of the muscle were stained for apoptotic cells according to manufacturer's instructions. Apoptotic nuclei stained brown, while other nuclei were counterstained with hematoxylin. Five to eight images were taken at ×400 magnification to quantitatively determine the localization of apoptotic cells (myofiber or interstitial space). Apoptotic nuclei were determined by an investigator blinded to the treatment group.
RNA isolation and real-time PCR.
RNA isolation, DNase treatment, cDNA synthesis, and real-time PCR were performed as previously described (7). Briefly, real-time PCR was performed with Taqman gene expression assays from Applied Biosystems (Foster City, CA). Gene expression for Bax was carried out in 25-μl reactions consisting of 12.5 μl of 2× Taqman Universal PCR master mix, 1.0 μl of cDNA, 1.25 μl of 20× primer, and RNase-free water. The 2−ΔΔCT method (24) (where CT is threshold cycle) was used to determine changes in gene expression between mice, with the 18S ribosomal RNA CT as the correction factor.
Citrate synthase assay.
Citrate synthase (CS) activity was determined in frozen gastrocnemius muscles as previously described (26). Briefly, the muscle was homogenized at a 1:21 dilution in homogenizing buffer (0.175 M KCl, 0.002 M EDTA, pH 7.4). CS activity was measured at 412 nm in a buffer containing (in mM) 100 Tris·HCl, pH 8.3 (0.700 ml), 1 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) (0.100 ml), 10 oxaloacetate (0.050 ml), and 3 acetyl-CoA (0.150 ml). Tissue homogenate was added (5–10 μl) to the cocktail, and the absorbance was recorded every 15 s for 3 min. CS activity was calculated based on the extinction coefficient for DTNB at 412 nm (13,600 M−1).
Statistical analyses.
Body mass loss, voluntary wheel running variables, and blood variables were analyzed with a two-way ANOVA (cachexia severity × time) with repeated measures. To determine the effect of fiber type on apoptosis, a two-way ANOVA (muscle phenotype × cachexia severity) was used. All other variables were analyzed by one-way ANOVA. Post hoc analyses were performed with Tukey's multiple comparison tests. If the assumption of normality failed, nonparametric tests were used. Linear regressions were performed to determine associations between variables. Data are presented as means ± SE. Significance was set at P < 0.05.
RESULTS
Loss of body mass in ApcMin/+ mice over time.
By retroactive examination of body mass, ApcMin/+ mice that ended up developing severe cachexia (25.1 ± 0.7 g) and moderate cachexia (25.6 ± 0.3 g) had peak body masses that were less than wild-type mice (28.4 ± 0.6 g; P = 0.005), but there were no differences between wild-type and mildly cachectic mice (26.9 ± 0.4 g). Both categories of cachectic mice also reached their peak body masses at an earlier age (15–21 wk of age) than wild-type mice (26 wk of age; P < 0.001), and wild-type mice did not differ from mice with mild cachexia (24 wk of age). However, body mass did not differ between any of the groups of mice at 15 wk of age (P = 0.564). Since losing >5% body mass was a criterion defining cachexia, we determined this to be the change in body mass from 15 wk of age (Fig. 1A; P < 0.001). Wild-type and mildly cachectic mice continued to gain body mass from 15 wk until 26 wk of age. This was in contrast to severely cachectic mice, which lost body mass and were different from wild-type mice beginning at 18 wk of age. Moderately cachectic mice delayed their decline in body mass, becoming different from wild-type mice at 25 wk of age. Between 15 and 26 wk of age, severely cachectic mice lost an average of 12% body mass and moderately cachectic mice lost an average of 6% body mass, fulfilling the definition of cachexia.
