Abstract
Physical contact between an inhaled particle and alveolar epithelium at the moment of particle deposition must have substantial effects on subsequent cellular functions of neighboring cells, such as alveolar type-I, type-II pneumocytes, alveolar macrophage, as well as afferent sensory nerve cells, extending their dendrites toward the alveolar septal surface. The forces driving this physical insult are born at the surface of the alveolar air-liquid layer. The role of alveolar surfactant submerging a hydrophilic particle has been suggested by Gehr and Schürch's group (e.g., Respir Physiol 80: 17–32, 1990). In this paper, we extended their studies by developing a further comprehensive and mechanistic analysis. The analysis reveals that the mechanics operating in the particle-tissue interaction phenomena can be explained on the basis of a balance between surface tension force and tissue resistance force; the former tend to move a particle toward alveolar epithelial cell surface, the latter to resist the cell deformation. As a result, the submerged particle deforms the tissue and makes a noticeable indentation, which creates unphysiological stress and strain fields in tissue around the particle. This particle-induced microdeformation could likely trigger adverse mechanotransduction and mechanosensing pathways, as well as potentially enhancing particle uptake by the cells.
Keywords: Zisman's plot, three-phase interfacial lines, ultra fine particles, contact angle, aerosols, mechanotransduction
interaction between inhaled particles and the lung is of great interest in lung physiology (13). It is likely that it triggers a variety of subsequent physiological and pathophysiological events, including physical and chemical insults on the epithelium, uptake by alveolar macrophage or by alveolar type-I, type-II pneumocytes, as well as transepithelial translocation, both transcellularly and pericellularly. The nature of this interaction depends on the physicochemical characteristics of particles (e.g., size, shape, surface characteristics, core properties).
As soon as inhaled particles touch the lung alveolar surface, the first event to occur is that the particles encounter surfactant at the air-liquid interface. Pulmonary surfactant, located in the liquid layer covering the alveolar walls, generally lowers surface tension; this, in turn, reduces the contact angle between the particle and the air-liquid interface. In the physiological range of surface tension (<30 dyn/cm2), most of particles are hydrophilic and submerged in the alveolar lining layer called hypophase (50, 51). If the particle diameter is greater than the thickness of the hypophase, the surface tension forces that act at the particle and air-liquid interface border push the particle toward the epithelium, as shown by Gehr and Schürch's and colleagues [e.g., Schürch et al. (51, 52), Gehr et al. (12), Geiser et al. (16, 18)]. As a result, submerged particles push against the epithelial surface creating, thereby, indentation on the soft alveolar septal tissue (Fig. 1). This indentation likely creates unphysiological stresses and strains, which may, in turn, cause important pathophysiological consequences. While numerous potential biological consequences of this phenomenon are listed and discussed in detail in the discussion section, an observation we have recently made, which motivates this study, is as follows. We recently visualized innervations of alveolar septa with sensory neurons (31) [and also by others (9, 25, 63)]; this leads to an idea that it is highly conceivable that even a microstress exerted by a particle deposited around sensory neurons could be large enough to trigger firing those afferent neurons, causing subsequent functional effects.
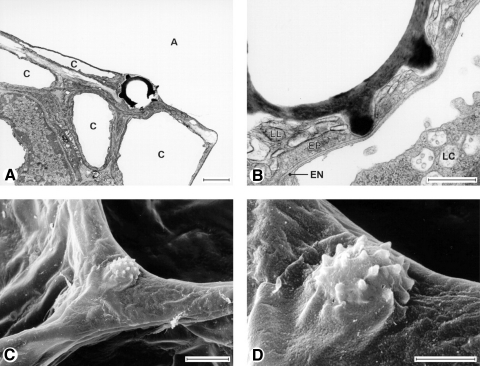
Fig. 1.
Transmission (A and B) and scanning (C and D) electron micrographs of puffball spores deposited on alveolar surfaces. Notice that the spore particle is totally covered by the surface lining layer and submerged. The epithelium was indented even by the particle's spiny protrusions (at these locations, the particle is separated from the capillary by 100 nm). A, alveolar lumen; C, capillary; EN, endothelial cell; EP, epithelial (type 1) cell; LC, leukocyte; LL, osmophilic lining layer material. Scale bars = 2 (A and D), 0.5 (B), or 5 μm (C). [From Geiser et al. (18)].
The objective of this study is to elucidate the mechanisms by which deposited particles exert mechanical forces on the septal tissue and to estimate the extent of physical insults exerted on the alveolar epithelium. The quantitative assessment of these forces and alveolar tissues deformation may give us an insight into new mechanosensing as well as mechanotransducting pathways.
METHODS
Surface tension is the key driving force to submerge a deposited particle into the alveolar liquid layer and to force the particle into alveolar tissue. The surface tension force depends on a number of factors, including 1) pulmonary surfactant concentration, 2) alveolar surface area, 3) the wettability of a particle, which is governed by the particle surface characteristics and liquid-air surface tension, 4) the thickness of an alveolar liquid layer called hypophase, and 5) the extent of particle indentation into the alveolar wall tissue, which depends on tissue stiffness. Because the surface tension forces, the extent of indentation, and the tissue restoring forces are all mechanically coupled, it is necessary to define the governing equations for the indentation process, which are based on the laws of interfacial phenomena and the laws of the solid mechanics.
Geometric and Material Model and Associated Assumptions
To keep the problem tractable without a loss of generality, we consider the following approximations. We assume that a particle is rigid and spherical with a radius of R ranging from 0.125 to 0.5 μm. We also assume that the indentation occurs orthogonal to the solid-liquid surface. The tissue is considered as a semi-infinite medium, and its mechanical behavior is simplified by assuming that material is linear elastic with elasticity modulus E, ranging from 10 to 30 kPa (11) and Poisson's ratio within the values from 0.1 to 0.5.
Throughout the analysis, it is assumed that surface tension γ is constant in each calculation, ranging from 10 to 50 dyn/cm. This range corresponds to conditions in the lung where the alveolar walls are covered by a liquid layer with normal or with dysfunctional lung surfactant. For this range of γ, the extent of tissue indentation is primarily determined by a balance between surface tension force, Fγ, which pushes the particle toward the tissue, and resisting tissue elastic force, Fel, due to the resulting tissue indentation. For a given particle size and characteristics of lung surfactant and tissue elasticity, there is a maximum particle indentation umax. Both forces can be expressed by complex nonlinear functions of umax; Fγ includes geometric parameters, as described below, while Fel depends on the tissue mechanical characteristics and the contact conditions between the particle and tissue. For simplicity, the contact is assumed to be frictionless during the indentation. We consider the process to be quasi-static; hence, inertial and viscous effects are neglected in the present analyses. Other forces, such as gravity and buoyancy forces, are small1 and they are considered negligible for the size of particles that we considered in this study (51).
Surface Tension Acting on a Particle
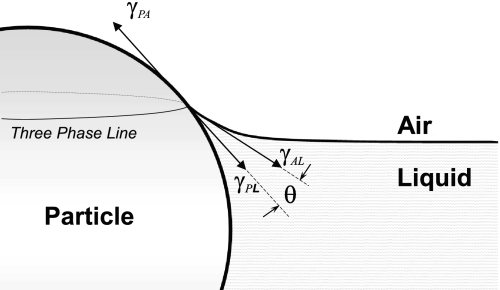
Because the alveolar surface is covered by a liquid layer, a particle forms a particle-air-liquid interface as soon as it lands on the alveolar surface. There are three interfaces: the particle (P)-liquid (L), the particle (P)-air (A), and air (A)-liquid (L) with three associated interfacial tensions, γPL, γPA, and γAL (Fig. 2). The air-liquid interfacial tension, γAL, is also called the surface tension and is denoted as γ for simplicity. The angle between γ and γPL at the contact point is called the contact angle, θ. At equilibrium (46),
| (1) |
Angle θ defines “wettability” of a particle; the particle is “wetted” by the liquid when 0 < θ < π/2, and “completely wetted” when θ = 0. On the other hand, the particle is “unwetted” when π/2 < θ ≤ π.
Fig. 2.
Particle-air-liquid interface. The interfacial tensions associated with the interfacial energies are denoted as follows: particle-air as γPA, particle-liquid as γPL, and air-liquid (i.e., the surface tension) as γAL, or simply as γ. The balance of the interfacial tensions at a common point where three surfaces meet defines Young's equilibrium equation. The three-phase line denotes the line where particle surface interfaces with air-liquid surface.
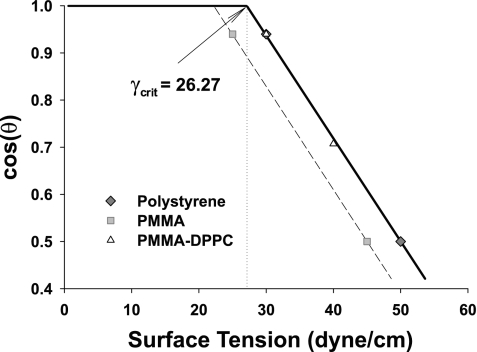
The magnitude of θ depends on surface characteristics (surface-free energy) of the particle and γ. For a latex (polystyrene) particle interacting with pulmonary surfactant (DPPC) (51), the relationship between cosθ and γ, spanning a representative physiological range, is shown in Fig. 3. A similar relationship between θ and γ can be obtained for a polymethylmethacrylate (PMMA) particle. The graphic representation of these relationships is called a Zisman plot (10, 68). This plot shows that the “wetting coefficient”, cosθ is equal to 1 at low surface tensions (i.e., γ < γcrit), denoting “complete wetting,” while for higher surface tension (γ > γcrit), cosθ decreases linearly with increasing γ. The nonlinear effects due to the line tension are negligible2 over the whole range of γ (37, 38). The typical range of alveolar air-liquid interfacial tension under normal breathing conditions is 0 < γ < 30 dyn/cm, where γ is modulated by the lung surfactant (64). The Zisman plot demonstrates that in this range, both latex beads and PMMA particles are completely wetted by the liquid layer.
Fig. 3.
