Abstract
The difference in effectiveness between volume-controlled ventilation (VCV) and pressure-controlled ventilation (PCV) on mechanically ventilated patients during bronchoconstriction is not totally clear. PCV is thought to deliver a more uniform distribution of ventilation than VCV, but the delivered tidal volume could be unstable and affected by changes in the degree of constriction. To explore the magnitude of these effects, we ran numerical simulations with both modes of ventilation in a network model of the lung in which we incorporated not only the pressure and flow dynamics along the airways but also the effect of cycling pressures and tissue tethering forces during breathing on the dynamic equilibrium of the airway smooth muscle (ASM) (Venegas et al., Nature 434: 777–782). These simulations provided an illustration of changes in airway radii, the total delivered tidal volume stability, and distribution of ventilation following a transition from VCV to PCV and during progressively increasing ASM activation level. These simulations yielded three major results. First, the ventilation heterogeneity and patchiness in ventilation during steady-state VCV were substantially reduced after the transition to PCV. Second, airway radius, tidal volume, and the distribution of ventilation under severe bronchoconstriction were highly sensitive to the setting of inspiratory pressure selected for PCV and to the degree of activation of the ASM. Third, the dynamic equilibrium of active ASM exposed to cycling forces is the major contributor to these effects. These insights may provide a theoretical framework to guide the selection of ventilation mode, the adjustment of ventilator settings, and the interpretation of clinical observations in mechanically ventilated asthmatic patients.
Keywords: tidal volume sensitivity, modeling, ventilation distribution, asthma
the two common ventilatory modes are volume-controlled ventilation (VCV) and pressure-controlled ventilation (PCV). Interestingly, experimental data suggest that PCV may have advantages on gas exchanges over VCV, which is a traditional mode of ventilation for patients with severe asthma. López-Herce et al. (7) reported two clinical cases showing significant and rapid improvements in blood gases of children with severe asthma when ventilatory mode was switched to PCV after ventilating with VCV for 6 h, during which López-Herce et al. observed no improvement in blood gases. Sarnaik et al. (14) published a study of 40 patients with severe asthma who showed significant improvements in blood gases as a result of mechanical ventilation with PCV. Understanding the theoretical basis of the regional behavior of a constricted lung could be helpful to interpret results of those studies.
We postulate that the significant improvement in blood gases observed experimentally during PCV may have resulted from changes in the distribution of ventilation caused by modifications in the interaction between a short-range positive and long-range negative feedback mechanism within the lung (19).
A positive feedback takes place when a small increase in local airway constriction leads to further constriction of that airway and those distal to it. Central to this mechanism is the response of the airway smooth muscle (ASM) to dynamic conditions. When ASM is activated, the airway narrows, reducing the amount of airflow through that airway. The reduction in airflow leads to reductions in regional tidal ventilation and thus the dynamic forces on the airway wall from parenchymal tethering and the transmural pressures. A reduction in these forces leads to shortening of the ASM and therefore further enhances the constriction of the airway. This mechanism is short ranged because it involves local interactions between airway and surrounding parenchyma and interconnected airways.
A long-range negative feedback takes place when airway constriction in one airway leads to airway dilatation of others far away in the tree. Central to this feedback mechanism is the maintenance of total tidal volume (Vt) such as in VCV. When an airway constricts, airflow through that airway is reduced. If total Vt is kept constant, a decrease in airflow along that airway subsequently causes an increase in driving pressure and airflow to the rest of the lung, which leads to airway dilatation by the mechanism described above. This mechanism is long range because it involves the interaction of airways far from each other.
Because in PCV driving pressure does not change in response to increased airway resistance, the negative feedback will be absent. This should affect the patchiness in ventilation distribution, and the instability of an unopposed positive feedback should rapidly lead to catastrophic closure of airways. In this paper, we investigate the impact of ventilatory mode on the distribution of ventilation during bronchoconstriction. Using a computational network model embodying the above-mentioned feedback mechanisms (19), we 1) examine the differences in spatial distribution of ventilation, total delivered Vt, and airway lumen in a bronchoconstricted lung between VCV and PCV, 2) investigate how these differences depend on the baseline ASM tone and ventilator settings, and 3) investigate whether the dynamic response of active ASM to tidal breathing is responsible for the differences in the ventilation distribution between VCV and PCV.
METHODS
Network model of the lung.