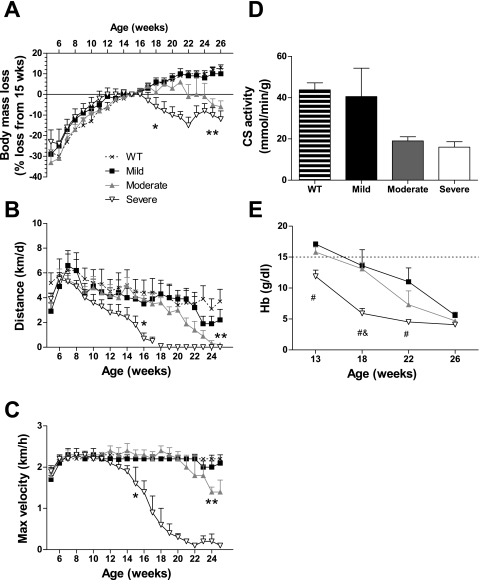
Fig. 1.
Changes in voluntary wheel running activity, anemia, and body mass loss. Wild-type (WT) and ApcMin/+ mice were given access to voluntary activity wheels and stratified by their severity of cachexia at 26 wk of age (mild, moderate, or severe). Body mass was measured weekly, and peak body mass occurred at 15 wk of age for mice with severe cachexia. A: body mass loss relative to 15 wk of age. B: average daily distance run. C: maximal running velocity. D: citrate synthase (CS) activity in gastrocnemius muscle. E: hemoglobin concentration. The dotted line represents WT values measured at 26 wk of age. Data are presented as means ± SE. *First time point at which mice with severe cachexia are different from WT mice; **first time point at which mice with moderate cachexia are different from WT mice; #different from mice with mild cachexia; &different from mice with moderate cachexia.
Changes in voluntary wheel running behavior during cachexia.
Wild-type and ApcMin/+ mice were given access to voluntary activity wheels starting at 5 wk of age. As shown in Fig. 1B, all mice covered less distance throughout the course of the study. However, the decline in wheel running distance was more prominent in mice that became severely cachectic by 16 wk of age compared with wild-type mice (P < 0.001). The same was true for mice that became moderately cachectic, except that this occurred at 25 wk of age. By 26 wk of age, the only differences in wheel running distance were that severely and moderately cachectic mice were running less than wild-type mice.
Since running distance decreased in healthy, wild-type mice, we looked at maximum running velocity to see how it changed with cachexia symptom severity and over time (Fig. 1C; P < 0.001). Unlike distance run, wild-type and mildly cachectic mice were able to maintain their maximum speed throughout the course of the study. However, maximum speed plummeted in severely cachectic mice, and they became different from wild-type mice starting at 15 wk of age. The same phenomenon occurred in mice with moderate cachexia, albeit at 24 wk of age. In fact, at 26 wk of age all groups were different from one another, except wild-type and mildly cachectic mice. This lack of volitional activity also pointed to physiological changes in the skeletal muscle, as there was a trend for CS activity levels to be affected by the severity of cachexia (Fig. 1D; P = 0.07). Also, there was a significant relationship between maximum running velocity at the study's end and CS activity among all ApcMin/+ mice (r2 = 0.465; P = 0.030).
Development of anemia during cachexia.
Blood draws were taken at 13, 18, 22, and 26 wk of age in all ApcMin/+ mice to determine whether anemia was associated with the development of cachexia. Wild-type values were assessed at the 26 wk time point only. There was a significant interaction between hemoglobin concentration and the severity of cachexia (Fig. 1E; P = 0.013). In mice that became severely cachectic by 26 wk of age, the concentration of hemoglobin was 30–59% less than that in mildly cachectic mice at 13, 18, and 22 wk of age. However, by 26 wk of age, none of these parameters was different with cachexia severity.
Apoptosis and skeletal muscle wasting in ApcMin/+ mice.
