Abstract
Insulin-mediated glucose disposal is dependent on the vasodilator effects of insulin. In type 2 diabetes, insulin-stimulated vasodilation is impaired as a result of an imbalance in NO and ET-1 production. We tested the hypothesis that chronic voluntary wheel running (RUN) prevents impairments in insulin-stimulated vasodilation associated with obesity and type 2 diabetes independent of the effects of RUN on adiposity by randomizing Otsuka Long Evans Tokushima Fatty (OLETF) rats, a model of hyperphagia-induced obesity and type 2 diabetes, to 1) RUN, 2) caloric restriction (CR; diet adjusted to match body weights of RUN group), or 3) sedentary control (SED) groups (n = 8/group) at 4 wk. At 40 wk, NO- and ET-1-mediated vasoreactivity to insulin (1–1,000 μIU/ml) was assessed in the presence of a nonselective ET-1 receptor blocker (tezosentan) or a NO synthase (NOS) inhibitor [NG-nitro-l-arginine methyl ester (l-NAME)], respectively, in second-order arterioles isolated from the white portion of the gastrocnemius muscle. Body weight, fasting plasma glucose, and hemoglobin A1c were lower in RUN and CR than SED (P < 0.05); however, the glucose area under the curve (AUC) following the intraperitoneal glucose tolerance test was lower only in the RUN group (P < 0.05). Vasodilator responses to all doses of insulin were greater in RUN than SED or CR in the presence of a tezosentan (P < 0.05), but group differences in vasoreactivity to insulin with coadministration of l-NAME were not observed. We conclude daily wheel running prevents obesity and type 2 diabetes-associated declines in insulin-stimulated vasodilation in skeletal muscle arterioles through mechanisms that appear to be NO mediated and independent of attenuating excess adiposity in hyperphagic rats.
Keywords: insulin sensitivity, vasodilation, exercise
insulin resistance is characterized by decreased sensitivity to the metabolic actions of insulin (glucose disposal) and is a hallmark of obesity and type 2 diabetes. Further, cardiovascular diseases characterized by endothelial dysfunction, including hypertension, coronary artery disease, and atherosclerosis are common morbidities associated with insulin resistance (34, 46). In the endothelium, insulin stimulates at least two signaling pathways. One produces the vasodilator nitric oxide (NO), while the other stimulates production of endothelin-1 (ET-1), a potent vasoconstrictor (15, 34, 37). Insulin-stimulated vasodilation enhances skeletal muscle perfusion and increases delivery of glucose and insulin to target tissues and is responsible for as much as 40% of insulin-stimulated glucose disposal following a meal (25). An imbalance in the vasodilator and vasoconstrictor actions of insulin has been described in obesity and type 2 diabetes and is proposed to contribute to diminished microvascular perfusion and skeletal muscle glucose delivery (8, 25, 26). Although improvements in skeletal muscle insulin sensitivity and blood flow responses to hyperemia or acetylcholine (ACh) in response to aerobic exercise are widely documented, the effects of exercise on insulin sensitivity of vascular endothelium are not fully understood.
Lifestyle modifications, including exercise, caloric restriction, and weight loss, are associated with improvements in insulin sensitivity and endothelial function (12, 18, 19). Exercise also enhances endothelial NO synthase (eNOS) expression in vessels that experience increases in blood flow during exercise (27, 31), an effect that is associated with improved endothelium-dependent dilation (EDD) (10, 11).
Although EDD is typically assessed by measuring blood flow responses to ACh or flow-mediated vasodilation (FMD), multiple redundant pathways mediate EDD (13, 40, 44). These pathways are often differentially affected by disease, and selective EDD dysfunction in response to specific signals may be compensated for by parallel pathways (39, 47). For example, ACh-induced vasodilation may be influenced by hyperpolarizing factors sometimes overexpressed in animal models of obesity and type 2 diabetes, whereas insulin-stimulated vasodilation is thought to be entirely NO dependent (7, 15). Further, recent evidence suggests FMD of conduit arteries is not a reliable measure of global endothelial function in type 2 diabetes (17, 32). Therefore, blood flow responses to FMD and ACh may not accurately reflect blood flow responses to insulin (2, 3). This is an important distinction given the critical role of insulin-mediated blood flow in insulin-stimulated glucose disposal and the strong association between type 2 diabetes and cardiovascular diseases.
The purpose of this study was to determine whether chronic daily physical activity (voluntary wheel running) enhances vascular reactivity to insulin in skeletal muscle arterioles of a rodent model of hyperphagia-induced obesity and type 2 diabetes [Otsuka Long-Evans Tokushima Fatty (OLETF) rats]. We also sought to determine whether these effects were exercise specific or the result of an exercise-induced attenuation of adiposity. This was accomplished by including a group of rats that were calorie restricted to match the body weight and composition of the rats given access to running wheels.
OLETF rats possess null expression of the cholecystokinin receptor 1 (CCK1) and are widely used to study interactions between obesity/type 2 diabetes and exercise (28, 36, 50). OLETFs develop obesity and display insulin resistance by 16 wk of age and develop overt type 2 diabetes by 28–30 wk of age (23); however, when given access to a running wheel, OLETF rats will voluntarily run, preventing the onset of obesity and type 2 diabetes (5). Our hypothesis was that daily wheel running and caloric restriction would prevent impairments in insulin-mediated vasodilation by attenuating adiposity and preventing whole body insulin resistance. We also hypothesized that daily wheel running would further enhance vasoreactivity to insulin through exercise-specific effects.