Simulations were run on the network model that was previously described in details (19, 22). The model consisted of a 12-generation airway tree with symmetric dichotomous branching. Airway sizes were taken from the morphological data from the fourth generation to the terminal bronchi of a human airway tree estimated by Weibel (21). The model of a single terminal airway published by Anafi and Wilson (1) was implemented for all airways. Flow through each airway driven by a pressure drop was assumed to be laminar (9), with negligible gas compressibility (12) and negligible entrance effect. Each terminal bronchiole was connected to an acinar unit of constant and linear compliance. The effects of pleural pressure, gravity, and chest wall mechanics were neglected. Additionally, we neglected the interdependence between neighboring acinar units, as well as the nonlinearities of parenchyma and chest walls. Airway length was independent of lung inflation, and the wall thickness was adjusted with changes in airway diameter to preserve the wall volume (15). To break the model-imposed functional and structural symmetry, a small random variation in wall thickness (1% coefficient of variation) was added to all airways.
In the model, the lumen of each airway was assumed to remain constant during the breath. However, in a breath-by-breath manner, airway luminal area was determined based on the airway wall area and the ASM length, which was assumed to be equal to the outer circumference of the airway. The ASM length was computed based on the transmural pressure across the airway wall and the dynamic properties of the ASM. To do this, transmural pressure as a function of time was estimated in time steps of 10 ms along the breath as the net sum of radial forces on the airway wall caused by the luminal pressure, tethering stress from the surrounding parenchyma, and average pressure within the subtended alveoli.
Dynamic equilibrium of active ASM.
For a given set of flow and pressure conditions within each breath, the ASM length expected for dynamic equilibrium was extrapolated from an empirical linear relationship between the peak ASM length and peak ASM tension for maximal ASM activation (1, 3, 5, 15). Such a peak ASM tension was calculated as that balancing the peak transmural pressure during the breathing cycle. We then compared the current ASM length of each airway with that expected for dynamic equilibrium, and, if different, ASM length was adjusted by a fraction of the difference. This algorithm, repeated over several breaths, led to steady-state conditions where ASM length of each airway was in dynamic equilibrium. This approach has been found to describe well the dynamic response of airways to a deep breath (2, 3) and is consistent with the force-velocity relationship (17) and the viscoelastic properties for active smooth muscle (20).
Relative level of ASM activation.
In the Anafi and Wilson model (1), the relationship between the peak ASM tension and peak ASM length at dynamic equilibrium was taken to be that for a maximally activated ASM. To simulate conditions with the ASM activation below maximum, the slope of that relationship was scaled by a factor of relative level of ASM activation (Tr). This factor ranged from Tr = 0 for fully relaxed to Tr = 1 for maximally activated ASM.
Alveolar ventilation was estimated as the Vt expansion of a terminal acinar unit during the breath as determined by the product of compliance and the alveolar pressure swing. Total lung Vt was computed as the difference between maximum and minimum total lung volume during each breath.
Simulation details.
We numerically solved the set of recursive state equations using MATLAB (Mathworks, Natick, MA) to determine the flow along each airway and the pressure at each branch point of the network. The model was driven with a constant breathing frequency of 12 breaths per minute, with inspiratory-to-expiratory time ratio of 1:1 and a positive end-expiratory pressure of 5 cmH2O. Our previous model (19) was modified so that a user could specify the ventilatory mode to VCV or PCV. For VCV, the model input function was a constant inspiratory flow providing a predefined Vt, followed by a passive exhalation in which airway opening pressure was kept constant at the positive end-expiratory pressure value. For PCV, the input function was a constant pressure during inspiration (Pi) followed also by a passive exhalation as in VCV. The mode of ventilation could be changed at the end of a given breath. For a transition from VCV to PCV, a specific value of Pi could be selected or a value estimated so that the Vt delivered during the first breath at PCV was equal to that during VCV. The value of Tr was set to be uniform throughout the airway tree and constant during each breath but could be varied breath by breath in a predefined manner.
Simulation protocols.