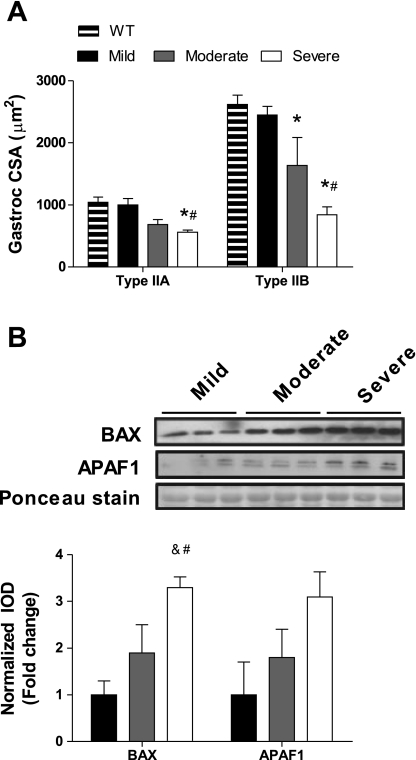
We determined skeletal muscle fiber CSA of the gastrocnemius type IIa and type IIb fibers. Muscle masses have been previously published from this set of mice, with severely cachectic mice having gastrocnemius muscle masses that were 61% less than mildly cachectic mice (7). Both type IIa (P = 0.001) and type IIb (P < 0.001) fibers demonstrated muscle atrophy in severely cachectic mice, but moderately cachectic mice demonstrated atrophy only of type IIb fibers (Fig. 2A). Severely cachectic type IIa gastrocnemius muscle fibers were 46% less and type IIb fibers were 68% less than wild-type mice, indicating the greater atrophy of glycolytic fibers. We used proapoptotic protein expression as a molecular marker of muscle wasting. BAX protein expression was increased (P < 0.001) with cachexia symptom severity, with severely cachectic mice having threefold more of the proapoptotic protein compared with mildly cachectic mice (Fig. 2B). There was also a trend for APAF1 protein expression to be threefold greater in the gastrocnemius muscle of severely cachectic mice compared with mildly cachectic mice (P = 0.056). BCL2 protein levels were undetectable.
Fig. 2.
Apoptotic proteins are induced in severely cachectic skeletal muscle. WT and ApcMin/+ mice were given access to voluntary activity wheels and stratified by their severity of cachexia at 26 wk of age (mild, moderate, or severe). A: type IIa and type IIb gastrocnemius (Gastroc) muscle fiber cross-sectional area (CSA) in WT and ApcMin/+ mice. B: BCL2-associated X protein (BAX) and apoptotic peptidase activating factor 1 (APAF1) protein expression in the gastrocnemius muscle. Data are presented as means ± SE. *Different from WT mice; #different from mice with mild cachexia; &different from mice with moderate cachexia. IOD, integrated optical density.
To determine the relationship between muscle size, exercise performance, and apoptosis, we performed linear regressions between gastrocnemius type II CSA, maximum running velocity, and BAX protein expression. There were significant relationships between muscle fiber CSA and maximum wheel running velocity (r2 = 0.899; P < 0.001), BAX protein expression (r2 = 0.867; P < 0.001), and hemoglobin concentration (r2 = 0.842; P = 0.017).
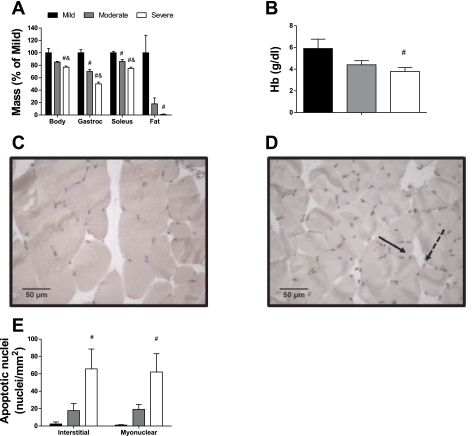
We followed up these markers by detecting apoptotic nuclei in a second study using sedentary mice. This new study also showed similar differences in body mass (−23%), gastrocnemius muscle mass (−50%), soleus muscle mass (−25%), and epididymal fat pad mass (−99%) between mildly and severely cachectic mice (Fig. 3A; P ≤ 0.001 for all variables). Mice in all categories demonstrated anemia, with severely cachectic mice having a hemoglobin concentration 36% less than mildly cachectic mice (Fig. 3B; P = 0.039). Severely cachectic ApcMin/+ mice had more interstitial and myonuclear apoptotic nuclei compared with mice with mild/no cachexia (Fig. 3, C–E; P ≤ 0.022). There were also significant relationships between gastrocnemius muscle mass and the number of apoptotic interstitial nuclei (r2 = 0.29; P = 0.040) and the number of apoptotic myonuclei (r2 = 0.28; P = 0.045).