METHODS
Protocol approval.
All experimental protocols were approved by the Animal Care and Use Committee at the University of Missouri.
Animals and housing.
Male OLETF rats (n = 24; Tokushima Research Institute, Otsuka Pharmaceutical; Tokushima, Japan) were obtained at 4 wk of age and were individually housed in cages maintained in temperature-controlled (21°C) animal quarters with 0600–1800 light and 1800–0600 dark cycles.
Experimental design.
At 4 wk of age, rats were randomized to one of three groups: 1) voluntary wheel running + ad libitum fed (RUN); 2) sedentary + caloric restriction (fed ∼70% of ad libitum-fed SED animals) (CR); or 3) sedentary + ad libitum-fed controls (SED) (n = 8 per group). Cages of the animals in the RUN group were equipped with running wheels connected to a Sigma Sport BC 800 bicycle computer (Cherry Creek Cyclery, Foster Falls, VA) for determination of daily running distance. All rats were provided with standard chow (Formulab 5008, Purina Mills, St Louis, MO) with approximately 26%, 18%, and 56% of energy derived from protein, fat, and carbohydrate, respectively. The SED and RUN groups were given ad libitum access to food, while the food provided to the CR group was modified weekly to ensure the mean body weights of the CR and RUN groups were closely matched. At 40 wk of age, rats were anesthetized with intraperitoneal administration of pentobarbital sodium (100 mg/kg). Tissues were harvested, and the animals were killed by exsanguination. Food was removed from the cages, and the wheels of the RUN group were locked 5 h before death.
Serum markers.
On the day animals were euthanized, whole blood was collected in EDTA tubes for analysis of glycosylated hemoglobin (HbA1c) by the boronate-affinity HPLC method (Primus Diagnostics, Kansas City, MO) in the Diabetes Diagnostics Lab at the University of Missouri. Additional blood was collected in separate EDTA tubes and centrifuged at 3,000 g for 5 min. The serum was removed and stored at −80°C until it was later analyzed for fasting glucose [glucose oxidase reagent kit (Sigma, St. Louis, MO)], insulin [radioimmunoassay (Linco, St. Charles, MO)], and ET-1 concentrations [radioimmunoassay (R&D Systems, Minneapolis, MN)].
Intraperitoneal glucose tolerance test (IPGTT).
One week before animals were euthanized, rats were given 2 g glucose/kg body wt ip following a 12-h fast. Blood was collected from a tail vein at 0, 15, 30, 45, 60, and 120 min and centrifuged at 3,000 g for 5 min. The serum was separated, stored, and analyzed as described above. The glucose area under the curve (AUC) was calculated using the trapezoidal method.
Body composition and adiposity.
The body mass of the rats was measured to the nearest 0.01 g and body composition was determined using dual-energy x-ray absorptiometry (Hologic QDR-1000/w DEXA calibrated for rats) under anesthesia on the day animals were euthanized. Following the DEXA, epididymal fat pads were removed and weighed to the nearest 0.01 g.
Vasoreactivity to insulin.
The medial head of the gastrocnemius muscles were excised and pinned to a dissecting dish containing cold (4°C) MOPS-buffered physiological saline solution (PSS) containing, in mM, 145.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 glucose, 2.0 pyruvate, 0.02 EDTA, and 3.0 MOPS (pH 7.4). Second-order arterioles (2As) were isolated from the white portion of the medial head of the gastrocnemius muscle, cannulated, and mounted on a pressure myograph containing MOPS-PSS (37°C) as previously described (1, 27). Continuous measurement of intraluminal diameter using a video camera mounted on a microscope was used to ascertain the effects of vasoactive substances added directly to the MOPS-PSS to attain desired concentrations. Vessels that did not develop spontaneous smooth muscle tone (<70% maximal diameter) were preconstricted with phenylephrine (PE; 10−8–10−4 M). Endothelial and smooth muscle integrity were assessed by measuring vasoresponses to ACh (10−8–10−6 M) and KCl (80 mM), respectively. Vessels that did not respond to ACh or KCl were considered unviable and discarded. Vasodilatory and vasoconstrictor responses to four concentrations (1, 10, 100, and 1,000 μIU/ml) of bovine insulin (Sigma, St. Louis, MO) were determined following incubation with tezosentan (3 μM), a nonselective ET-1 receptor blocker, or NG-nitro-l-arginine methyl ester (l-NAME; 0.1 mM), a NOS inhibitor, to assess vasoresponsiveness to insulin when ET-1 or NO are inhibited, respectively. The insulin was dissolved in 0.01 N HCl and diluted 1:10 with PSS containing 1% BSA. Maximal vessel diameter was determined following incubation of the vessels in calcium-free PSS containing 30 μg/ml papaverine for >30 min.
Immunohistochemistry.