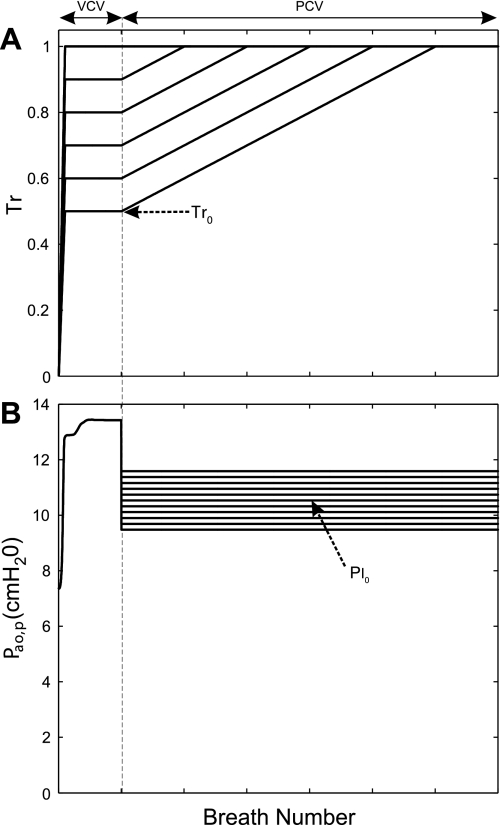
All simulations started with VCV (constant Vt of 650 ml) until a steady state was established at baseline (Tr0). In simulation protocol 1, we maintained VCV at a constant Vt = Vt0. In simulation protocol 2, a ventilatory mode was changed from VCV to PCV, keeping the respiratory frequency and inspiratory-to-expiratory time ratio. For PCV, value of Pi = Pi0 was defined such that the total Vt for the first breath following the transition of ventilatory mode equaled Vt0 (Fig. 1). After a steady state was achieved, a ramp increase in Tr from Tr0 to Tr = 1 with a slope of 0.0002 per breath was applied to simulate a slow and progressive exposure to a bronchoconstrictor stimulus (Fig. 1). The following three sets of simulations were run to investigate the three aims listed in the Introduction.
Fig. 1.
Simulation protocols. A: relative smooth muscle activation (Tr) plotted breath by breath. Tr increases to a baseline value (Tr0) and is kept constant to ascertain a steady state during volume-controlled ventilation (VCV). From that point, Tr progressively increased to maximum activation (Tr = 1) (slope of 0.0002 unit per breath). B: response of peak airway opening pressure (Pao,p) relative to positive end-expiratory pressure (PEEP). The dashed line divides the 2 simulation protocols. The simulation protocol 1 starts from the beginning and ends at the dashed line. The simulation protocol 2 begins from the dashed line onward. During the first breath in simulation protocol 2, the ventilatory mode was transitioned to pressure-controlled ventilation (PCV) with the inspiratory pressure (Pi) set at a fraction of Pi0. Pi0 is the Pi that yields the tidal volume (Vt) immediately after the transition equal to Vt0 during VCV.
Effects of ventilatory mode on airway lumen, delivered Vt, and the distribution of ventilation.
Both simulation protocols were run using Tr0 = 0.5. This value was chosen to be 10% less than the estimated Tr0 reported previously (22). Two hundred fifty-six terminal airways were randomly selected, and their radii were normalized by the fully dilated respective radius at Tr = 0 plotted as a function of time. The fraction of closed terminal airways, at a given time, was computed as the fraction of airways with radii less than 0.5% of their fully dilated radii. Values of Pi during PCV and Vt/Vt0 and the peak airway opening pressure during VCV were plotted as a function of time. Finally, the distribution of ventilation was represented by a Mandelbrot-like tree, where ventilation to each terminal unit corresponded to a data point in the 64 × 64 grid. The color scale corresponded to the ventilation normalized by the average ventilation during VCV. Throughout this paper, this plot is referred to as a pseudo-ventilation image.
Influence of the baseline ASM activation and ventilator setting.
Both simulation protocols were conducted with Tr0 set at a value ranging from 0.5 to 1.0 in increments of 0.1. In simulations with protocol 2, PCV was given with values of Pi/Pi0 ranging from 0.90 to 1.10 in increments of 0.01. Differences in steady-state airway radii, steady-state Vt (Vtss), and the spatial distribution of ventilation were noted. The sensitivity of Vtss to the selection of Pi was calculated as the slope of a plot between Vtss/Vt0 and Pi/Pi0. The spatial distributions of ventilation in steady state were plotted for VCV before the transition to PCV and in PCV for all Pi settings.
Contribution of the dynamic behavior of ASM to the distribution of ventilation in PCV.
Simulation protocol 2 was run with Tr0 = 1.0 with a transition to PCV using Pi/Pi0 = 1.031. This number was derived iteratively so that Vt during steady-state condition in PCV equaled Vt0. Note that this value of Pi is not the same as that which gives a first-breath Vt = Vt0. The simulation was then repeated, but this time with a model that did not include the dynamic properties of ASM; instead, airway radii during PCV were fixed to the values obtained during steady-state VCV. Ventilation histograms and pseudo-ventilation images during steady state in PCV were generated for each simulation. The degree of ventilation heterogeneity was quantified by the coefficient of variation (equal to SD/mean) (10, 18, 19). The simulations were also run with a value of Pi/Pi0 = 1.04, a condition where the effect of the ASM dynamic behavior on the distribution of ventilation was exaggerated.