Fig. 3.
Anemia and apoptosis occur in severely cachectic muscle of sedentary ApcMin/+ mice stratified by cachexia symptom severity. A: body, gastrocnemius muscle (Gastroc), soleus, and epididymal fat pad mass (Fat) relative to mice with mild cachexia. B: hemoglobin concentration. C: representative image of a mouse with mild cachexia. D: representative image of a mouse with severe cachexia. Apoptotic nuclei are brown, and nonapoptotic nuclei are in blue. Solid arrow points to apoptotic myonucleus. Dashed arrow points to apoptotic interstitial nucleus. E: apoptotic myonuclei and interstitial nuclei in cachectic ApcMin/+ mice. Data are presented as means ± SE. #Different from mice with mild cachexia; &different from mice with moderate cachexia.
Apoptosis occurs independently of muscle's oxidative phenotype.
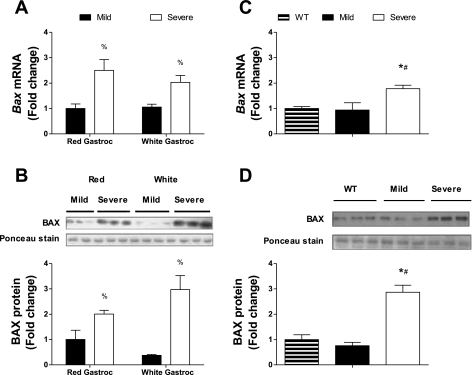
In study 3, the gastrocnemius muscle was dissected into its red and white portions, representing the oxidative and glycolytic parts of the muscle, in sedentary mice. Gene expression for Bax was induced in severely cachectic mice, independent of the muscle's oxidative capacity (Fig. 4A; P < 0.001). These results were confirmed by measuring BAX protein levels (Fig. 4B; P < 0.001), with severely cachectic mice having two- to threefold more BAX protein expression in both the red and white portions of the gastrocnemius muscle. We found similar results in the soleus muscle, with severely cachectic mice having greater expression of Bax mRNA (Fig. 4C; P = 0.008) and BAX protein (Fig. 4D; P = 0.001) compared with both mildly cachectic and wild-type mice.
Fig. 4.
Apoptotic signaling is induced in severely cachectic muscle independent of the muscle's oxidative capacity. Sedentary ApcMin/+ mice were stratified by their severity of cachexia. The gastrocnemius muscle was dissected into its red and white portions and assessed for Bax gene expression (A) and BAX protein expression (B). The soleus muscle was examined for Bax gene expression (C) and BAX protein expression (D) in WT and mild and severely cachectic ApcMin/+ mice. Data are presented as means ± SE. %Main effect of cachexia symptom severity; *different from WT mice; #different from mice with mild cachexia.