Zisman plot. The Zisman plot defines a characteristic relationship between contact angle and surface tension. Both a latex (polystyrine) particle and a polymethylmethacrylate (PMMA) particle interact similarly with monolayer of 1,2-dipalmitoyl-sn-3-glycerophosphorylcholine pulmonary surfactant (DPPC). Thus, the Zisman plots of those particles almost coincide, and they both reach perfect wetting (θ = 0) at the critical surface tension of γcrit = 26.27 dyn/cm. However, a PMMA particle interacting with aqueous solution reaches θ at lower γcrit = 22.25 dyn/cm.
Knowing the relationship between θ and γ, we are now in a position to estimate the normal surface tension force Fγ acting on the particle (47, 48, 51),
| (2) |
where ϕ is the polar angle, denoting the position of the particle-air-liquid contact point on the surface (Fig. 4), which is given as
| (3) |
where δhypo and zo denote the thickness of the hypophase [∼0.1 μm on average or much less depending on the location in the alveolus, according to P. Gehr (personal communication)] and a capillary rise, respectively.
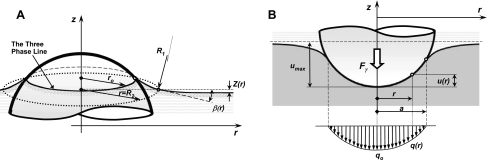
Fig. 4.
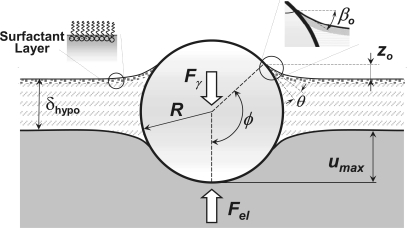
A schematic view of particle-hypophase-tissue interactions. At the equilibrium the surface tension force Fγ is balanced with the elastic tissue restoring force Fel, and this equilibrium force causes a deformation in tissue with the maximum indentation depth of umax. The increase in thickness of hypophase in the proximity of the particle (comparing to far-field thickness δhypo) is caused by capillary rise, zo. The angle ϕ measured with respect to the vertical axis defines the angular position of the point where three surfaces meet, where tan(βo) is the slope (in vertical plane) of the air-liquid interface at the particle surface, and R is the particle radius. The wetting angle is denoted as θ.
The a priori unknown values of ϕ, zo, and umax are determined iteratively, following the computational steps as follows: 1) for a given ϕ, Fγ is calculated from Eq. 2, and zo is calculated from the solution of the Young-Laplace equation (46) (see Eq. 4 below); 2) for known Fγ, umax is calculated form the analytical solution (see Eq. 14, below) of the Hertz problem (21); and 3) by varying ϕ, the solution for zo and umax is found iteratively to fully satisfy the Eq. 3 within a prescribed tolerance.
Capillary Rise and Menisci Profile Around Small Spherical Particles
At the surface of a spherical particle, the three-phase line is a circle that is uniquely defined by the polar angle, ϕ (Fig. 5A). The equilibrium value ϕ is a priori unknown, and it can be calculated from Eq. 3 once zo and umax are determined. The capillary rise, zo, is obtained from the hydrostatic Young-Laplace equation for the meniscus (46):
| (4) |
where Δp denotes the pressure difference across the liquid-air interface (we set it to be equal to zero because the system is assumed to be at equilibrium and there is no lateral flow), ρ is the mass density of the liquid,3 g is the gravity acceleration, and z(r) is the capillary rise of an axisymmetric surface at distance r from the (vertical) z-axis. We showed above that gravity effects are negligible for submicron particles. There are two finite radii of curvature involved at the spherical particle-fluid-air-interface in the three-dimensional problem: one denoted as R1 in the plane shown in Fig. 5A, and the other denoted as R2 in the orthogonal plane. Both radii R1 and R2 depend on the shape of the axisymmetric liquid-air surface; they can be expressed as functions of z(r), as
| (5) |
where z′ = dz/dr and z″ = d2z/dr2. The boundary conditions refer to the slopes, which are defined at the three-phase line and in the far field: 1) at radius r0 = Rsinϕ, as the slope of the air-liquid interface at the sphere surface (Fig. 4); and 2) at r >> R, the slope of the air-liquid interface in the vertical plane approaches to zero. These boundary conditions in mathematical form are
| (6) |
where βo = θ + ϕ − π (Fig. 4). The vertical position of the three-phase line on the surface of the spherical particle is not a priori known and is implicitly defined by Eq. 3.
Fig. 5.
The geometry of the interface between air-liquid and particle surfaces, and the geometry of the particle indentation into alveolar wall tissue. A: capillary rise. At the radius, r, the air-liquid surface is elevated by z(r) above the surface where pressure across the surface is zero. The capillary elevation z(r) is function of two radii of curvature of air-liquid surface, R1 and R2 (see Eqs. 5 and 6). The capillary rise has a maximum value at the particle surface, denoted in Fig. 4 as zo, where zo is the vertical distance from the free surface at far field to the three-phase line. The capillary rise is due to a net upward force produced by the attraction of the liquid to a solid surface. B: deformation of tissue caused by particle indentation. The spherical contact pressure distribution, q(r), reaches a maximum value qo at the vertical axis of symmetry where the maximum indentation umax occurs. The radius of the contact surface is denoted as a, and the indentation force is equal to surface tension force Fγ.
Substituting Eq. 5 into Eq. 4, defining the capillary constant c2 = 2γ/ρg, and normalizing r and z by the capillary length, c, (i.e., x = r/c and y = z/c), Eq. 4 can be transformed into a nondimensional form (47, 48):
| (7) |
where y′ = dy/dx, and y″ = d2y/dx2. The boundary conditions (Eq. 6) can also be transformed into a nondimensional form:
| (8) |
where xo = ro/c and βo is an angle between air-liquid surface and horizontal plane at the three-phase line (Fig. 4).
The profile of the meniscus and, thus, zo can now be calculated from the solution of Eq. 7 with boundary conditions given in Eq. 8. Since there is no analytical solution of this problem, we further simplified Eq. 7 by assuming that the dimensionless variable x is small so that xy ≪ sinβ (47). Here, β is an angle (in radial plane) between air-liquid interface and the horizontal plane at an arbitrary radius r (see Fig. 5A). Also, tanβ = y′ is a slope of the air-liquid surface. For submicron spherical particles, this condition is always satisfied because the deformation of the liquid-air interface around submicron particles is small. This assumption implicitly implies that the gravity effect is negligible,4 thus the term 2y in Eq. 7 can be dropped out. Therefore, in the case of submicron particles, Eq. 7 can be transformed into a system of two simple equations (47):
| (9a) |
| (9b) |
The solution of Eq. 9a for the known initial condition β = βo at x = xo, is
| (10) |
Substituting Eq. 10 into Eq. 9b and satisfying boundary condition: y = yo at β = βo, the approximate solution for the profile of air-liquid interface is obtained as
| (11) |
The only remaining unknown is the value of the dimensionless capillary rise yo = zo/c, which is not known a priori. To determine yo, we use the following approach.
Whereas Eq. 11 accurately describes the shape and position of the air-liquid interface in the proximity of three-phase line (i.e., the particle surface), the accuracy of this so-called “the outer solution” decreases with increasing x (away from a particle).5 Thus, we need some other asymptotic solution to satisfy boundary condition in far field to precisely determine yo and, therefore, the position of the three-phase line (via Eq. 3). One convenient method for approximately determining yo is the shooting method6 (47). With this method, the value of yo is determined by matching the above outer solution, which satisfies boundary conditions at the three-phase line, with so-called “inner solution,” which satisfies the boundary condition in the far field (29). This solution yields to an approximate analytical expression of yo, in the limit of the small Bond number εB2 = 2r02 /c2→ 07 as
| (12) |
where λ is the Euler constant equal to 0.57721.
Deformation in Alveolar Tissue Caused by Particle Indentation
Under equilibrium conditions, umax (schematically shown in Fig. 4) is calculated from a force balance between Fγ and Fel under quasi-static conditions:
| (13) |
The force Fel is evaluated as a function of umax using the classical Hertz solution (34, 56), which assumes no friction between the indenter and the elastic medium:
| (14) |
The tissue deformation and stress distribution can be calculated according to the Hertzian solution for a spherical particle, in which the contact pressure q(r) is obtained in a form of a spherical distribution:
| (15) |
where a is the radius of contact,
| (16) |
and qo is the maximum contact pressure:
| (17) |
This solution is accurate only for the small deformations, i.e., when the indentation is within 10% of the particle diameter. For larger indentations, the pressure distribution significantly deviates from the spherical, leading to the solution error. This will be analyzed in detail in the discussion section.