RESULTS
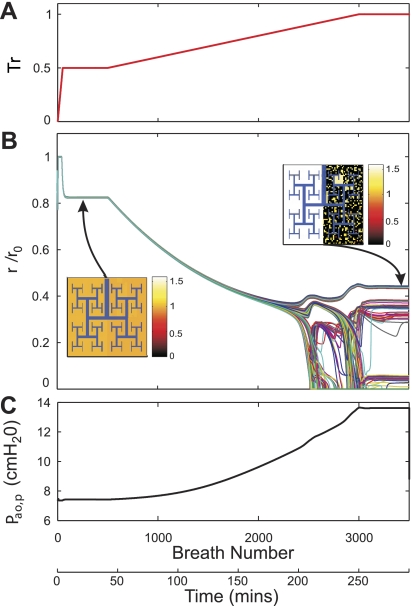
As observed before, in VCV, the constriction of airways was homogenous at low levels of ASM activation. A slow increase in Tr over time caused a reduction in airway radii and a concomitant increase in peak airway opening pressure. As Tr reached a critical point (Tr > 0.8), groups of airways rapidly constricted, whereas others expanded, resulting in a combination of airway constriction and dilation (19) and in patchy ventilation distribution characterized by large size ventilation defects (22) (Fig. 2). As Tr continued to increase above that critical point, ventilation defects continued to emerge with a concomitant redistribution of ventilation away from ventilation defects. During PCV, low levels of Tr resulted in a uniform airway constriction similar to that in VCV as long as Vt remained unchanged (up to ∼1,000 breaths in Fig. 3). However, as Tr continued to increase, we observed a progressive drop in Vt and an accelerated airway constriction that led to full closure of all terminal airways (Fig. 3). Note that the timing for full airway closure was not uniform.
Fig. 2.
Airway response and changes in airway opening pressure with progressive bronchoconstriction during VCV. A: Tr plotted breath by breath. Tr increased to 0.5 and was kept constant to ascertain a steady state (1st to 500th breath). From that point, Tr progressively increased to maximum activation (Tr = 1). B: response to the changes in Tr of lumen radii of terminal airways normalized by their corresponding fully dilated radii (r/r0). Plots for 256 representative airway radii are presented in different colors for illustrative purposes. In the pseudo-ventilation images at 2 steady conditions, the values of acinar ventilation normalized by total ventilation were represented in a color scale. C: response of Pao,p relative to PEEP. For explanation, see text.
Fig. 3.
Airway response and changes in airway opening pressure during progressive bronchoconstriction with PCV with Tr0 = 0.5. A: in this simulation, Tr was initially increased to 0.5 and kept constant to ascertain a steady state (1st to 200th breath). At that point, the ventilatory mode was transitioned to PCV with a value of Pi chosen such so that Vt of the breath immediately after a transition equaled the Vt during VCV (Vt0). Tr was progressively increased to a maximum activation value (Tr = 1). B: response to the changes in Tr of lumen radii of terminal airways normalized by their corresponding fully dilated radii (r/r0). Plots for 256 representative airway radii are presented in different colors for illustrative purposes. In the pseudo-ventilation images at 2 steady conditions, the values of acinar ventilation normalized by total ventilation were represented in a color scale. C: response of Vt normalized by Vt0. D: response of Paop relative to PEEP. For explanation, see text.
Transition from VCV to PCV.
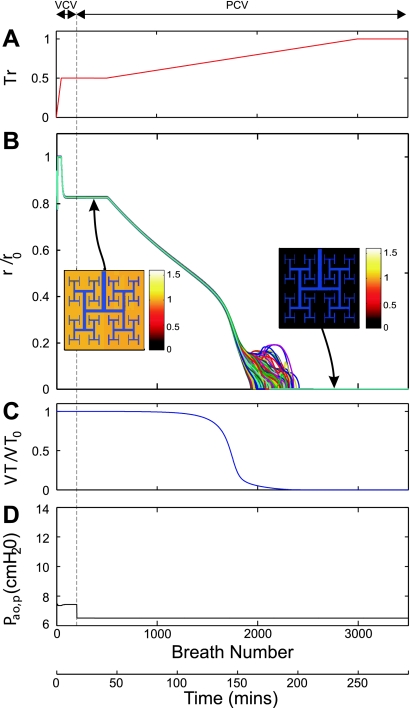
For transitions from VCV to PCV taking place with Tr0 ≤ 0.8, airway radii were uniformly constricted and remained constant before, during, and after the transition (Fig. 3). However, for Tr0 > 0.8, i.e., 0.9 (Fig. 4) or 1.0 (Fig. 5), transitions from VCV to PCV led to Pi-dependent dynamic changes in radius and Vt until a new steady state was reached. Note that even when Pi was set such that the first-breath Vt in PCV was equal to that during VCV (Vt = Vt0), when distribution of ventilation was heterogeneous, the new steady state reached involved a substantially reduced Vt with a ventilation distribution that was also heterogeneous but no longer patchy. Further increasing Tr during PCV led to rapid closure of most terminal airways (fraction of closed airways was 0.96) (Fig. 4).