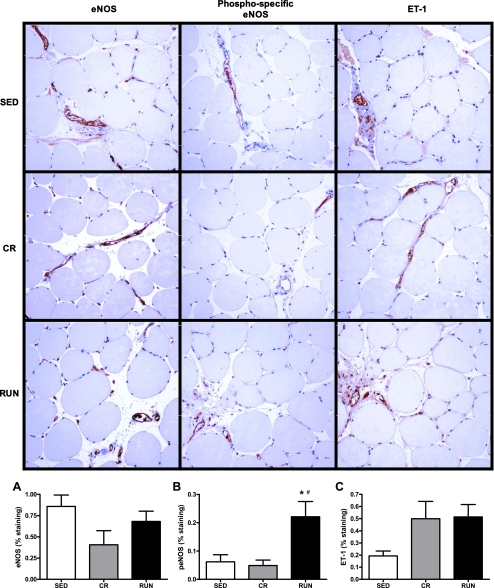
Sections of the white portion of the gastrocnemius muscle were dissected and immersed in neutral-buffered 10% formalin for ≥24 h using standard techniques (21). The sections were processed to paraffin embedment, and 5-μm sections were cut with an automated microtome (Microm, Thermo Fischer Scientific, Bellefonte, PA), floated onto positively charged slides (Thermo Fischer Scientific, Bellefonte, PA), and deparaffinized. The slides were then steamed in citrate buffer at pH 6.0 (Dako target retrieval solution S1699, DAKO, Carpenteria, CA) for 30 min to achieve antigen retrieval and subsequently cooled for 30 min. Next, the slides were stained manually with sequential Tris buffer and water wash steps performed after each protocol step. Sections were incubated with avidin biotin two-step blocking solution (Vector SP-2001, Vector Laboratories, Burlingame, CA) to inhibit background staining and in 3% hydrogen peroxide to inhibit endogenous peroxidase. Nonserum protein block (Dako X909, DAKO) was applied to inhibit nonspecific protein binding. Immunostaining was performed using mouse anti-human eNOS (1:800 dilution, BD Transduction Laboratories, San Diego, CA), mouse phospho-specific anti-eNOS (1:800 dilution, BD Transduction), or rabbit anti-human porcine ET-1 (1:800 dilution, Penninsula Labs, Belmont, CA) incubated with the tissue sections overnight at 4°C. Sections were examined using an Olympus BX60 photomicroscope (Olympus, Melville, NY) and photographed with a Spot Insight digital camera (Diagnostic Instruments, Sterling Heights, MI) at 20× magnification. Image Pro Plus software (Media Cybernetics, Silver Spring, MD) was used to quantify the positive area of staining. The staining was specific to the endothelium of vessels within the muscle (expressed as percent of total slide area, shown in red).
Statistical analysis.
One-way ANOVA was used to detect significant group effects for body weight, body composition, fasting insulin, glucose and triglycerides, glucose responses to the IPGTT, and heart-to-body weight ratio. Repeated-measures ANOVA was used to detect group effects for percent relaxation in response to graded doses of insulin in the presence of either tezosentan or l-NAME. Where significant group effects were detected, univariate analyses were performed to detect specific between-group differences, and Tukey-Kramer post hoc comparisons were performed. All analyses were performed using SAS Version 9.1 (SAS Institute, Cary, NC). Data are presented as means ± SE, and significance was set at P < 0.05.
RESULTS
Daily running distance.
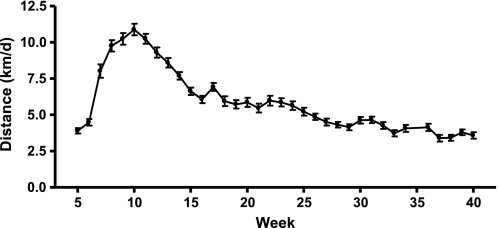
Animals given access to running wheels progressively increased daily running distance between weeks 4 (3.9 ± 0.2 km/day) and 10 (10.9 ± 4 km/day), after which running distance gradually declined to 3.6 ± 0.2 km/day at 40 wk (Fig. 1). Voluntary wheel running provided sufficient stimulus to produce exercise training adaptations as evidenced by the greater heart weight-to-body weight ratio observed in the RUN group (3.2 ± 0.1 mg/g) relative to the SED (2.55 ± 0.1, P = 0.001 vs. RUN) and CR (2.7 ± 0.1, P = 0.01 vs. RUN) groups.
Fig. 1.
Daily running distance. Voluntary wheel running distances of Otsuka Long Evans Tokushima Fatty (OLETF) rats given access to a running wheel at 4 wk of age. Values are means ± SE (n = 7).
Food intake, body weight, and body composition.