The alveolar tissue, and especially epithelial cell layer, is very soft; thus, the particle indentation could be larger than the particle radius. When the radius of contact, a, reaches the sphere radius, R, further indentation does not increase the contact area and, therefore, the indentation depth increases linearly with the additional force increase. The excess force, Fplunge, with respect to the maximum Hertzian force FHertz,max = Fel (a = R), is
| (18) |
The linear increase in Fplunge for the force exceeding FHertz,max, i.e., for umax, is given as
| (19) |
and therefore umax is also linearly related to the total force Fγ > FHertz,max. Finally, the pressure distribution can be approximated by using the solution for the flat indenter:
| (20) |
and the reference pressure (at the axis of symmetry) is
| (21) |
The total pressure distribution for Fγ > FHertz,max is equal to the sum of q(r) and qplunge(r). For known normal traction (pressure) distribution imposed by the indented particle (Eqs. 15 and 20), the stress, strain, and displacement fields can be calculated analytically by employing the Boussinesq solution (56) for the force acting on a semi-infinite body. This includes evaluation of the convolution integral over the spherical contact pressure distribution, which is described in the appendix. Because of the tensorial nature of stresses and strains, we also calculated the equivalent stress and the effective strain, which are convenient in displaying the tensorial stress and strain fields as scalar fields. The equivalent (or effective) stress used here is the von Mises stress, which in the cylindrical coordinates is
| (22) |
Similarly, the effective strain is defined as
| (23) |
RESULTS
Degree of Particle Indentation into Alveolar Tissue by Surface Tension Forces
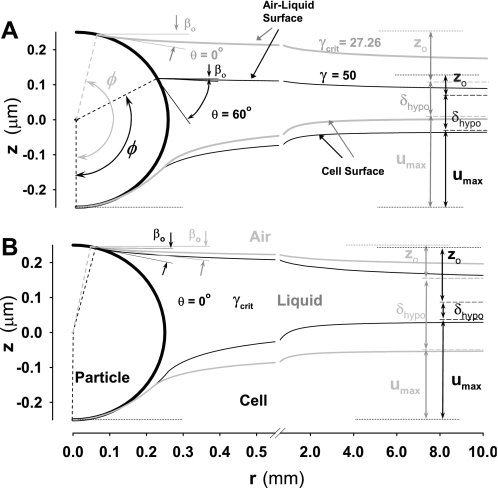
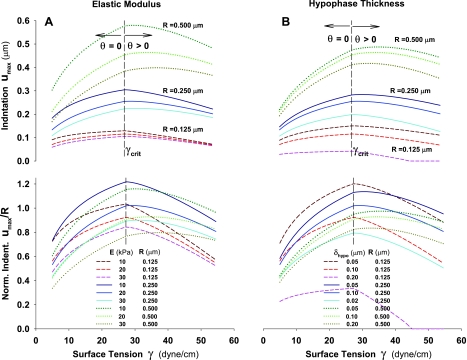
The degree of the particle indentation depends primarily on surface tension, alveolar tissue elasticity, particle size, and thickness of hypophase. These factors significantly change shape of air-liquid and liquid-solid interfaces and consequently, the degree of indentation. The effects of some of the factors are straightforward to predict. For instance, a decrease in the tissue elastic modulus, increases the degree of particle indentation (see Figs. 6 and 7), or when a particle is completely wetted (θ = 0, i.e., Fγ < Fγcrit), an increase in surface tension increases the particle indentation too (Figs. 6A and 7A). On the other hand, the effects of some other factors are counterintuitive. For example, the particle indentation decreases with an increase of γ when surface tension is above γcrit (i.e., γ > γcrit). This occurs because of a strong effect of increase of θ with increasing γ [see Zisman's plot (Fig. 3), where cosθ decreases with increasing γ]. In Fig. 6A, it is demonstrated how an increase in θ causes a decrease in ϕ and most importantly decrease in βo. Consequently, the vertical component of surface tension force (∝ γsinβo) sharply decreases. Thus, according to Eq. 2, the decrease in Fγ is caused by a larger decrease in βo compared to smaller increase in the product of γ and the length of the three-phase line, 2πRsinϕ. In other words, the effects of an increase in θ on Fγ is strong so that even a large decrease in ϕ (i.e., a large increase in the length of the three-line phase line), cannot compensate for a large decrease of βo and zo (Fig. 6A). Since Fγ is a strong function of these geometric factors (see Eq. 2), an increase of γ above γcrit would result in decreasing Fγ and, consequently, a decrease in the degree of particle indentation (Fig. 6A). In this analysis, the thickness of hypophase δhypo is taken to be constant.
Fig. 6.
Air-liquid and liquid-solid interfaces after particle indentation by surface tension forces. A: particle is indented more at critical surface tension, γcrit = 26.27 dyn/cm and wetting angle θ = 0° (gray lines), than at much higher surface tension of 50 dyn/cm because of large wetting angle θ of 60°. Increase in θ excessively reduces the effect of large increase in surface tension (black lines). The thickness of hypophase, δhypo is 0.1 μm. B: decrease of the thickness of hypophase from 0.2 μm (gray lines) to 0.05 μm (black lines) significantly increases indentation because the thinner hypohase increases capillary rise due to decrease in angle ϕ, and increase in angle βo. The surface tension is taken to be equal to γcrit and θ = 0°. In all calculations, particle diameter is 0.5 μm, and alveolar wall Young's modulus and Poisson's ratio are taken to be 20 kPa and 0.5, respectively.
Fig. 7.
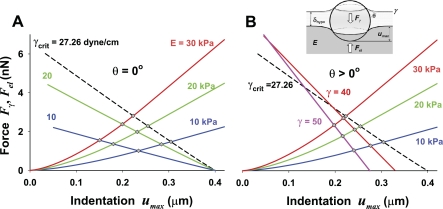
Depth of particle indentation as a function of surface tension, and the Young elastic modulus of the alveolar wall tissue. The equilibrium force, Fγ = Fel, and the corresponding maximum indentation, umax, are obtained at the intersection of the lines for Fγ(umax) the fixed surface tension, γ, and those for Fel(umax) fixed elastic moduli, E and v = 0.5. The particle diameter is 0.5 μm. The diamond symbols denote points of static equilibrium and hence values of umax,eq for that particular pair of forces. The increase of Young's elastic modulus, E, always increases the equilibrium force and decreases umax,eq. A: for γ < γcrit, both the equilibrium force and umax,eq increase with increase of γ, while in B: for γ < γcrit, both the equilibrium force and umax,eq decrease.
When δhypo decreases (at constant γ and θ), Fγ increases, and consequently the degree of particle indentation increases (Fig. 6B). In this case, an increase in Fγ is caused by a decrease in ϕ as a consequence of larger capillary rise, zo, for thinner hypophase. Since smaller ϕ increases the length of the three-phase line and, in addition, increases magnitude of βo (i.e., the vertical component of the surface tension force), the increase in Fγ is amplified by these two synergetic effects. The cumulative effect, therefore, increases Fγ and the depth of particle indentation, umax.
Because of the fact that both Fel and Fγ are nonlinear functions of the maximum particle indentation umax, the equilibrium indentation, umax,eq, is determined iteratively, and is represented graphically as a crossover point of the Fel–umax and Fγ–umax curves. Here, the Fγ–umax relationship is obtained from Eqs. 2 and 3, while the Fel–umax relationship is derived from the Hertzian contact (Eqs. 14 and 19). In Figs. 7 and 8, A and B, the equilibrium indentation, umax,eq, is denoted as diamond symbols at the crossover points.
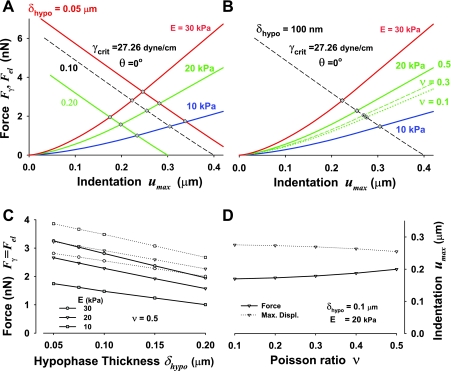
Fig. 8.
Depth of particle indentation as a function of the elastic moduli of the alveolar wall tissue and thickness of the hypophase, δhypo, at γcrit (A and B), and the quantitative effects of δhypo on the equilibrium force, Fγ = Fel, and indentation umax,eq (C and D). The diamond symbols denote the equilibrium force, Fγ = Fel, and indentation umax,eq. A: force and indentation cross-over points for three thicknesses of hypophase (0.05, 0.1, and 0.2 μm), three Young's elastic moduli (E = 30, 20, and 10 kPa), and v = 0.5. The particle diameter is 0.5 μm. The decrease of the thickness of the hypophase increases both Fγ and umax,eq. B: force and indentation cross-over points, denoted as circles and a diamond, for three Poisson's ratios (0.1, 0.3, and 0.5), E =20 kPa and δhypo = 0.2 μm. Increase in Poisson's ratio increases Fγ and decreases umax, having similar quantitative effect as modest increase in Young's elastic modulus. Note that for simplicity, umax here and in all following figures denotes equilibrium indentation umax,eq. C: effect of the thickness of hypophase on Fγ and umax for three Young's elastic moduli E, and v = 0.5. D: effect of Poisson's ratio for the range of v = 0.1-0.5 and for E = 20 kPa on Fγ and umax. In C and D, Fγ = Fel vs. δhypo is denoted by solid lines, and the symbols indicate which Young's modulus was used; the indentation umax vs. v is denoted by dashed lines.
In Fig. 7, umax is plotted vs. Fγ and Fel for a 0.5-μm diameter particle (R = 0.25 μm), in the physiologically relevant range of surface tension (0 < γ < 50 dyn/cm), and the mechanical properties of the alveolar tissue (Young's modulus 10 < E > 30 kPa and Poisson's ratio of 0.5). When γ ≤ γcrit (Fig. 7A), the extent of particle indentation, umax,eq, increases with increasing γ for a fixed E or with decreasing E for a fixed γ. When γ > γcrit (Fig. 7B), however, the situation is different: umax,eq decreases with increasing γ for a fixed E and with increasing E for a fixed γ. As we discussed above, the difference between these cases is due to the difference in the γ vs. cosθ relationship depending on whether γ is below or above its critical value γcrit (see Fig. 3, Zisman plot).
When the particle is completely wetted (θ = 0), for γ ≤ γcrit (Fig. 7A), the force Fγ nearly linearly decreases with the degree that the particle submerges. It is interesting to notice that for any γ ≤ γcrit, the indentation always increases up to umax = 0.4 μm (i.e., up to the diameter of the particle of 0.5 μm minus δhypo = 0.1 μm) while Fγ decreases to 0. Thus, when Fγ = 0 at umax = 0.4 μm, the particle is completely submerged and ϕ = 180°. On the other hand, when γ > γcrit [i.e., a particle is partially wetted (θ > 0)] (Fig. 7B), the slope of Fγ vs. umax relationship increases (in absolute value) much faster with increasing γ, leading to a progressively lower value of umax when Fγ = 0. In this last case, increasing θ (with increasing γ) causes βo to decrease to 0 (see Fig. 6A), and consequently, Fγ = 0 occurs at ϕ < 180°. This, in turn, indicates that the particle is only partially submerged, even with no resistance from tissue. For simplicity, in the text that follows (and figures, other than Figs. 7 and 8, A and B), the equilibrium indentation, umax,eq, is denoted as umax.
Effects of Hypophase Thickness, Poisson Ratio, and Particle Size on the Degree of Particle Indentation
The effect of the thickness of hypophase δhypo on particle indentation is not negligible, which is demonstrated in Fig. 8A for θ = 0 and γcrit. Decrease in δhypo increases both Fγ and the degree of particle indentation. This is because the decrease of δhypo (i.e., thinner hypophase) leads to a combined effect of an increase of the length of the three-phase line (via decreasing ϕ) and an increase of βo, which is caused by larger capillary rise (see Fig. 6B). Altogether, these two synergetic effects result in higher equilibrium forces and thus, deeper indentation. The quantitative effects of the thickness of hypophase are summarized in Fig. 8C. Both, the equilibrium force and equilibrium indentation (denoted here as umax instead of umax,eq for simplicity) decrease approximately linearly with increasing δhypo in the range of δhypo from 0.05 to 0.2 μm. The force decreases faster than umax due to a nonlinear effect of force-indentation relationship of the Hertzian contact of a rigid sphere with an elastic medium (Eq. 14).