Fig. 4.
Airway response and changes in airway opening pressure during progressive bronchoconstriction with PCV with Tr0 = 0.9. A: in this simulation, Tr was initially increased to 0.9 and kept constant to ascertain a steady state (1st to 900th breath). At that point, the ventilatory mode was transitioned to PCV with a value of Pi chosen such so that Vt of the breath immediately after a transition equaled the Vt during VCV (Vt0). Tr was progressively increased to a maximum activation value (Tr = 1). B: response to the changes in Tr of lumen radii of terminal airways normalized by their corresponding fully dilated radii (r/r0). Plots for 256 representative airway radii are presented in different colors for illustrative purposes. In the pseudo-ventilation images at 2 steady conditions, the values of acinar ventilation normalized by total ventilation were represented in a color scale. C: Response of Vt normalized by Vt0. D: response of Paop relative to PEEP. For explanation, see text.
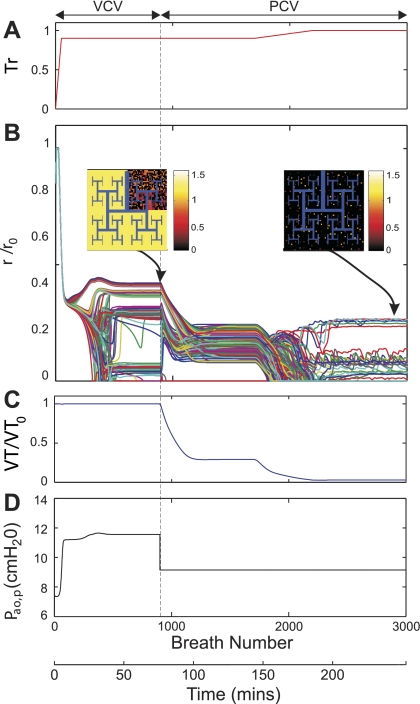
Fig. 5.
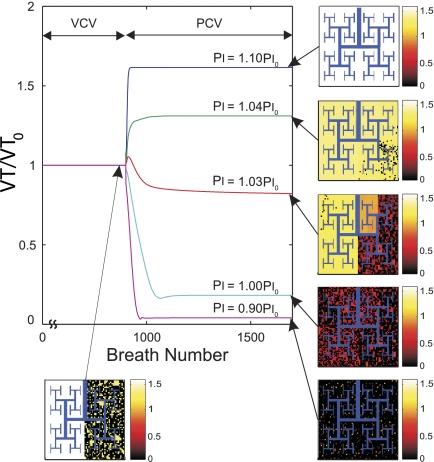
Effect of the setting of Pi during PCV on the Vt normalized by Vt0, the Vt during VCV, and ventilation distribution during maximal ASM activation (Tr0 = 1). Tr was initially increased to 1.0 and kept constant to ascertain a steady state (1st to 900th breath). At that point, the ventilatory mode was transitioned to PCV with Pi set at different values as a fraction of Pi0. Pi0 was the value of Pi such that Vt of the breath immediately after the transition to PCV remained Vt0. The pseudo-ventilation images illustrate the effect of different Pi on ventilation distribution. Note that slight variations in Pi can have dramatic effects on both the spatial distribution of ventilation and the total Vt delivered during steady state.
Sensitivity of Vt and regional ventilation to selection of Pi.
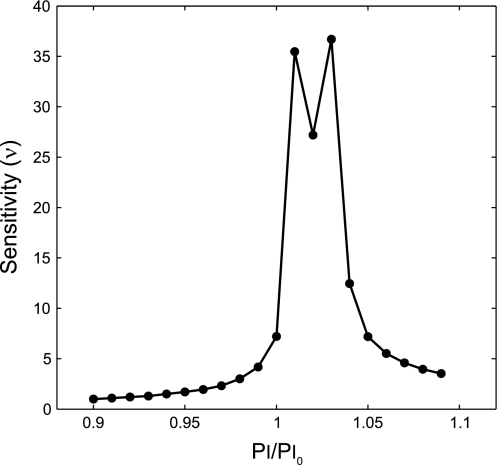
After the transition from VCV to PCV in conditions of Tr0 ≤ 0.8, both Vt and the regional distribution of ventilation remained steady, with Vtss linearly related to Pi. However, for Tr0 > 0.8, Vtss and the distribution of ventilation at steady state were highly and nonlinearly dependent on the chosen value of Pi (Fig. 6). Indeed, small differences in the setting of Pi led to large differences in the resultant Vtss and the distribution of ventilation (Fig. 5). For example, for Pi just 10% greater than Pi0, Vtss increased by 68% relative to Vt0 with PCV and all airways reopened to yield a uniform distribution of ventilation. Conversely, for Pi just 10% less than Pi0, there was a reduction of Vtss of more than 95% relative to Vt0 and virtual closure of most (78%) terminal airways. The maximum sensitivity of Vtss to the selection of Pi for PCV at Tr0 = 1 (Fig. 7) was almost 40 times greater than the sensitivity of the system with Tr0 ≤ 0.8.