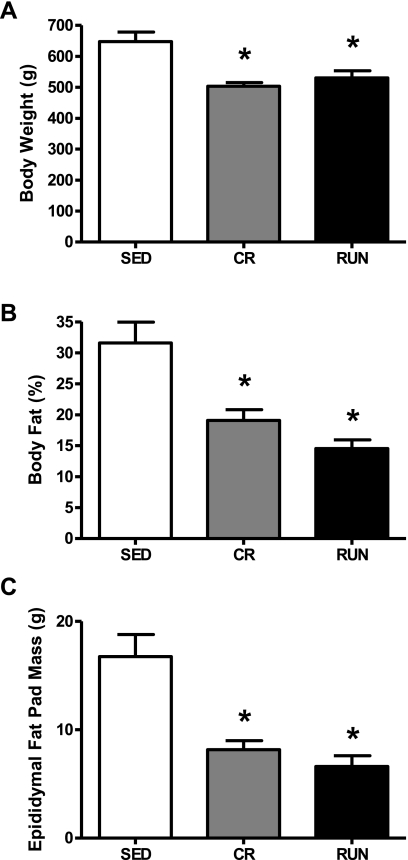
Average weekly food intake was significantly higher in the SED group (220.6 ± 5.3 g/wk) than the CR group (150.8 ± 0.1g/wk; P = 0.001), and greater in the RUN (237.3 ± 5.0) than SED (P = 0.007) or CR groups (P = 0.001). The mean body weights, percent body fat, and epididymal fat pad mass of the RUN and CR groups were similar, and were significantly lower than the SED group (P = 0.006; Fig. 2, A–C) at 40 wk of age.
Fig. 2.
Body weight (A), percent body fat (B), and epididymal fat pad mass (C) in OLETF rats randomized to voluntary wheel running (RUN), caloric restriction (CR), or sedentary control (SED) groups. Values are means ± SE (n = 6–8). *Significantly different from SED (P < 0.01).
Insulin sensitivity.
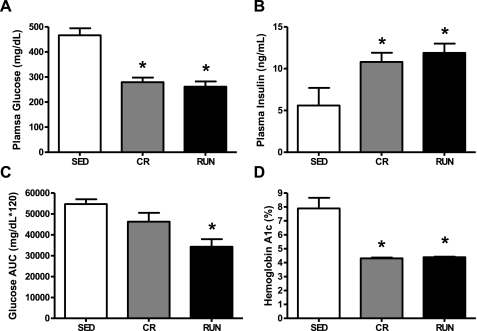
Consistent with previous reports, the 40-wk-old sedentary, ad libitum-fed OLETF rats studied here displayed characteristics of overt type 2 diabetes, including two-fold higher HbA1c levels and reduced fasting insulin levels (43). Additionally, both daily wheel running and caloric restriction protected against the development of type 2 diabetes. Fasting blood glucose and HbA1c were significantly higher in the SED group than either the CR or RUN groups (P = 0.001; Fig. 3, A and D). The glucose AUC following the IPGTT was significantly higher in the SED group than the RUN group (P = 0.006; Fig. 3C). Although the glucose AUC was 40% lower in the RUN than the CR group, this difference was not statistically significant (P = 0.09). Conversely, fasting insulin concentrations were higher in the CR and RUN groups relative to the SED group (P = 0.05; Fig. 3B), suggesting pancreatic β-cell function was severely impaired in the SED animals at 40 wk of age.
Fig. 3.
Fasting blood glucose (A), insulin (B), glucose area under the curve (AUC) (C), and hemoglobin A1c (HbA1c) (D) in OLETF rats randomized to voluntary wheel running (RUN), caloric restriction (CR), or sedentary control (SED) groups. Values are means ± SE (n = 6–8). *Significantly different from SED (P < 0.05).
Circulating ET-1.
Fasting plasma ET-1 concentrations did not differ between groups (3.22 ± 0.22, 2.88 ± 0.12, and 3.2 ± 0.80 pg/ml for SED, CR and RUN groups, respectively).
Vessel characteristics.
In second-order arterioles taken from the white portion of gastrocnemius muscles of OLETF rats at 40 wk of age, maximal vessel diameter was not different among SED, CR, and RUN groups (116.4 ± 6.4, 110.1 ± 10.4, and 131.2 ± 13.4 μm, respectively). The number of vessels requiring preconstriction also did not differ significantly among groups (n = 6, 7, and 6 for SED, CR, and RUN, respectively), nor did the concentration of PE used for preconstriction (1.35e−6 ± 2.17e−6, 1.60e−7 ± 2.51e−6, and 4.48e−6 ± 2.32e−6 M for SED, CR, and RUN groups, P for group effect = 0.46).
Vascular function.
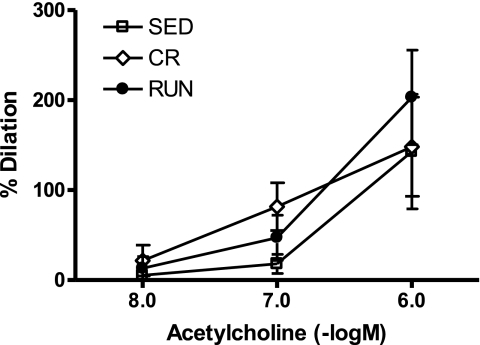
Group differences were not detected in response to any dose of ACh (10−8–10−6 M) (P for group effect = 0.60; Fig. 4). These data provide evidence that ACh-induced endothelial dependent vasodilation is not impaired in the SED compared with RUN and CR animals.
Fig. 4.
Vasodilator responses to acetylcholine in 2nd-order arterioles from the white gastrocnemius muscle of OLETF rats randomized to voluntary wheel running (RUN; n = 5), caloric restriction (CR; n = 6), or sedentary control (SED; n = 4) groups. %Dilation = [(diameter − initial diameter)/initial diameter] × 100. Values are means ± SE. Significant between-group differences were not observed.