DISCUSSION
Understanding the early mechanisms that initiate skeletal muscle wasting remains an important underlying question of cancer cachexia research. Although physical activity is routinely mentioned with cachexia in review articles, there are far fewer studies examining how physical activity levels and function are affected in cachectic humans (18, 28). We monitored ApcMin/+ mice from 5 to 26 wk of age that were provided unlimited access to voluntary wheel running to determine whether changes in activity patterns precede muscle mass loss. Related to this question, this study presents two important findings. First, in the mouse cachexia model examined, maximum wheel running velocity was a better predictor of future cachexia severity than the accumulation of total daily distance run. Maximal running velocity demonstrated alterations at an earlier age than wheel running distance. Mild, moderate, and severely cachectic ApcMin/+ mice had different maximum running velocities by the study's end. The second important finding was that the loss of body mass with cachexia occurred after the changes in running performance. These data would indicate that reduced physical performance could be a prognosticator of future cancer-induced wasting. We report that 89% of the variability in gastrocnemius muscle fiber CSA could be explained by differences in maximum running velocity. We should acknowledge that maximum speed was computed as the highest speed obtained for at least 3 s over a 24-h period. This is important to note since mice are intermittent runners, averaging 100–200 bouts of activity that last ∼2–3 min per 24-h period (13). This parameter may not be sensitive for the onset of cachexia if maximum speed is computed over longer time intervals.
Cachexia is also associated with alterations in circulating markers related to inflammation, metabolism, and anemia. Anemia is an important component of the wasting process, since erythropoietin administered to tumor-bearing mice can decrease circulating IL-6 levels, improve anemia, and delay the wasting process (21). Since maximal running velocity appeared to be an important predictor of future muscle mass loss, we looked to see whether there was a relationship with the development of anemia. The definition of anemia in humans with cachexia is a hemoglobin level of <12 g/dl (15). Normal hemoglobin concentrations in mice range from 10 to 17 g/dl (3). With the definition of anemia scaled to <10 g/dl in mice, our severely cachectic mice would be considered anemic by 18 wk of age, our moderately cachectic mice at 22 wk of age, and our severely cachectic mice at 26 wk of age. Our data do not suggest that anemia is causing the initial reduction in voluntary activity. For example, our severely cachectic mice show decrements in maximal running speed at 15 wk of age but do not show anemia until 18 wk of age. In addition, our moderately and mildly cachectic mice become anemic at 22 wk and 26 wk of age, respectively, but still continue to maintain maximal running speeds similar to wild-type mice at these time points. Similar to our results, erythropoietin given to healthy mice did not increase voluntary wheel running activity (22). The importance of anemia may be related to the duration of the anemic condition. While all ApcMin/+ mice in our study became anemic regardless of weight loss or activity level, the mice developing severe cachexia developed it at an earlier age.
Alternatively, circulating IL-6 may also be a factor that contributes to changes in voluntary activity. We have previously reported (7) that the circulating IL-6 values in these mice are directly related to the degree of weight loss and vary from ∼3 pg/ml in mildly cachectic mice to ∼30 pg/ml in severely cachectic mice. We found a relationship between plasma IL-6 levels and distance run (r2 = 0.481; P = 0.012). IL-6 can also affect activity levels, since IL-6 knockout mice have increased volitional fatigue during treadmill running (16). There could also be an interaction between anemia and IL-6 that affects activity. While anemia appears to be a good early indicator of whether mice will become severely cachectic, it is not as clear whether it could be responsible for affecting volitional physical activity, and anemia's interaction with elevated circulating IL-6 may be worthy of further investigation. More work is necessary to understand the relationship between elevated IL-6 and changes in voluntary wheel running performance.