Fig. 6.
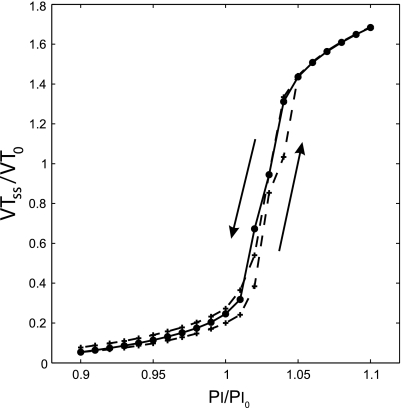
Steady-state Vt (Vtss) during PCV as a function of Pi for simulations with maximal ASM activation (Tr0 = 1). Vtss was normalized by the Vt during VCV (Vt0) and Pi by the Pi yielding a first-breath Vt following the transition equal to Vt0 (Pi0). The solid line connects data points obtained from different simulations that were run with different values of Pi/Pi0. The dashed line connects data points obtained from a single simulation that was run with successive step increases in Pi/Pi0 from 0.9 to 1.1 and successive step decreases from 1.1 back to 0.9 (the direction of change is indicated by arrows). Note that Vtss/Vt0 varies nonlinearly with Pi/Pi0 and changes in Pi particularly around Pi = 1.03 Pi0 lead to a very large change in total Vtss.
Fig. 7.
The sensitivity of Vtss to the selection of Pi was estimated from multiple simulation runs with independent changes in Pi (solid line in Fig. 6). Note that the sensitivity of Vtss to the values of Pi is exceptionally high around Pi = 1.03 Pi0.
Ventilation heterogeneity and ASM dynamic behavior.
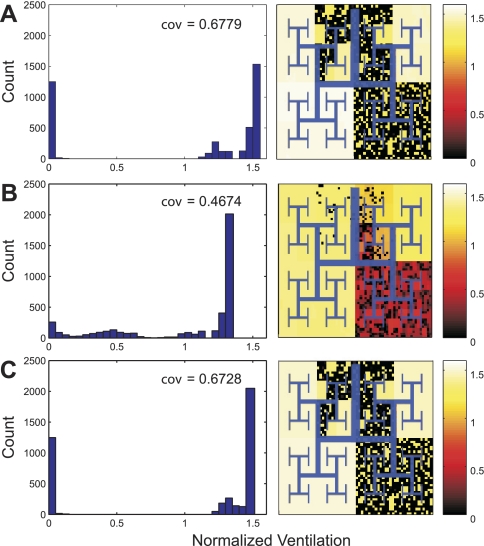
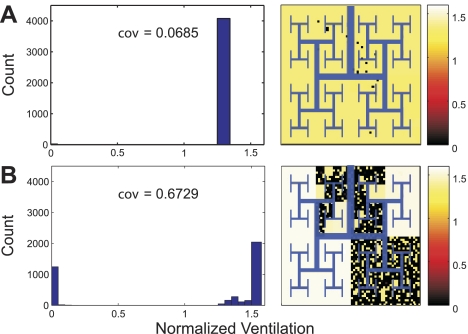
When airway radii were allowed to change according to the dynamic properties of active ASM, the ventilation heterogeneity was higher during VCV (Fig. 8A) than during PCV with Pi selected for equivalent Vtss (Pi = 1.031 Pi0) (Fig. 8B). Indeed, the coefficient of variation of regional ventilation during PCV was reduced by 31.05% compared with VCV, and the ventilation to severely hypoventilating units substantially decreased (left shift of the low ventilation mode in the histogram of Fig. 8B). In contrast, the distribution of ventilation remained virtually unchanged, and the ventilation heterogeneity was only minimally reduced when the dynamic properties of active ASM were ignored by keeping the airway radii constant during and after the transition from VCV to PCV (Fig. 8C). In this case, there was only a minor right shift of the middle mode of the ventilation histogram (Fig. 8C). Note also that the ventilation to severely constricted regions was virtually identical between VCV (Fig. 8A) and PCV when the dynamic properties of active ASM were ignored (Fig. 8C).