Vasoreactivity to insulin.
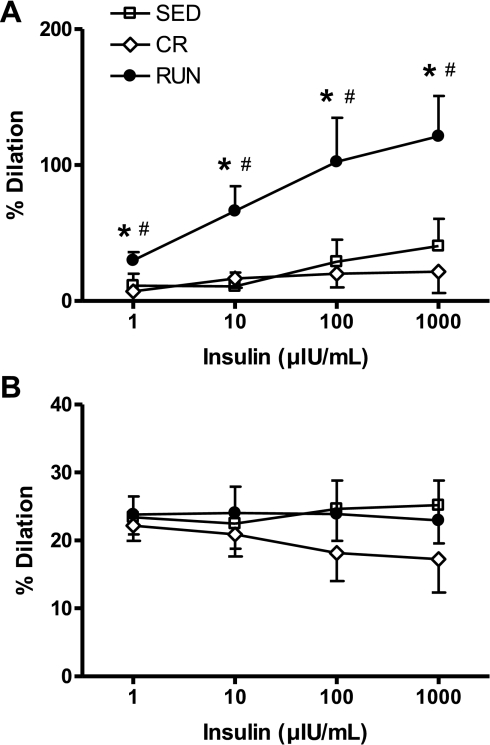
In the presence of the tezosentan, insulin-stimulated vasodilation (NO-mediated) was greater in the RUN group at all doses of insulin (1, 10, 100, and 1,000 μIU/ml) relative to the CR (P = 0.01) and SED groups (P = 0.02; Fig. 5A). Conversely, in the presence of l-NAME, vessel diameter did not differ among the groups in response to any dose of insulin (P for group effect = 0.47; Fig. 5B). Collectively, these data suggest daily voluntary wheel running enhances insulin-mediated vasodilation in second-order arterioles of the white gastrocnemius muscle through mechanisms that appear to be NO mediated and not reproduced by CR, i.e., these effects are exercise specific and not due to reduced adiposity.
Fig. 5.
Vasodilator responses to graded doses of insulin in the presence of the ET-1 receptor blocker, tezosentan (A), or the nitric oxide synthase (NOS) inhibitor NG-nitro-l-arginine methyl ester (l-NAME) (B) in 2nd-order arterioles from the white gastrocnemius muscle of OLETF rats randomized to voluntary wheel running (RUN), caloric restriction (CR), or sedentary control (SED) groups. %Dilation = [(diameter − initial diameter)/initial diameter] × 100. Values are means ± SE (n = 6–8). *Significantly different from SED (P < 0.05). #Significantly different from CR (P < 0.05).
Immunohistochemistry.
Observations in sections of white gastrocnemius muscle stained for eNOS, phospho-specific eNOS, and ET-1 support our findings from the functional studies (vasoreactivity to insulin) described above. Percent staining for eNOS and ET-1 did not differ among the groups (P for group effect = 0.12 and 0.13, respectively; Fig. 6, A and C); however, staining for phospho-specific eNOS was significantly higher in animals from the RUN group than the SED (P = 0.03) and CR groups (P = 0.01; Fig. 6B). These data indicate elevated basal levels of phospho-eNOS may contribute to the enhanced NO-mediated vasodilation observed in response to insulin in animals given access to running wheels and provide further evidence that these effects are exercise specific and independent of the attenuation of adiposity.
Fig. 6.
Representative images and quantification of immunostaining (shown here in red) for endothelial NOS (eNOS) (A), phospho-specific eNOS (peNOS; B), and ET-1 (C) in cross sections of the white portion of the gastrocnemius muscle of OLETF rats randomized to voluntary wheel running (RUN), caloric restriction (CR), or sedentary control (SED) groups. %Staining is percent of slide area positive for staining. Values are means ± SE (n = 7–8). *Significantly different from SED (P < 0.05). #Significantly different from CR (P < 0.05).
DISCUSSION
Here we report novel findings that daily voluntary wheel running enhanced insulin-stimulated vasodilation in skeletal muscle arterioles of hyperphagic rats prone to obesity and diabetes, an effect that appears to be NO mediated and specific to exercise. Daily voluntary wheel running and caloric restriction resulted in a comparable attenuation of body weight and adiposity and similarly maintained several measures of insulin sensitivity (fasting blood glucose and insulin, and HbA1c) compared with sedentary OLETF rats. However, only voluntary wheel running produced a glucose AUC in response to the glucose tolerance test that was significantly lower than that of sedentary animals. Furthermore, in the presence of an ET-1 inhibitor, insulin-stimulated vasodilation was greater in second-order arterioles from white gastrocnemius muscle of animals given access to running wheels than those with restricted food intake, suggesting daily physical activity exerts specific insulin-sensitizing effects on the arterioles of skeletal muscles likely recruited during exercise that are independent of attenuating adiposity.