Finally, the effect of Poisson's ratio8 on indentation is shown to be relatively small as demonstrated in Fig. 8B for θ = 0 and γcrit. A decrease of the equilibrium force, Fγ = Fel, for a decrease of v from 0.5 to 0.1 is roughly equivalent to 5 kPa decrease in E. This change in Fel–umax relationship only slightly increases the particle indentation, showing that the overall effect of v is modest. In fact, the quantitative effect of Poisson's ratio on the equilibrium force is about 16%, for a four-fold increase in v, and this increase is more pronounced at higher values of v, while the decrease in displacement is only 8% (Fig. 8D).
The effects of particle size on the equilibrium indentation umax are examined for various tissue elastic moduli (E = 10, 20, and 30 kPa) with constant δhypo = 0.1 μm (Fig. 9A, Table 1) and for various hypophase thicknesses (= 0.1, 0.15, and 0.2 μm) with constant E = 20 kPa (Fig. 9B, Table 1). One of the remarkable features common in the results is that umax behaves differently for γ < γcrit than for γ > γcrit, suggesting a strong influence of geometric factors, such as contact angle θ, as discussed above. It is noteworthy that the maximum indentation occurs at γ = γcrit. As the size of the particle decreases, the magnitudes of Fγ = Fel and umax all dramatically decrease. Also for smaller particles, the effect of E variation on umax is dramatically reduced (Fig. 9A, top), while the effect of δhypo variation is amplified (Fig. 9B, top). Normalizing particle indentation umax with particle radius R shows that the largest relative indentation umax/R occurs for an intermediate particle size (e.g., R = 0.25 μm) at E = 10 kPa and δhypo = 0.1 μm (Fig. 9A, bottom). Interestingly, a much larger effect of decreased particle size on change in umax/R is caused by decrease of hypophase thickness. Although the smallest particle causes much smaller indentation, the decrease of δhypo causes the largest relative imbedding of smaller compared with larger particles (Fig. 9B, bottom). In contrast, the smallest particles tested (R = 0.125 μm) with thicker δhypo (= 0.20 μm) does not show any indentation when γ > 45 dyn/cm.
Fig. 9.
The quantitative affects of the elastic modulus of alveolar tissue, thickness of hypophase, and the particle size on maximum indentation, umax, as a function of surface tension γ. A: effect of Young's elastic modulus and particle size. B: effect of the thickness of the hypophase and particle size. Top: absolute value of displacements. Bottom: displacements normalized to the particle size. All calculations are performed for Fγ at γcrit.
Table 1.
The effect of particle size, Young's elastic modulus, and the thickness of the hypophase on the equilibrium indentation force and displacement at γcrit = 26.27 dyn/cm2
| R, μm | E, kPa | δhypo, μm | umax, μm | F, nN | umax/R | F/E, μm2 | F/(EumaxR) | γcrit, dyn/cm |
|---|---|---|---|---|---|---|---|---|
| 0.125 | 10.0 | 0.10 | 0.1291 | 0.2915 | 1.0330 | 0.0292 | 1.8061 | 27.260 |
| 0.125 | 20.0 | 0.10 | 0.1154 | 0.4930 | 0.9235 | 0.0247 | 1.7084 | 27.260 |
| 0.125 | 30.0 | 0.10 | 0.1053 | 0.6444 | 0.8425 | 0.0215 | 1.6318 | 27.260 |
| 0.250 | 10.0 | 0.10 | 0.3044 | 1.4734 | 1.2174 | 0.1473 | 1.9365 | 27.260 |
| 0.250 | 20.0 | 0.10 | 0.2546 | 2.2834 | 1.0184 | 0.1142 | 1.7938 | 27.260 |
| 0.250 | 30.0 | 0.10 | 0.2229 | 2.8063 | 0.8916 | 0.0935 | 1.6787 | 27.260 |
| 0.500 | 10.0 | 0.10 | 0.5767 | 5.4672 | 1.1534 | 0.5467 | 1.8960 | 27.260 |
| 0.500 | 20.0 | 0.10 | 0.4533 | 7.6742 | 0.9067 | 0.3837 | 1.6928 | 27.260 |
| 0.500 | 30.0 | 0.10 | 0.3831 | 8.9437 | 0.7663 | 0.2981 | 1.5562 | 27.260 |
| 0.125 | 20.0 | 0.05 | 0.1501 | 0.7229 | 1.2008 | 0.0361 | 1.9264 | 27.260 |
| 0.125 | 20.0 | 0.10 | 0.1154 | 0.4930 | 0.9235 | 0.0247 | 1.7084 | 27.260 |
| 0.125 | 20.0 | 0.20 | 0.0420 | 0.1080 | 0.3356 | 0.0054 | 1.0286 | 27.260 |
| 0.250 | 20.0 | 0.05 | 0.2822 | 2.6519 | 1.1289 | 0.1326 | 1.8794 | 27.260 |
| 0.250 | 20.0 | 0.10 | 0.2546 | 2.2834 | 1.0184 | 0.1142 | 1.7938 | 27.260 |
| 0.250 | 20.0 | 0.20 | 0.1983 | 1.5704 | 0.7934 | 0.0785 | 1.5839 | 27.260 |
| 0.500 | 20.0 | 0.05 | 0.4740 | 8.2041 | 0.9480 | 0.4102 | 1.7308 | 27.260 |
| 0.500 | 20.0 | 0.10 | 0.4533 | 7.6742 | 0.9067 | 0.3837 | 1.6928 | 27.260 |
| 0.500 | 20.0 | 0.20 | 0.4113 | 6.6326 | 0.8227 | 0.3316 | 1.6126 | 27.260 |
Stress-Strain Fields
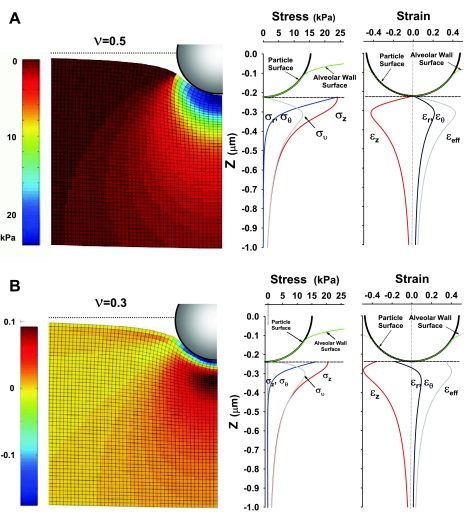
When a particle is indented into alveolar tissue, the tissue deforms, and consequently, a unique stress-strain field is created in the vicinity of the particle (Fig. 10). Generally, both the stress and strain diminish sharply with the distance from the particle surface. The axisymmetric formulation of the problem in cylindrical coordinates provides that σr ≈ σθ and εr ≈ εθ, where σr and σθ are radial and hoop stresses, and εr and εθ are radial and hoop strains, respectively; subscript θ denotes the hoop direction, which is different from contact angle θ. The maximum compressive stress can reach up to 25 kPa for R = 0.25 μm, and the maximum strain ∼0.4 for v = 0.5 or 0.3. The axial stress in the z-direction, σz, and stresses σr (≈σθ) rapidly decay from the maximum values at the particle-tissue interface, while the effective (von Mises) stress, σv, peaks at about 0.1 μm from the particle-tissue interface and then also diminishes sharply with the distance from the particle surface. It is interesting to notice how change in v from 0.5 to 0.3 affects the stress and strain distributions in the vicinity of the indentation surface of the particle. For v = 0.5, both σz and σr (≈σθ) start from the same value (∼25 kPa), whereas for v = 0.3, σz and σr (≈σθ) start from the different values. Similarly, for v = 0.5, both strains εz and εr (≈εθ) start from zero at the particle-tissue interface, whereas for v = 0.3, εz and εr (≈εθ) start from negative values denoting contraction of the particle-tissue interface. Especially, εr (≈εθ) switches the sign (from negative to positive, i.e., from compression to extension) with the distance from the particle surface in case of v = 0.3. These large changes in the distributions of stresses and strains result in minimal change in Fel = Fγ and umax. The stress field for v = 0.5 shows spherical iso-σz distribution, which decay fast with distance from the particle-tissue interface (Fig. 10A, left). The strain field for v = 0.3 shows a complex pattern of strain sign change; for example, εr (≈ εθ) starts from a minimum negative value of approximately −0.2 at the particle-tissue interface, but soon becomes positive value of ∼0.1 at the distance of about 0.1 μm (∼0.4 R) from the surface (Fig. 10B, left).
Fig. 10.
Analytically calculated tissue deformation, stress, and strain fields under an indented particle of 0.5 μm in diameter, and for Fγ at γcrit, and E = 20 kPa. A: deformed shape and stress and strain distributions for ν = 0.5. Stress field of σz (left) (blue = higher compressive stress); Stress components σz, σr, σθ, and effective stress, σv, along vertical axis of symmetry (middle); Strain components and effective strain along a vertical axis of symmetry (right). B: deformation and strain and stress distributions for ν = 0.3. Strain field of εr = εθ (left) (blue = higher compressive strain); Stress components and effective stress along a vertical axis of symmetry (middle). Strain components and effective strain along a vertical axis of symmetry (right).