Fig. 8.
Histograms and spatial distributions of ventilation normalized by the average ventilation during VCV at maximal ASM activation (Tr0 = 1). Histograms and pseudo-ventilation images from 3 simulations with identical Vtss were compared. Each simulation was run under each of the following 3 conditions. A: during steady state, VCV before the transition to PCV. B: during steady state, after the transition from VCV to PCV with Pi selected so that Vtss equaled Vt0 (Pi = 1.031 Pi0) and the dynamic properties of active ASM were present. C: during steady state after the transition from VCV to PCV with Pi selected so that Vtss equaled Vt0 (Pi = 1.031 Pi0) and the dynamic properties of active ASM were absent. Note that the virtual patchiness and ventilation heterogeneity as measured by coefficient of variation (cov) are reduced during steady-state PCV (B and C), particularly when the dynamic properties of active ASM were present (B).
When Pi is set to a slightly higher value (Pi = 1.04 Pi0), an even greater difference in ventilation heterogeneity was observed between the model that included (Fig. 9A) and excluded (Fig. 9B) the dynamic properties of ASM. During steady state, the coefficient of variation of ventilation was nearly 10 times lower in the model that included the dynamic properties of active ASM (Fig. 9).
Fig. 9.
Histograms and spatial distributions of ventilation normalized by the average ventilation during VCV at maximal ASM activation (Tr0 = 1). Histograms and pseudo-ventilation images from 3 simulations of the identical Vtss were compared. Each simulation was run under each of the following 3 conditions. A: during steady state after the transition from VCV to PCV with Pi = 1.04 Pi0 and the dynamic properties of active ASM were present. B: during steady state, after the transition from VCV to PCV with Pi = 1.04 Pi0 and the dynamic properties of active ASM were absent. Note that, when the dynamic properties of active ASM were present (A), the ventilation heterogeneity as measured by cov was markedly lower than those when the dynamic properties of active ASM were absent (B).
DISCUSSION
Our simulations suggest that the total ventilation and spatial distribution during PCV could be substantially more sensitive to changes in smooth muscle activation level (Fig. 3) and Pi settings (Figs. 5 and 6) than previously anticipated. Such a high sensitivity was the result of the properties of the active ASM under dynamic forces and was much greater than that attributable to temporal differences predicted by models that ignore ASM dynamic properties. In practical terms, our findings imply that small changes in the settings of the mechanical ventilator or in the degree of bronchoconstrictive stimulus to the lung of asthmatic patients ventilated with PCV could result in unexpectedly large changes in the magnitude of the delivered Vt (increase or decrease), in airway behavior (full reopening of closed airways or catastrophic closure), and in ventilation distribution (elimination of patchiness).
Methodological considerations.
We used a computational network model of the lung that used realistic anatomic and physiological parameters for a healthy human (19). The model incorporated Weibel's (21) symmetric airway tree structure and virtually uniform anatomic and functional parameters for each generation of the airway tree. This idealized structure-function architecture served to demonstrate that the severe heterogeneity in ventilation during bronchoconstriction in asthma could be explained by interactions of positive and negative feedbacks among the system's components without invoking intrinsic component heterogeneity. Such a model was previously run with VCV, using a constant inspiratory flow and passive exhalation, to simulate an idealized spontaneously breathing person with enough respiratory drive and respiratory muscles capable of maintaining his/her ventilatory requirements during bronchoconstriction. Here, we took advantage of the same model but ran it with PCV to explore the sensitivity of the model when Pi, and not Vt, was kept constant during bronchoconstriction. To establish an initial state in the model, we first ran the simulations with VCV while Tr was progressively increased from a fully relaxed state to a baseline muscle tone. Once a steady state was achieved, ventilatory mode was switched to PCV with Pi set to deliver the next breath Vt equal to that during VCV and observed the dynamic changes in airway radii and ventilation to a new steady state. This simulated the case where Pi for PCV was set to keep ventilatory levels at transition equivalent to those with VCV. Transitioning to PCV such that Vt was similar to that during VCV helped us to eliminate the possibility that the known effect of Vt on bronchoconstriction and on the size of ventilation defects (19) was the cause of observed differences in airway behavior between ventilatory modes. However, note that because of the difference in the input function in both modes of ventilation, during heterogeneous conditions, Vt and its regional distribution were not at steady state after the transition to PCV but underwent a transient period before settling into a new steady state at a substantially reduced value (Figs. 4 and 5).