During disease, apoptosis of skeletal muscle myonuclei is an established regulatory component of skeletal muscle mass loss (29). Both tumor-bearing animals and humans demonstrate activation of apoptotic pathways during cachexia (11, 17, 20, 33). It has also been suggested that apoptosis is initiated early in the wasting process (6). In the present study, severely cachectic ApcMin/+ mice with access to activity wheels demonstrated more proapoptotic protein expression at 26 wk of age than mildly cachectic mice. Although our study demonstrated a relationship between skeletal muscle apoptosis and physical activity levels, the increase in apoptotic nuclei does not appear to be caused by physical inactivity. We also report that severely cachectic sedentary mice, with no access to activity wheels, also underwent atrophy and skeletal muscle apoptosis. Another interesting observation was that both oxidative and glycolytic portions of the gastrocnemius muscle were susceptible to apoptosis. Primarily glycolytic muscle fibers have been shown to undergo greater wasting than oxidative fibers, and this also holds true for glycolytic and oxidative muscles. Our data suggest that myonuclear apoptosis is not the leading candidate for the limiting mechanism related to the greater glycolytic fiber atrophy with cancer cachexia. In fact, it has been reported that oxidative fibers are actually protected from atrophy during some types of cachexia (23, 30). Our data also demonstrate that inherent oxidative capacity of muscle does not prevent or protect muscle fibers from myonuclear apoptosis. It remains to be determined whether increasing oxidative capacity through increased contractile activity would allow for repression of apoptosis during cancer cachexia. It also remains to be determined whether apoptosis is an early or late event in muscle wasting processes during cancer cachexia.
In summary, the ability to reverse severe cachexia in the clinical setting is rare, and interest has shifted to understanding the initial events during precachexia, when patients have lost <5% of body mass. This appears to be the opportune time for interventions to benefit clinical outcomes. We have demonstrated that a decrement in volitional activity precedes any changes in body mass and the onset of anemia during cachexia in the ApcMin/+ mouse. Since we only studied male mice, future studies are necessary to evaluate whether this phenomenon also occurs in female mice, since they typically run further distances than males. We also studied apoptotic markers, and they were elevated in severely cachectic muscle and these effects were independent of oxidative metabolism. Further studies will delineate the physiological events related to wheel running performance that foretell the development of severe skeletal muscle wasting with cancer.
GRANTS
The research described in this report was supported by National Institutes of Health (NIH) Grants 1RO1-CA-121249 to J. A. Carson and P20 RR-017698. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Julie Clements, Tia Davis, and Valerie Kennedy for technical assistance.
REFERENCES
- 1. Adhihetty PJ, O'Leary MF, Hood DA. Mitochondria in skeletal muscle: adaptable rheostats of apoptotic susceptibility. Exerc Sport Sci Rev 36: 116–121, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Allen DL, Roy RR, Edgerton VR. Myonuclear domains in muscle adaptation and disease. Muscle Nerve 22: 1350–1360, 1999 [DOI] [PubMed] [Google Scholar]
- 3.American College of Laboratory Medicine. The Mouse in Biomedical Research: Normative Biology, Husbandry, and Models. New York: Academic, 2006 [Google Scholar]
- 4. Ardies CM. Exercise, cachexia, and cancer therapy: a molecular rationale. Nutr Cancer 42: 143–157, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Argiles JM, Busquets S, Lopez-Soriano FJ. Cytokines in the pathogenesis of cancer cachexia. Curr Opin Clin Nutr Metab Care 6: 401–406, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Argiles JM, Lopez-Soriano FJ, Busquets S. Apoptosis signalling is essential and precedes protein degradation in wasting skeletal muscle during catabolic conditions. Int J Biochem Cell Biol 40: 1674–1678, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol 294: R393–R401, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Baltgalvis KA, Berger FG, Pena MM, Davis JM, White JP, Carson JA. Muscle wasting and interleukin-6-induced atrogin-I expression in the cachectic ApcMin/+ mouse. Pflügers Arch 457: 989–1001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Belizario JE, Lorite MJ, Tisdale MJ. Cleavage of caspases-1, -3, -6, -8 and -9 substrates by proteases in skeletal muscles from mice undergoing cancer cachexia. Br J Cancer 84: 1135–1140, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Booth FW, Gollnick PD. Effects of disuse on the structure and function of skeletal muscle. Med Sci Sports Exerc 15: 415–420, 1983 [PubMed] [Google Scholar]