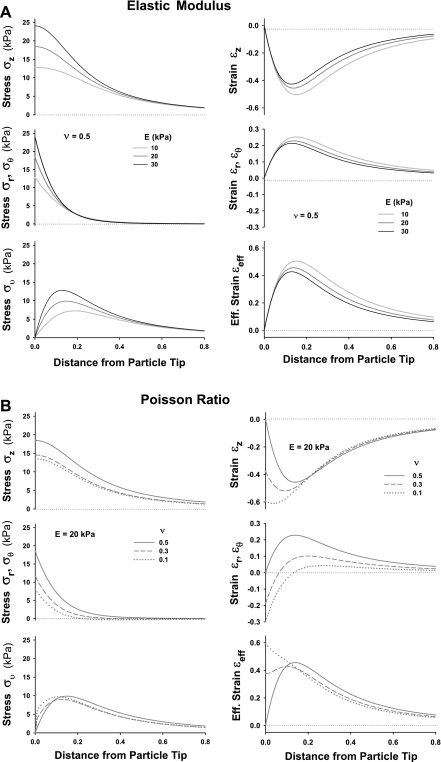
The effects of E and v on stress and strain distributions in the tissue along the z-axis are shown in Fig. 11, A and B, respectively. An increase in E from 10 to 30 kPa (Fig. 11A) results in an appreciable increase in the magnitude of σz, σr ≈ σθ, and σv right beneath the particle (z < 0.4 μm) and decrease in εz, εr ≈ εθ, and εeff, especially for z >0.1 μm. The increase in stress with an increase in E appears to be much larger than the decrease in strains due to nonlinear nature of the Hertz problem. A decrease of v from 0.5 to 0.1 (Fig. 11B) changes not only the magnitude, but also the shape and the spatial distribution of the stress and strain. The most significant deviation caused by a change in v is seen in the shape of the distributions of σv and εeff, showing the strong effects of Poisson ratio on tissue shear deformation.
Fig. 11.
Effects of elastic modulus and Poisson's ratio: the stress and strain distribution along vertical axis of symmetry for an indented particle of 0.5 μm in diameter. The indentation force corresponds to Fγ at γcrit. A: effect of Young's elastic modulus, E, for Poisson ratio v = 0.5 on axial stress, σz, a radial/hoop stress, σr, and von Mises stress, σθ (left); and on axial strain, εz, a radial/hoop strain, εr ≈ εθ, and effective strain, εeff (right). B: effect of Poisson ratio, v, for E = 20 kPa on stresses σz, σr, ≈ σθ, and σv (left); and on strains εz, εr ≈ εθ and εeff (right).
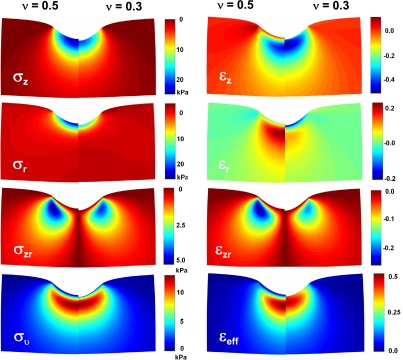
The difference in Poisson ratio (e.g., v = 0.5 vs. 0.3) results in quite different distributions in stress and strain fields (Fig. 12, left and right, respectively) not only along the z-axis (Fig. 11B). These distributions are contrasted for each component of stress and strain: on the left half for v = 0.5 and on the right half for v = 0.3. In some cases, both stress and strain change the sign in areas close to particle-tissue interface when v changes from 0.5 to 0.3, while the change in umax is insignificant. These small differences in umax are hardly visible as a small shift of the particle-tissue interface at the vertical axis of symmetry (Fig. 12). In contrast, the magnitude and spatial stress and strain distributions are significantly affected by the same change in v. These altered stress and strain distributions could be important for mechanotransduction and biological response of the epithelial cells to indentation of the particles in the alveolar tissue.
Fig. 12.
Stress and strain fields of the components of the stress and strain tensors under an indented particle (0.5 μm in diameter) into alveolar tissue for Fγ at γcrit, E = 20 kPa, and Poisson ratios of v = 0.5 (left halves) and v = 0.3 (right halves).
DISCUSSION
The principal findings of this study are as follows: 1) an inhaled particle deposited on the alveolar surface can be forced toward the epithelium by surface tension forces, Fγ, to produce an indentation on alveolar septal wall. The degree of indentation can be primarily estimated from a force balance between Fγ and tissue elastic resistance to deformation, Fel. 2) The nonlinear behavior of Fγ with respect to the degree of indentation is determined by geometric factors, such as the size and shape of a particle, the thickness of hypophase, and the interfacial tensions acting on the three-phase line on the surface of the particle. 3) The magnitude of Fγ is strongly dependent on a relationship between surface tension γ and contact angle θ, represented graphically as Zisman's plot (10, 68), with Fγ usually having the maximum at γcrit. 4) The degree of indentation is related inversely (nonlinearly) to the tissue elastic modulus, E, while it modestly (also nonlinearly) depends on the tissue Poisson ratio, v. 5) Stress and strain fields generated beneath the indented particle show complex patterns. These patterns are self-similar for different E, but they are quite different for different Poisson ratios, v. On the other hand, the magnitudes of the stresses and strains strongly depend on E, but only moderately on v.
Surface Tension Force
The surface tension force acting on a particle, Fγ, pushes the particle toward the alveolar epithelial cell surface. This force can be calculated as a function of the surface tension, γ, contact (or wetting) angle, θ, and the length of the three-phase interface line. The contact (or wetting) angle θ, can be obtained from a balance of interfacial tensions, γPL, γPA, and γAL ≈ γ [see Fig. 2 and Eq. 1]. Because the surface energy of a given particle is fixed as a result of the constant excess energy at the surface of a particle compared with the bulk, the associated interfacial tensions, γPL and γPA, are also constant. Therefore, for a given particle, θ essentially depends solely on the strength of surface tension, γ. The relationship between γ and cos(θ) is characterized by two distinctly different linear regimes (Fig. 3): 1) for γ < γcrit, where θ = 0, denoting complete wetting; and 2) for γ > γcrit, where θ > 0, denoting partial wetting. It should be noted that this relationship holds for any particle; only the value of γcrit (= γPA − γPL) is different, and this value depends on the particle surface energy (19). Gehr's group [e.g., Schürch et al. (51) and Gehr et al. (12)] studied latex (polystyrene) and PMMA particles. From their data, we determined γcrit = 27.26, and 22.25 dyn/cm for latex and PMMA particles, respectively. The fact that these two different particles have the different values of γcrit is consistent with the observation that polystyrene particles are less hydrophilic compared with PMMA (51). This is because the surface free energy of polystyrene is about 33 erg/cm2 while the surface free energy of PMMA is ∼40 erg/cm2 (19).
The air-liquid interfacial tension, γ, varies during breathing, while particle interfacial tensions γPL and γPA are constant throughout a breathing cycle because they solely depend on the surface characteristics of the particles. When the alveoli expand during inhalation, the alveolar walls stretch and the alveolar surface area increases. The increase in the area increases surface tension, γ, due to a reduction in concentration of lung surfactant at the air-liquid interface. Under normal breathing conditions of healthy subjects, surface tension γ is typically well below 30 dyn/cm2, and at the end of the exhalation, the alveolar surface tension can even reach values close to zero (50, 51). This indicates that the physiologically relevant range of values of γ is likely below γcrit for the most of common particles; thus, an inhaled particle most likely interacts with alveolar liquid phase in a perfect wetting condition. For instance, relatively hydrophilic inhaled particles, such as dust particles, pollen, spores, or even hydrophobic9 particles, such as Teflon particles (15), may be able to submerge below the air-liquid surface during the exhalation because the surface tension can reach values close to zero. Thus, even the particles that usually float on the water surface can be submerged into lung lining layers at low surface tension and indented into the alveolar epithelial cells. On the other hand, the surface tension, γ, in some pathological cases with dysfunctional surfactant may rise up to 50 dyn/cm2 (51). In this case, a particle would not be completely wetted; hence, the contact angle θ needs to be obtained using the γ vs. cos(θ) relationship described in Zisman's plot (Fig. 3) to obtain the equilibrium force Fγ and umax.
The position of the three-phase line (on the surface of the sphere, Fig. 5), defined by angle ϕ, is determined by Eq. 3; it depends on thickness of hypophase δhypo, capillary rise zo, maximum depth of particle indentation umax, and particle size. The effect of the reduction of δhypo on an increase in umax is demonstrated in Figs. 6 and 8. This behavior cannot be explained straightforwardly because the capillary rise, zo, depends intrinsically on angles θ and ϕ. Thus the magnitude of zo is directly associated with the position and length of the three-phase line (Fig. 5). Because zo is essential for assessing Fγ and umax, and, in turn, umax is also intrinsically linked to the position of the three-phase line, the indentation umax is modulated, in complex fashion, by both δhypo and the particle size.
Particle Indentation and Tissue Resistance to Deformation
For a particle larger than δhypo, the surface tension force, Fγ, pushes the particle into the alveolar wall, which results in deforming the tissue. Because Fγ depends on γ and the magnitude of umax, and conversely umax depends on Fel = Fγ and alveolar wall tissue elasticity, the interplay between these factors defines the Fγ-umax-γ relationship. The solution of this implicit relationship is found iteratively as described in Surface Tension Acting on a Particle. The overall results showed that the maximum values of Fγ and umax are usually observed in our calculations at γcrit (Fig. 9).
The particle size has diverse effects on the particle indentation. The plot of the raw value of indentation, umax, vs. particle size clearly shows that smaller particles generally make smaller indentations due to weaker indentation force (Fig. 9, top). This tendency can be seen by varying the thickness of the hypophase and elasticity, E, of alveolar wall tissue (Fig. 9). However, the plot of indentation normalized by particle size, umax/R (Fig. 9, bottom), shows that umax/R depends very little on particle size and points out more complex behavior. For example, within the physiological range of E, umax/R is the largest for a particle of ∼0.5 μm in diameter. However, in thin hypophase, umax/R displays the largest relative imbedding for the smallest particle (but larger than δhypo). This is somewhat consistent with the experimental observation by Schürch and colleagues (18), who showed that smaller particles submerge more rapidly in the liquid layer than larger particles. The fact that smaller particles make indentations more rapidly may partially explain why smaller (submicron/ultrafine) particles are taken up by the underlining epithelial cells at a greater rate than larger (>1 μm) particles (17). As soon as particles land on the alveolar surface, they encounter both epithelial cells and alveolar macrophages. Thus, these two types of cells compete for engulfing the particles. The fact that surface force enhances particle indentation into epithelial cells may potentially diminish (or change) the rate of macrophage-mediated clearance of smaller particles. In addition, for small particles (R = 0.125 μm), there is a significant dependency of umax/R on thickness of the hypophase (Fig. 9B, bottom). This suggests that the thickness of the hypophase plays an important role in indentation of nanoparticles into underling tissues (42). Also, the reduction in thickness of hypophase may contribute to slowing macrophage-mediated clearance on nanoparticles.