Second-order arterioles isolated from the white gastrocnemius muscle of OLETF rats given access to a running wheel exhibited enhanced vasoreactivity to insulin when treated with an ET-1 receptor blocker, indicating that NO-mediated vasoreactivity to insulin is increased by daily wheel running. Although it is possible that these findings are the result of an increase in insulin-stimulated production of or sensitivity to alternative vasodilator substances or decreased responsiveness to ET-1, treatment of the same arterioles with a NOS inhibitor abolished the vasoactive response to insulin, suggesting the effects of exercise on insulin-mediated vasodilation are likely NO mediated. Further, these observations coincided with higher concentrations of phospho-specific eNOS in the white gastrocnemius of the animals given access to a running wheel relative to their sedentary counterparts.
Insulin-stimulated vasodilation accounts for ∼40% of the increase in skeletal muscle glucose disposal during postprandial conditions by enhancing delivery and clearance of circulating insulin and glucose (8, 25, 41). This process is impaired in obesity and type 2 diabetes (4, 9, 25, 26) due to an imbalance in the NO and ET-1 branches of the insulin signaling pathway (14, 16, 29, 30). Interestingly, despite similar body weight and adiposity as well as fasting glucose and insulin levels in the CR and RUN animals at 40 wk, the glucose AUC in the RUN animals was 40% lower than that of the CR animals, a difference that approached significance (P = 0.09). Although not directly tested in these experiments, it is plausible that the greater insulin sensitivity of skeletal muscle arterioles in the RUN group played a role in the more favorable IPGTT glucose and insulin responses in the RUN animals.
Contrary to previous reports of enhanced ACh responses in thoracic aortas of exercise-trained OLETF rats (33), we did not observe greater vasodilation in response to ACh in second-order arterioles from the white gastrocnemius muscle of OLETF rats given access to a running wheel relative to sedentary or weight-matched animals at 40 wk of age. While these data should be interpreted with caution given our small sample size for this measure, our data suggest sedentary OLETF rats do not display global dysfunction of endothelial cell signaling pathways but rather impairments specific to insulin signaling in the endothelium of skeletal muscle arterioles.
Systemic factors linked to obesity and insulin resistance, including lipotoxicity, glucotoxicity, and inflammation, are often implicated in endothelial dysfunction, including impairments in insulin-stimulated vasodilation (6, 20, 45). Although food restriction significantly abated lipotoxicity and glucotoxicity in the OLETF rat, unlike voluntary wheel running, food restriction did not improve insulin-stimulated vasodilation in skeletal muscle arterioles over that observed in the sedentary animals, suggesting physical activity may play a more powerful role in modifying vascular reactivity to insulin than dietary modification (food restriction).
Nonetheless, outcomes relating to the effects of caloric restriction in this study should be interpreted with caution. The purpose of the CR group in this setting was not to test the effect of caloric restriction per se, but rather to differentiate the effects of daily physical activity from those of attenuated adiposity on insulin-mediated vasodilation. Accordingly, the food intake of the CR group was restricted to ensure the mean body weight and adiposity of the CR animals were closely matched to those in the RUN group. It is possible that a “true” caloric restriction may produce effects different from those described here.
An additional limitation of the present study is the absence of data examining vasoresponses to insulin alone, leaving uncertainty as to the aggregate effects of insulin stimulation of the ET-1 and NO pathways. Preliminary work in our lab (data not shown) supported previous findings from others suggesting that vasoresponsiveness to insulin alone is modest in isolated microvessels, and assessments of the aggregate effect of insulin may best be explored using in vivo methodologies.
Despite overwhelming evidence that a sedentary lifestyle and low cardiorespiratory fitness are positively associated with risk of developing insulin resistance and type 2 diabetes, independent of body mass index, the majority of Americans fail to meet national physical activity guidelines (22, 24, 48), and individuals with type 2 diabetes report lower levels of leisure time physical activity than their nondiabetic counterparts (35). Alarmingly, projections indicate that rates of both physical inactivity (38) and type 2 diabetes (49) will continue to rise. While others have demonstrated improvements in insulin-stimulated capillary recruitment and glucose uptake in response to exercise training in previously sedentary, lean rats (41), little work has been done to specifically assess the effects of daily physical activity on NO- and ET-1 mediated vasoreactivity to insulin.
Consistent with previous reports from our group, daily exercise in the form of voluntary wheel running was associated with favorable metabolic outcomes in the hyperphagic OLETF rat (36, 42). Here we report that daily wheel running is also associated with improved insulin-stimulated vasodilation in the presence of an ET-1 receptor blocker in skeletal muscle arterioles from a rodent model prone to obesity and type 2 diabetes. Maintaining or enhancing insulin-stimulated vasodilation and the resulting increase in blood flow may be one mechanism by which daily exercise preserves or improves insulin-stimulated glucose uptake and cardiovascular health. Future work is needed to definitively determine the effect of physical activity on microvascular responses to insulin and the role of these processes in the protection against the development of type 2 diabetes.
GRANTS
This work was supported with resources and the use of facilities at the Harry S Truman Memorial Veterans Hospital in Columbia, MO. This work was supported by the College of Veterinary Medicine, Department of Internal Medicine, and Institute for Clinical and Translational Sciences at the University of Missouri, the Department of Veterans Affairs (Career Development Award, J. P. Thyfault), and National Institutes of Health Grants T32-AR-048523 (C. R. Mikus), HL-36088 (M. H. Laughlin), and F32-DK-83182 (R. S. Rector).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Pam Thorne, Grace Uptergrove, Jennifer Casati, and Alexa Bermudez for technical assistance and Whitney Collins and Aaron Bunker for help with animal husbandry. We also thank the Diabetes Diagnostics Lab at the University of Missouri for performing hemoglobin A1c measurements.