- 11. Busquets S, Deans C, Figueras M, Moore-Carrasco R, Lopez-Soriano FJ, Fearon KC, Argiles JM. Apoptosis is present in skeletal muscle of cachectic gastro-intestinal cancer patients. Clin Nutr 26: 614–618, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Cheek DB. The control of cell mass and replication. The DNA unit—a personal 20-year study. Early Hum Dev 12: 211–239, 1985 [DOI] [PubMed] [Google Scholar]
- 13. De Bono JP, Adlam D, Paterson DJ, Channon KM. Novel quantitative phenotypes of exercise training in mouse models. Am J Physiol Regul Integr Comp Physiol 290: R926–R934, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, Price SR, Mitch WE. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest 113: 115–123, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr 27: 793–799, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Faldt J, Wernstedt I, Fitzgerald SM, Wallenius K, Bergstrom G, Jansson JO. Reduced exercise endurance in interleukin-6-deficient mice. Endocrinology 145: 2680–2686, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Figueras M, Busquets S, Carbo N, Barreiro E, Almendro V, Argiles JM, Lopez-Soriano FJ. Interleukin-15 is able to suppress the increased DNA fragmentation associated with muscle wasting in tumour-bearing rats. FEBS Lett 569: 201–206, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Fouladiun M, Korner U, Gunnebo L, Sixt-Ammilon P, Bosaeus I, Lundholm K. Daily physical-rest activities in relation to nutritional state, metabolism, and quality of life in cancer patients with progressive cachexia. Clin Cancer Res 13: 6379–6385, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Giordano A, Calvani M, Petillo O, Carteni M, Melone MR, Peluso G. Skeletal muscle metabolism in physiology and in cancer disease. J Cell Biochem 90: 170–186, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Ishiko O, Sumi T, Yoshida H, Hyun Y, Ogita S. Expression of apoptosis regulatory proteins in the skeletal muscle of tumor-bearing rabbits compared with diet-restricted rabbits. Int J Mol Med 8: 279–283, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Kanzaki M, Soda K, Gin PT, Kai T, Konishi F, Kawakami M. Erythropoietin attenuates cachectic events and decreases production of interleukin-6, a cachexia-inducing cytokine. Cytokine 32: 234–239, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Kolb EM, Kelly SA, Middleton KM, Sermsakdi LS, Chappell MA, Garland T., Jr Erythropoietin elevates VO2,max but not voluntary wheel running in mice. J Exp Biol 213: 510–519, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Li P, Waters RE, Redfern SI, Zhang M, Mao L, Annex BH, Yan Z. Oxidative phenotype protects myofibers from pathological insults induced by chronic heart failure in mice. Am J Pathol 170: 599–608, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Mehl KA, Davis JM, Berger FG, Carson JA. Myofiber degeneration/regeneration is induced in the cachectic ApcMin/+ mouse. J Appl Physiol 99: 2379–2387, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Mehl KA, Davis JM, Clements JM, Berger FG, Pena MM, Carson JA. Decreased intestinal polyp multiplicity is related to exercise mode and gender in ApcMin/+ mice. J Appl Physiol 98: 2219–2225, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247: 322–324, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Moses AW, Slater C, Preston T, Barber MD, Fearon KC. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br J Cancer 90: 996–1002, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sandri M, Carraro U. Apoptosis of skeletal muscles during development and disease. Int J Biochem Cell Biol 31: 1373–1390, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 103: 16260–16265, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stevenson EJ, Koncarevic A, Giresi PG, Jackman RW, Kandarian SC. Transcriptional profile of a myotube starvation model of atrophy. J Appl Physiol 98: 1396–1406, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 89: 381–410, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Yoshida H, Ishiko O, Sumi T, Honda KI, Hirai K, Ogita S. Expression of apoptosis regulatory proteins in the skeletal muscle of tumor-bearing rabbits. Jpn J Cancer Res 92: 631–637, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]