The force normalized by product EumaxR, showed much smaller variation with respect to large changes in Young's elastic modulus and particle size (Table 1). In general, increase in Young's elastic modulus only modestly decreased the normalized force. Similarly, the large change in particle size changed the normalized force by at most 10%. The effect of a four-fold change in the thickness of the hypophase was also significantly reduced, having the normalized force in the range between 1 and 2.
Model Assumptions and Simplifications
In this study, we used the semianalytic approach to elucidate basic physics involved in particle indentation when an inhaled particle deposited on the alveolar septal surface interacts with alveolar tissue. As the process is complex because of the interplay of many different variables, we had to adopt several assumptions and simplifications to keep the analysis simple and tractable. A point-by-point critical evaluation of the assumptions is given below.
Particle shape.
We studied spherical, smoothly surfaced, and well-characterized (e.g., latex or PMMA) particles. The geometric simplification and the knowledge of particle surface characteristics were necessary to employ our semianalytic approach, which focuses on elucidating basic physics operating at the three-phase interfacial lines, as well as tissue resistive forces in the vicinity of particle-tissue interface. Although real ambient particles are certainly more complex in their shapes, our approach—surface microscopic geometry and the surface characteristics—albeit highly simplified, can serve as the basis for further detailed analysis.
Capillary rise.
On the basis of the boundary condition at the three-phase line and the angle βo, the capillary rise zo can be assessed reasonably accurately by using an approximate formula (Eq. 12) developed by Rapacchietta and Neumann (47). In particular, it is known that this solution is sufficiently accurate for the small particles (<1 μm), which, indeed, are of main interest here. James (29) analyzed multiple approaches with increasing complexity for determining capillary rise, zo. He claims that the prediction of yo = zo/c (from Eq. 12) is accurate when the parameter c/ro > 10. The smallest c/ro considered here is larger than 200 for the size of particles and the range of physiological surface tensions. Taken together, this paper was primarily concerned with sufficiently accurate calculations of zo, and to a lesser degree, regional profiles of the interface proximate to a submicron particle.
If of interest for further mathematical clarity, the profile of the entire interface may be obtained from the solution of the Laplace's equation, in which the boundary conditions are simultaneously satisfied at the three-phase line and in the far field. Alternatively, the profile of the entire interface can be calculated using an approximate equation based on an additive composite expansion of the outer and inner solutions (60). In the latter approach, the approximate analytical solution is uniformly valid as εB → 0 and involves Bessel function Ko(rc)≡Ko(εBr/ro). The limit εB → 0 signifies that the effect of gravity is small. However, this solution is of little practical interest for submicron particles because in real geometry of the lungs, the alveolar walls are of finite dimensions, for which the boundary conditions in the far field may not be important, except for the mathematical clarity.
Quasi-static analysis.
The surface tension γ and the thickness of hypophase, δhypo, are known to vary during the breathing cycle. For simplicity, we performed a quasi-static analysis, in which we assessed the degree of particle indentation from a force balance between the instantaneous values of Fγ and Fel. Using this approach, we found that the indentation during breathing cycle can also be evaluated by the above quasi-static approach using the instantaneous values for γ, δhypo, and alveolar wall material characteristics as the model parameters. In these stepwise calculations, time is considered only as a parameter. This analysis takes partially into account the hysteresis of the surface tension during expansion and contraction of the alveolar surface (64). However, the truly dynamic nature of the process, such as viscoplastic dissipation effects in alveolar wall tissues (11, 40) and the dynamic changes in γ and δhypo during breathing cycle cannot be fully accounted by the quasi-static analysis. Nevertheless, our quasi-static analysis captures the important basic features of the particle indentation process, consistent with experimental data of Gehr and colleagues (12, 16, 18, 51, 52), permitting quick and effective analysis of the effect of the variation of the numerous model parameters.
Hertzian contact problem.
To describe the mechanics of particle indentation into the alveolar wall, we used the simplest solution of the contact problem—the Hertzian contact (21, 34). This simplification was necessary because the analysis described in this study involves variation of a large number of parameters with a complex geometry at the particle-tissue contact and, therefore, a large number of simulations. The Hertzian solution is accurate for small indentations, say, umax/R < 0.2, where the pressure distribution in the tissue beneath a particle remains approximately spherical. For larger indentations, on the other hand, this analysis may yield an error that manifests as a deviation of indentation pressure distribution shape from the spherical one. Note that the error also depends on the Poisson ratio. We estimate that the error would be of the order of 30% when umax/R ≈ 1. In that case, a numerical approach, such as a finite element analysis, would be appropriate to obtain more accurate solution.
Tissue stress-strain field.
Tissue deformation and stress and strain fields in the tissue induced by the particle indentation were calculated by convolution integrals of the contact pressure distributions using the Boussinesq analytical solution (2, 56). These integrals are obtained numerically. Since the Boussinesq solution is valid and accurate for a semi-infinite solid, our results (Figs. 10–12) are reasonably accurate in the proximity of the particle-tissue interface. We also limited our solution to the frictionless contact. The advantage of using this semianalytical approach is the simple and quick way to analyze large number of possible scenarios and quantitative assessment of the effect of the variation of a large number of the model parameters on the system behavior.
Despite many restricting assumptions and simplifications, the present approach conveniently provided a fast and effective tool for elucidating effects of model parameter variation on the degree of particle indentation (sensitivity analysis). This provides a valuable contribution to unlocking the principal mechanisms driving the indentation of particles into the alveolar wall by the surface tension forces.
Physiological Implication and Future Direction
Potential physiological consequences of our findings are as follows. First, the particle indentation may trigger mechanotransduction pathways, either by directly deforming epithelial cells (3, 5, 6, 8, 20, 26–28, 35, 44, 45, 49, 55, 61, 62), physically insulting cell surface molecules (e.g., 4, 14, 54, 57, 65), and by remodeling of the intracellular cytoskeleton. The activation of these pathways may alter cellular biochemistry (1, 7, 23, 36, 41) and thereby the normal cell function. For instance, we have shown previously that physical contact between particles and cell surface adhesion molecules under cyclic (tidal) motion results in a profound secretion of proinflammatory cytokine (39, 59). Second, particle indentation increases the contact area between the particle and cell surface. This may trigger biochemical pathways directly and enhance the pathogenic response to toxic and allergenic particles, as well as particle internalization (17, 53, 66). Enhanced particle uptake by epithelial cells may indirectly alter the rate of particle clearance from the lung periphery, as discussed in Particle Indentation and Tissue Resistance to Deformation. Third, particle indentation may trigger signals, which activate afferent nerve fibers. Innervations of alveolar septa with sensory neurons have been visualized by us (31) and others (9, 25, 63). Because the afferent fibers can locate very close to the alveolar surface, particle-induced unphysiological stresses and strains in the proximity of afferents may mechanically stimulate afferent fibers and trigger neuronal responses. Similar mechanosensing phenomena have been recently studied in many biological models (67).
Regarding further model refinements, there are many additional details and factors, such as realistic shape and surface characteristics of (ambient) particles, viscoelastic nature of alveolar tissue characteristics, and dynamic changes of the surface tension imposed by breathing, all of which can be considered for a more realistic model analysis. Among all model parameters, the consideration of realistic structure and composition of the alveolar wall might be the most important. In the current model, we treated the alveolar wall as a whole, using a range of effective Young's modulus and Poisson's ratio measured by Fukaya et al. (11). However, the alveolar walls, in reality, are made of several different components with different mechanical properties, such as soft epithelial, endothelial, and other cellular components, relatively stiff collagen-based basement membrane, as well as other residual connective tissues. Furthermore, the alveolar walls are typically formed in a thin multiple-layered structure (i.e., epithelial cells, basement membrane, interstitium, endothelial cells). In addition, this detailed overall analysis could also include the lateral alveolar boundaries [e.g., other particles (32), alveolar shape, and airway bifurcations] and the effect of gravity, which, for particles significantly larger than 1 μm, becomes progressively important. Although the inclusion of these additional factors in the model would certainly make the analysis more complicated, the consideration of those factors might be necessary when one aims to more precisely model the specific effects of the particle indention on biological consequences.
Summary
We have developed a mathematical model to study mechanisms of the particle indentation into alveolar tissue. The analysis reveals that these mechanisms are centered on a mechanical balance between surface tension forces and tissue elastic forces; the former push the particle against the alveolar epithelial surface; the latter resist alveolar tissue deformation. The model describes in detail how various factors are involved in the indentation process. The quantitative model predictions can be used for understanding of mechanisms associated with mechanotransducting pathways triggered by indentation of the alveolar septa. For simplicity and mathematical transparency, several idealizations were employed in the model. Nevertheless, the model is capable of capturing principal features of the particle indentation process and can be used as the basis for the further detailed analysis.
GRANTS
This work was supported by Grants National Institutes of Health R01 AR048776 (to S. M. Mijailovich), National Heart, Lung, and Blood Institute HL054885 (to A. Tsuda), HL070542 (to A. Tsuda), HL074022 (to A. Tsuda), and Mijailovich Family Foundation. This work was also supported by National Aeronautics and Space Administration Grant NNJ06HE06A and State of Texas, Emerging Technology Fund and the Ministry of Science and Technological Development of Serbia (Grant OI-144028) (to M. Kojic).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We gratefully acknowledge Drs. P. Gehr and M. Geiser for useful discussions, Dr. D. Stamenović for critical review, A. Marinkovic for graphical design, and Dr. A. Perin for proofreading of this article.
APPENDIX
Calculation of the Displacement Vector and the Components of the Stress and Strain Tensors
The indentation of a spherical particle in a compliant solid causes symmetrical deformation with respect to the axis of revolution, i.e., the z-axis. In this case, all stress components are independent of the circumferential angle θ, all derivatives with respect to θ disappear, and the displacements in θ direction are equal to zero. This symmetry significantly simplifies the calculations of the stress, strain, and displacement fields. Assuming that the alveolar tissue is linearly elastic further simplifies the problem; thus the stress, strain, and displacement fields can be conveniently calculated using Boussinesq analytical solution (2) for a concentrated force acting on the boundary of a semi-infinite solid. According to the principle of superposition, the Boussinesq analytical solution can be applied on distributed loads by using infinitesimal force acting on an infinitesimally small area and integrating it over the whole area of the contact between the particle and alveolar tissue. Thus, from known Boussinesq formulas, for displacements and stresses, a convolution integral can be constructed (Fig. 13), and the components of the stress tensor can be determined at any point of the deformed semi-solid.