The OLETF rats were a generous gift from the Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan).
REFERENCES
- 1. Aaker A, Laughlin MH. Differential adenosine sensitivity of diaphragm and skeletal muscle arterioles. J Appl Physiol 93: 848–856, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Andrews TJ, Laight DW, Anggard EE, Carrier MJ. Investigation of endothelial hyperreactivity in the obese Zucker rat in-situ: reversal by vitamin E. J Pharm Pharmacol 52: 83–86, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Bakker EN, Sipkema P. Components of acetylcholine-induced dilation in isolated rat arterioles. Am J Physiol Heart Circ Physiol 273: H1848–H1853, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Baron AD, Brechtel G. Insulin differentially regulates systemic and skeletal muscle vascular resistance. Am J Physiol Endocrinol Metab 265: E61–E67, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Bi S, Scott KA, Hyun J, Ladenheim EE, Moran TH. Running wheel activity prevents hyperphagia and obesity in Otsuka Long-Evans Tokushima Fatty rats: role of hypothalamic signaling. Endocrinology 146: 1676–1685, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA 286: 1218–1227, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Chen YL, Messina EJ. Dilation of isolated skeletal muscle arterioles by insulin is endothelium dependent and nitric oxide mediated. Am J Physiol Heart Circ Physiol 270: H2120–H2124, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Clark MG. Impaired microvascular perfusion: a consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am J Physiol Endocrinol Metab 295: E732–E750, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 55: 1436–1442, 2006 [DOI] [PubMed] [Google Scholar]
- 10. De Filippis E, Cusi K, Ocampo G, Berria R, Buck S, Consoli A, Mandarino LJ. Exercise-induced improvement in vasodilatory function accompanies increased insulin sensitivity in obesity and type 2 diabetes mellitus. J Clin Endocrinol Metab 91: 4903–4910, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Delp MD, McAllister RM, Laughlin MH. Exercise training alters endothelium-dependent vasoreactivity of rat abdominal aorta. J Appl Physiol 75: 1354–1363, 1993 [DOI] [PubMed] [Google Scholar]
- 12. Dengel DR, Kelly AS, Olson TP, Kaiser DR, Dengel JL, Bank AJ. Effects of weight loss on insulin sensitivity and arterial stiffness in overweight adults. Metabolism 55: 907–911, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Dusting GJ, Moncada S, Vane JR. Prostacyclin (PGX) is the endogenous metabolite responsible for relaxation of coronary arteries induced by arachidonic acid. Prostaglandins 13: 3–15, 1977 [DOI] [PubMed] [Google Scholar]
- 14. Elgebaly MM, Kelly A, Harris AK, Elewa H, Portik-Dobos V, Ketsawatsomkron P, Marrero M, Ergul A. Impaired insulin-mediated vasorelaxation in a nonobese model of type 2 diabetes: role of endothelin-1. Can J Physiol Pharmacol 86: 358–364, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eringa EC, Stehouwer CD, Merlijn T, Westerhof N, Sipkema P. Physiological concentrations of insulin induce endothelin-mediated vasoconstriction during inhibition of NOS or PI3-kinase in skeletal muscle arterioles. Cardiovasc Res 56: 464–471, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Eringa EC, Stehouwer CD, Roos MH, Westerhof N, Sipkema P. Selective resistance to vasoactive effects of insulin in muscle resistance arteries of obese Zucker (fa/fa) rats. Am J Physiol Endocrinol Metab 293: E1134–E1139, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Green DJ, Maiorana AJ, Tschakovsky ME, Pyke KE, Weisbrod CJ, O'Driscoll G. Relationship between changes in brachial artery flow-mediated dilation and basal release of nitric oxide in subjects with Type 2 diabetes. Am J Physiol Heart Circ Physiol 291: H1193–H1199, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 107: 3152–3158, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Hamdy O, Ledbury S, Mullooly C, Jarema C, Porter S, Ovalle K, Moussa A, Caselli A, Caballero AE, Economides PA, Veves A, Horton ES. Lifestyle modification improves endothelial function in obese subjects with the insulin resistance syndrome. Diabetes Care 26: 2119–2125, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Hammer S, Snel M, Lamb HJ, Jazet IM, van der Meer RW, Pijl H, Meinders EA, Romijn JA, de Roos A, Smit JW. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol 52: 1006–1012, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Homma H, Takahashi T, Seki H, Ohtani M, Kondoh T, Fukuda M. Immunohistochemical localization of inducible nitric oxide synthase in synovial tissue of human temporomandibular joints with internal derangement. Arch Oral Biol 46: 93–97, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Hu G, Lindstrom J, Valle TT, Eriksson JG, Jousilahti P, Silventoinen K, Qiao Q, Tuomilehto J. Physical activity, body mass index, and risk of type 2 diabetes in patients with normal or impaired glucose regulation. Arch Intern Med 164: 892–896, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Ishida K, Mizuno A, Min Z, Sano T, Shima K. Which is the primary etiologic event in Otsuka Long-Evans Tokushima Fatty rats, a model of spontaneous non-insulin-dependent diabetes mellitus, insulin resistance, or impaired insulin secretion? Metabolism 44: 940–945, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care 30: 744–752, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest 85: 1844–1852, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laakso M, Edelman SV, Brechtel G, Baron AD. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes 41: 1076–1083, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Laughlin MH, Woodman CR, Schrage WG, Gute D, Price EM. Interval sprint training enhances endothelial function and eNOS content in some arteries that perfuse white gastrocnemius muscle. J Appl Physiol 96: 233–244, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Laye MJ, Rector RS, Borengasser SJ, Naples SP, Uptergrove GM, Ibdah JA, Booth FW, Thyfault JP. Cessation of daily wheel running differentially alters fat oxidation capacity in liver, muscle, and adipose tissue. J Appl Physiol 106: 161–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lteif A, Vaishnava P, Baron AD, Mather KJ. Endothelin limits insulin action in obese/insulin-resistant humans. Diabetes 56: 728–734, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Lteif AA, Fulford AD, Considine RV, Gelfand I, Baron AD, Mather KJ. Hyperinsulinemia fails to augment ET-1 action in the skeletal muscle vascular bed in vivo in humans. Am J Physiol Endocrinol Metab 295: E1510–E1517, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McAllister RM, Newcomer SC, Laughlin MH. Vascular nitric oxide: effects of exercise training in animals. Appl Physiol Nutr Metab 33: 173–178, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meyer MF, Lieps D, Schatz H, Pfohl M. Impaired flow-mediated vasodilation in type 2 diabetes: lack of relation to microvascular dysfunction. Microvasc Res 76: 61–65, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Minami A, Ishimura N, Harada N, Sakamoto S, Niwa Y, Nakaya Y. Exercise training improves acetylcholine-induced endothelium-dependent hyperpolarization in type 2 diabetic rats, Otsuka Long-Evans Tokushima fatty rats. Atherosclerosis 162: 85–92, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Montagnani M, Quon MJ. Insulin action in vascular endothelium: potential mechanisms linking insulin resistance with hypertension. Diabetes Obes Metab 2: 285–292, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care 30: 203–209, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Morris RT, Laye MJ, Lees SJ, Rector RS, Thyfault JP, Booth FW. Exercise-induced attenuation of obesity, hyperinsulinemia, and skeletal muscle lipid peroxidation in the OLETF rat. J Appl Physiol 104: 708–715, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Muniyappa R, Quon MJ. Insulin action and insulin resistance in vascular endothelium. Curr Opin Clin Nutr Metab Care 10: 523–530, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Nelson KM, Reiber G, Boyko EJ. Diet and exercise among adults with type 2 diabetes: findings from the third national health and nutrition examination survey (NHANES III). Diabetes Care 25: 1722–1728, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Nishikawa Y, Stepp DW, Chilian WM. Nitric oxide exerts feedback inhibition on EDHF-induced coronary arteriolar dilation in vivo. Am J Physiol Heart Circ Physiol 279: H459–H465, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524–526, 1987 [DOI] [PubMed] [Google Scholar]
- 41. Rattigan S, Wallis MG, Youd JM, Clark MG. Exercise training improves insulin-mediated capillary recruitment in association with glucose uptake in rat hindlimb. Diabetes 50: 2659–2665, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Rector RS, Thyfault JP, Laye MJ, Morris RT, Borengasser SJ, Uptergrove GM, Chakravarthy MV, Booth FW, Ibdah JA. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. J Physiol 586: 4241–4249, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rector RS, Uptergrove GM, Borengasser SJ, Mikus CR, Morris EM, Naples SP, Laye MJ, Laughlin MH, Booth FW, Ibdah JA, Thyfault JP. Changes in skeletal muscle mitochondria in response to the development of type 2 diabetes or prevention by daily wheel running in hyperphagic OLETF rats. Am J Physiol Endocrinol Metab 298: E1179–E1187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Taylor SG, Weston AH. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol Sci 9: 272–274, 1988 [DOI] [PubMed] [Google Scholar]
- 45. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344: 1343–1350, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Vincent MA, Montagnani M, Quon MJ. Molecular and physiologic actions of insulin related to production of nitric oxide in vascular endothelium. Curr Diab Rep 3: 279–288, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Waldron GJ, Ding H, Lovren F, Kubes P, Triggle CR. Acetylcholine-induced relaxation of peripheral arteries isolated from mice lacking endothelial nitric oxide synthase. Br J Pharmacol 128: 653–658, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee CD, Blair SN. The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Ann Intern Med 130: 89–96, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047–1053, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Yasuda K, Adachi T, Kikuchi N, Tsujimoto G, Aoki N, Tsuda K, Ishihara A. Effects of running exercise on fibre-type distribution of soleus and plantaris muscles in diabetic Otsuka Long-Evans Tokushima fatty rats. Diabetes Obes Metab 8: 311–321, 2006 [DOI] [PubMed] [Google Scholar]