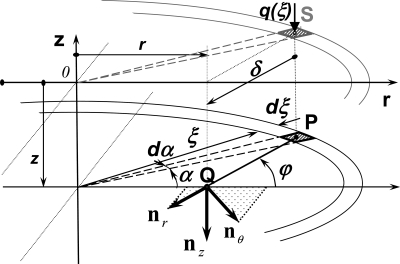
Fig. 13.
Construction of the convolution integral for displacements, stress and strains from analytical solution of concentrated force on boundary of a semi-infinite elastic solid integrated over the distributed contact load q(r) (Fig. 5). The Boussinesq solution for displacements, stress and strains for an infinitesimal force applied to an infinitesimal area, i.e., contact pressure, are integrated over the area of the contact between particle and elastic substrate. Integration domains are for radial direction (along ξ) from 0 to a, and for circumferential direction a is from 0 to 2π. For q(ξ) acting at point S on the surface, the Boussinesq solution provides functions for displacements, stresses, and strains at the point Q at the depth z, at radial distance δ rotated by angle ϕ relative to the zr plane from point P. The point P is a projection of point S to the plane on depth z, i.e., to the plane of integration. The directions of components of the Boussinesq displacement vector and stress and strain tensors are denoted as init vectors nz, nr, and nθ. The calculation of radial and hoop components of displacements, stresses, and strains takes into account the Boussinesq solutions for all values of α.
Calculation of the stress field in semi-infinite solid.
The stress components at a point Q with coordinates β and z of a semi-infinite solid per unit of normal force acting on the plane boundary surface in point S (see Fig. 13) are (2, 56)
| (A1a) |
| (A1b) |
| (A1c) |
| (A1d) |
| (A1e) |
Here, δ is the distance between the infinitesimal force dP = q(ξ) ξdξdα at the position S (ξ,α) on the surface of the semi-infinite solid (i.e., at z = 0) and the point Q (r, α = 0, z) at which the stress σz is calculated (Fig. A1):
| (A2) |
The stress component σz at arbitrary point Q of the semi-infinite solid with coordinates r and z, produced by traction distribution (Eqs. 15 and 20) over the entire circular area of contact of radius a is calculated from the following convolution integral:
| (A3) |
The calculation of stresses σr and σθ is more complicated because the Boussinesq stresses σ̂nrBouss and σ̂nθBouss act in the plane of integration; thus, both components contribute simultaneously for both σr and σθ (Fig. 13). Therefore, the convolution integrals of σr and σθ at a point Q(r, α = 0, z) requires recalculation of the Boussinesq stresses (per unit force) σ̂nrBouss, σ̂n0Bouss, and σ̂nrnθBouss using Mohr circle (56) to obtain the component of the stresses, σ̂rBouss* and σ̂θBouss*, in directions r and θ at in the horizontal plane at height z. The Boussinesq stresses in r and θ directions from the Mohr circle are:
| (A4a) |
| (A4b) |
where ϕ = atan[ξsinα/(ξcosα − r)] is the angle between plane rz of the Boussinesq stresses and the plane in which the stress is calculated at point Q(r, α = 0, z). Becauseτ̂rθBouss = 0, it is omitted in Eqs. A4a, b. To calculate stress produced by the entire contact pressure distributed over the contact circular area with the radius a, we must integrate Eqs. A4a, b. It is interesting that Eqs. A4a and A4b represent vectors shifted by π/2 (56). Thus, after integration over the full circle, the convolution integral gives the resulting values for the stresses in r and θ directions, at any radius r and depth z:
| (A5) |
The only nonzero shear stress, τrz, is obtained from the integral of the projection of the vector τ̂nr,nzBouss, which rotates with α (Fig. 13), is
| (A6) |
Calculation of the strain field in semi-infinite solid.
Using the stress-strain relationship of a linear elastic solid in cylindrical coordinates (56), we can calculate the strains from known stresses at the position (r,z) as
| (A7a) |
| (A7b) |
| (A7c) |
| (A7d) |
| (A7e) |
Calculation of the displacement field in semi-infinite solid.
Since the indentation of a spherical particle into a compliant solid causes symmetrical deformation with respect to the z-axis, the displacements in θ direction, as well as the derivatives with respect to θ are equal to zero, and the strains are related to the displacements by simple relationships (56). Because we are interested in the simplest way to determine nonzero displacements in z and r directions, denoted as u and w, respectively, we only need the following Boussinesq strains per unit force:
| (A8a) |
| (A8b) |
Substituting appropriate Eqs. A1 into Eqs. A7b, c provides the analytical formulae for ε̂nθBouss and ε̂nzBouss from which ŵ can be obtained directly as:
| (A9a) |
and û after integration as (56):
| (A9b) |
The following convolution integrals provide the displacements u and w at any radius r and depth z:
| (A10a) |
| (A10b) |
Footnotes
1For submicron particles, buoyancy force and particle weight are more than two orders of magnitude smaller than either Fel or Fγ. For example, the weight for typical 1-μm particle with density of 2-3 g/cm3 is about 200 times smaller than the smallest equilibrium forces Fel = Fγ (calculated for the highest elastic modulus of alveolar wall tissue and the largest thickness of hypophase). The effect of gravity decreases with third power with respect to the particle diameter and for diameters <1 μm, this effect is negligible, i.e., spatial orientation of alveolar surface with respect to gravity vector affects very little the degree of particle indentation. However, the asymptotic solution for capillary rise (Eq. 12) contains a term that weakly depends on gravity. Therefore, inclusion of gravity in these calculations is necessary, but overall impact of this term on the equilibrium indentation of the particle is small.
2The effect of line tension on the contact angle, θ, can be large for the small droplets in the flat surface especially for θ ∼ 0 (37, 38). However, for a small sphere in a liquid layer, as we considered here, this effect is negligible, because the critical three phase contact radius, rc, is of the order or smaller than an average distance between the solid and liquid molecules, δmol. The rc, is calculated from modified Young equation cosθi = cos(θ) − τ/(γro), where θi is contact angle modulated by the line tension, τ, and ro is the radius of the three-phase line. For a small sphere partially submerged in liquid layer, the term τ/(γro) is approximately equal to −sinθi, yielding to rc/δ = |sin(θ)/[cos(θ) − cos(θ − π)]|. For θ < 65°, the critical radius rc/δmol < 1, and it is below the lower limit of experimental resolution in contact angle measurements (37, 38) and, therefore, the effect of line tension on ω is negligible.
3Strictly speaking, the density in Eq. 4 should be ρ = ρL − ρA, where ρL and ρA are densities of liquid and air, respectively. However, because ρL >> ρA, we assume ρ ≈ ρL.
4Multiplying Eq. 7 by x provides the term 2xy, which is of much smaller magnitude than sinβ for small particles. For y′ = tanβ ≪ 1, the second term in Eq. 7 can be approximated as y′/(1 + y′2)1/2 ≈ tanβ ≈ sinβ. Because |xy| ≪ |sinβ|, the third term, which includes the gravity effect can be neglected and the first term is of approximately equal magnitude as the second term. Because y rapidly decays with x, for large x, the product |xy| → 0.
5For example, the predicted values of x and y from Eqs. 10 and 11 as a function of β are within 2% for x = 2.0 (i.e., r > 2 mm) at β = 0.5° compared with the tabulated values of Huh and Scriven (24). For larger values of r > 2 mm, the error increases and the solution diverges for large values of x.
6The method of solving the boundary-value problem, which involves transforming it into an initial-value problem is called the shooting method, because this technique “shoots” from a point where one of the initial values is a guess to another point where the effect of that guess may be judged owing to the known conditions at the final point of the calculations.
7The limit of εB → 0 is equivalent to one in which there is no effect of the hydrostatic pressure in the liquid. In other words, the pressure difference across the interface is zero (33), which is valid for submicron particles.
8Although it is widely accepted that Poisson's ratios of cells and tissues are close to 0.5 [e.g., Fukaya et al. (11), Ofek et al. (43)], the local Poisson's ratio can be much lower as observed in cartilage (22, 30, 58) due to local compressibility of the cell cytoskeleton immersed in cytosol.
9The particles with low γcrit are considered as hydrophobic. These particles have large θ at air-water interface if the surfactant is not present.
REFERENCES
- 1. Bohmer RM, Scharf E, Assoian RK. Cytoskeletal integrity is required throughout the mitogen stimulation phase of the cell cycle and mediates the anchorage-dependent expression of cyclin d1. Mol Biol Cell 7: 101–111, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boussinesq J. Applications des potentiels a l'tude de l'equilibre et du mouvem des solides elastiques. Paris, France: Gauthier-Villars, 1885 [Google Scholar]
- 3. Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science 276: 1425–1428, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Clark CB, McKnight NL, Frangos JA. Strain and strain rate activation of g proteins in human endothelial cells. Biochem Biophys Res Commun 299: 258–262, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Dike LE, Chen CS, Mrksich M, Tien J, Whitesides GM, Ingber DE. Geometric control of switching between growth, apoptosis, and differentiation during angiogenesis using micropatterned substrates. In Vitro Cell Dev Biol Anim 35: 441–448, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature 463: 485–492, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flusberg DA, Numaguchi Y, Ingber DE. Cooperative control of akt phosphorylation, bcl-2 expression, and apoptosis by cytoskeletal microfilaments and microtubules in capillary endothelial cells. Mol Biol Cell 12: 3087–3094, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Folkman J, Moscona A. Role of cell shape in growth control. Nature 273: 345–349, 1978 [DOI] [PubMed] [Google Scholar]
- 9. Fox B, Bull TB, Guz A. Innervation of alveolar walls in the human lung: An electron microscopic study. J Anat 131: 683–692, 1980 [PMC free article] [PubMed] [Google Scholar]
- 10. Fox HW, Zisman WA. The spreading of liquids on low energy surfaces, I. Polytetrafluoroethylene. J Coll Sci 5: 514–531, 1950 [Google Scholar]
- 11. Fukaya H, Martin CJ, Young AC, Katsura S. Mechanial properties of alveolar walls. J Appl Physiol 25: 689–695, 1968 [DOI] [PubMed] [Google Scholar]
- 12. Gehr P, Geiser M, Im Hof V, Schurch S, Waber U, Baumann M. Surfactant and inhaled particles in the conducting airways: Structural, stereological, and biophysical aspects. Microsc Res Tech 26: 423–436, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Gehr P, Hyder J. Particle-Lung Interactions. New York: Marcel Dekker, 2000 [Google Scholar]
- 14. Geiger B, Bershadsky A. Exploring the neighborhood: Adhesion-coupled cell mechanosensors. Cell 110: 139–142, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Geiser M, Gerber P, Maye I, Im Hof V, Gehr P. Retention of teflon particles in hamster lungs: A stereological study. J Aerosol Med 13: 43–55, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Geiser M, Im Hof V, Gehr P, Schürch S. Structure and interafactial aspects of particle retention In Particle lung interactions, edited by Gehr P, Heyder J. New York: Marcel Dekker, 2000, vol. 143, chapt. 6, p. 291–321 [Google Scholar]
- 17. Geiser M, Rothen-Rutishauser B, Kapp N, Schurch S, Kreyling W, Schulz H, Semmler M, Im Hof V, Heyder J, Gehr P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect 113: 1555–1560, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geiser M, Schurch S, Gehr P. Influence of surface chemistry and topography of particles on their immersion into the lung's surface-lining layer. J Appl Physiol 94: 1793–1801, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Gerson DF. An empirical equation-of-state for solid-fluid interracial free energies. Colloid Polymer Sci 260: 539–544, 1982 [Google Scholar]
- 20. Gieni RS, Hendzel MJ. Mechanotransduction from the ecm to the genome: Are the pieces now in place? J Cell Biochem 104: 1964–1987, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Hertz H. [Über die berührung fester elastischer körper]. J MAth (Crelle's J) 92: 156–171, 1881 [Google Scholar]
- 22. Hu K, Radhakrishnan P, Patel RV, Mao JJ. Regional structural and viscoelastic properties of fibrocartilage upon dynamic nanoindentation of the articular condyle. J Struct Biol 136: 46–52, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Huang S, Chen CS, Ingber DE. Control of cyclin d1, p27(kip1), and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol Biol Cell 9: 3179–3193, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huh C, Scriven LE. Shapes of axisymmetric fluid interfaces of unbound extent. J Colloid Interface Sci 30: 323–337, 1969 [Google Scholar]
- 25. Hung KS, Hertweck MS, Hardy JD, Loosli CG. Innervation of pulmonary alveoli of the mouse lung: An electron microscopic study. Am J Anat 135: 477–495, 1972 [DOI] [PubMed] [Google Scholar]
- 26. Ingber DE. Cellular basis of mechanotransduction. Biol Bull 194: 323–325; discussion 325–327, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med 35: 564–577, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Ingber DE. The mechanochemical basis of cell and tissue regulation. Mech Chem Biosyst 1: 53–68, 2004 [PubMed] [Google Scholar]
- 29. James DF. The meniscus on the outside of a small circular cylinder. J Fluid Mech 63: 657–664, 1974 [Google Scholar]
- 30. Jin H, Lewis JL. Determination of poisson's ratio of articular cartilage by indentation using different-sized indenters. J Biomech Eng 126: 138–145, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Kodera K, Matsui Y, Sakamoto H, Kawata M, Tsuda A. Immunohistochemical staining of sensory nerves around pulmonary alveoli in mouse (Abstract). Am J Respir Crit Care Med 177: A858, 2008 [Google Scholar]
- 32. Kralchevsky PA, Nagayama K. Capillary interactions between particles bound to interfaces, liquid films and biomembranes. Adv Colloid Interface Sci 85: 145–192, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Landau LD, Lifshitz EM. Fluid Mechanics. London: Pergamon Press, 1959 [Google Scholar]
- 34. Landau LD, Lifshitz EM. Theory of Elasticity. Oxford, UK: Butterworth-Heinemann, 1986 [Google Scholar]
- 35. Lauffenburger DA, Horwitz AF. Cell migration: A physically integrated molecular process. Cell 84: 359–369, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Mammoto A, Huang S, Moore K, Oh P, Ingber DE. Role of rhoa, mdia, and rock in cell shape-dependent control of the skp2-p27kip1 pathway and the g1/s transition. J Biol Chem 279: 26323–26330, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Marmur A. Line tension and the intrinsic contact angle in solid-liquid-fluid systems. J Colloid Interface Sci 186: 462–466, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Marmur A, Krasovitski B. Line tension on curved surfaces: Liquid drops on solid micro- and nanospheres. Langmuir 18: 8919–8923, 2002 [Google Scholar]
- 39. Mijailovich SM, Hamada K, Tsuda A. Il-8 response of cyclically stretching alveolar epithelial cells exposed to non-fibrous particles. Ann Biomed Eng 35: 582–594, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Mijailovich SM, Stamenovic D, Brown R, Leith DE, Fredberg JJ. Dynamic moduli of rabbit lung tissue and pigeon ligamentum propatagiale undergoing uniaxial cyclic loading. J Appl Physiol 76: 773–782, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Numaguchi Y, Huang S, Polte TR, Eichler GS, Wang N, Ingber DE. Caldesmon-dependent switching between capillary endothelial cell growth and apoptosis through modulation of cell shape and contractility. Angiogenesis 6: 55–64, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113: 823–839, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ofek G, Wiltz DC, Athanasiou KA. Contribution of the cytoskeleton to the compressive properties and recovery behavior of single cells. Biophys J 97: 1873–1882, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, Geisse NA, Adams JC, Whitesides GM, Ingber DE. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB J 16: 1195–1204, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Polte TR, Eichler GS, Wang N, Ingber DE. Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. Am J Physiol Cell Physiol 286: C518–C528, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Probstein RF. Physicochemical Hydrodynamics: An Introduction (2nd ed.). New York, NY: John Wiley, 1994 [Google Scholar]
- 47. Rapacchietta A, Neumann A. Force and free-energy analyses of small particles at fluid interfaces - ii. Spheres J Coll Interf Sci 59(3): 555–567, 1977 [Google Scholar]
- 48. Rapacchietta A, Neumann A, Omenyi S. Force and free-energy analyses of small particles at fluid interfaces- I. Cylinders J Coll Interf Sci 59: 541–554, 1977 [Google Scholar]
- 49. Roskelley CD, Desprez PY, Bissell MJ. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc Natl Acad Sci USA 91: 12378–12382, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schürch S, Bachofen H, Weibel ER. Alveolar surface tensions in excised rabbit lungs: Effect of temperature. Respir Physiol 62: 31–45, 1985 [DOI] [PubMed] [Google Scholar]
- 51. Schürch S, Gehr P, Im Hof V, Geiser M, Green F. Surfactant displaces particles toward the epithelium in airways and alveoli. Respir Physiol 80: 17–32, 1990 [DOI] [PubMed] [Google Scholar]
- 52. Schürch S, Lee M, Gehr P. Pulmonary surfactant: Surface properties and function of alveolar and airway surfactant. Pure Appl Chem 64: 1745–1750, 1992 [Google Scholar]
- 53. Semmler-Behnke M, Takenaka S, Fertsch S, Wenk A, Seitz J, Mayer P, Oberdorster G, Kreyling WG. Efficient elimination of inhaled nanoparticles from the alveolar region: Evidence for interstitial uptake and subsequent reentrainment onto airways epithelium. Environ Health Perspect 115: 728–733, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res 91: 769–775, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DI, Whitesides GM, Ingber DE. Engineering cell shape and function. Science 264: 696–698, 1994 [DOI] [PubMed] [Google Scholar]
- 56. Timoshenko S, Goodier JN. Theory of Elasticity. New York, NY: McGraw-Hill Book, 1951 [Google Scholar]
- 57. Traub O, Berk BC. Laminar shear stress: Mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol 18: 677–685, 1998 [DOI] [PubMed] [Google Scholar]
- 58. Trickey WR, Baaijens FP, Laursen TA, Alexopoulos LG, Guilak F. Determination of the poisson's ratio of the cell: Recovery properties of chondrocytes after release from complete micropipette aspiration. J Biomech 39: 78–87, 2006 [DOI] [PubMed] [Google Scholar]
- 59. Tsuda A, Stringer BK, Mijailovich SM, Rogers RA, Hamada K, Gray ML. Alveolar cell stretching in the presence of fibrous particles induces interleukin-8 responses. Am J Respir Cell Mol Biol 21: 455–462, 1999 [DOI] [PubMed] [Google Scholar]
- 60. Van Dyke M. Perturbation Methods in Fluid Mechanics. New York: Academic, 1964 [Google Scholar]
- 61. Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science 260: 1124–1127, 1993 [DOI] [PubMed] [Google Scholar]
- 62. Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol 10: 75–82, 2009 [DOI] [PubMed] [Google Scholar]
- 63. Watanabe N, Horie S, Michael GJ, Keir S, Spina D, Page CP, Priestley JV. Immunohistochemical co-localization of transient receptor potential vanilloid (trpv)1 and sensory neuropeptides in the guinea-pig respiratory system. Neuroscience 141: 1533–1543, 2006 [DOI] [PubMed] [Google Scholar]
- 64. Wilson TA. Surface tension-surface area curves calculated from pressure-volume loops. J Appl Physiol 53: 1512–1520, 1982 [DOI] [PubMed] [Google Scholar]
- 65. Wirtz HR, Dobbs LG. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science 250: 1266–1269, 1990 [DOI] [PubMed] [Google Scholar]
- 66. Yacobi NR, Malmstadt N, Fazlollahi F, Demaio L, Marchelletta R, Hamm-Alvarez SF, Borok Z, Kim KJ, Crandall ED. Mechanisms of alveolar epithelial translocation of a defined population of nanoparticles. Am J Respir Cell Mol Biol 42: 604–614, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yu J. Airway mechanosensors. Respir Physiol Neurobiol 148: 217–243, 2005 [DOI] [PubMed] [Google Scholar]
- 68. Zisman WA. Relation of equilibrium contact angle to liquid and solid constitution. In: Contact Angle, Wettability, and Adhesion, edited by Gould R. F. Washington DC: American Chemical Society, 1964 [Google Scholar]