For the smooth muscle activation beyond criticality (Tr > 0.8), a transition from VCV to PCV caused major changes in ventilation. Delivered Vt during PCV could not be maintained equal to that during VCV, even though Pi was selected to deliver equal Vt in the breath immediately after the transition to PCV. In fact, a slightly larger value of Pi had to be chosen so that Vtss during PCV would equal Vt0.
Transition from VCV to PCV.
After the transition from VCV to PCV, the system behaved differently depending on the baseline level of smooth muscle activation (Tr0).
For Tr0 ≤ 0.8, the system started off constricting uniformly. After the transition from VCV to PCV, the system remained uniform with a stable Vt. As Tr was further increased during PCV, airways continued to constrict uniformly with constant Vt, and, at a certain point, Vt rapidly dropped as accelerated airway constriction led to full airway closure (Fig. 3). This behavior can be explained based on the positive feedback mechanism implicit in the basic building block of our model (1). To understand this behavior, let us consider first an equivalent lung model of parallel pathways where each path to a terminal unit is represented by resistance element connected to a compliant alveolar element. Assuming this model is driven at constant frequency (f), the flow rate and volume delivered into each branch (i) at a given pressure amplitude depends on the magnitude of the pathway impedance |Zi| = Ri2 + (1/2πfCi)2 where Ci and Ri are the equivalent compliance and resistance for each pathway, respectively. For low levels of Tr and ventilation at normal f in this network, since Ri ≪ 1/2πfCi, Zi is mostly dependent on Ci, and thus Vt and its distribution among branches are relatively independent of Tr. This behavior is apparent during the period of the breaths 500–1,000 in Fig. 3. However, as Tr is further increased and airway caliber reduced, Ri becomes an increasingly important contribution to pathway impedance and Vt begins to drop. As Vt is reduced, the tidal tethering forces on the airways decreased, further constricting them, increasing R and reducing Vt. Eventually, ventilation delivered to each of the branches in the network catastrophically collapses. This effect is manifested differently during VCV. In VCV, total Vt is kept constant, and, as Tr is increased above a critical level, the reduction of flow to a slightly overconstricted branch results in an increase in pressure gradient and a redistribution of flow toward slightly less constricted branches. This shift in regional expansion affected both parent and daughter branches, resulting in the patchiness of ventilation shown in Fig. 2 as previously described (19).
In simulations with Tr0 > 0.8, the system under VCV started in a highly heterogeneous state with a patchy distribution of ventilation. Under this condition, the transition to PCV was not stable and was highly dependent on the value of Pi selected for PCV (Figs. 4 and 5). Indeed, the total Vt and the distribution of ventilation changed in the subsequent breaths following the transition, even though PCV was set to provide a first-breath Vt equal to that during VCV and Pi was kept constant. This behavior is not entirely surprising since, in contrast to the case of Tr0 ≤ 0.8, the distribution of ventilation before the transition was not uniform and thus expected to change due to the difference in the driving signal (16). PCV maintains airway opening pressure at a high constant level during inspiration, whereas in VCV airway pressure is initially low and increases progressively with lung volume inhalation. Therefore, the driving signal in PCV should promote more flow to regions with prolonged time constant (high RiCi) than that in VCV. Moreover, in contrast with VCV, in PCV as some airways constricted, flow is not redirected to less constricted airways, potentially reducing ventilation heterogeneity relative to that during VCV (Fig. 5). However, the reasoning based exclusively on linear networks (4, 8, 11) failed to explain the magnitude of the response of our model. In fact, when airways were kept frozen in the model during the transition from VCV to PCV, the resulting reduction in ventilation heterogeneity was substantially less than that observed when airway dimensions were dynamically determined by the properties of active ASM (Figs. 8 and 9).
The simulation data also showed that the sensitivity of Vt to Pi during PCV was almost 40 times greater in the model with the dynamic properties of ASM than in the model with no dynamic properties of ASM. As a result, differences in Pi of ±10% during PCV could lead to dramatic differences in both Vt and the distribution of ventilation from severe hypoventilation to hyperventilation. Worth noticing was the fact that a change of only 1% in Pi (from 1.03 Pi0 to 1.04 Pi0) resulted not only in an elevation of 30% in Vt but also in the virtual elimination of patchiness in ventilation (Fig. 5).
This model behavior could explain clinical observations by López-Herce and colleagues (7, 14) of rapid and significant improvements in arterial Pco2 and oxygenation during PCV. They found in two children with severe asthma, whose level of gas exchange had not improved during 6 h of VCV, that a change to PCV resulted in fast improvements in blood gases (7). The results from our simulations might explain those improvements as resulting from an improvement in ventilation distribution. Motivated by that case report work, Sarnaik et al. studied 40 patients with severe asthma who were intubated and mechanically ventilated with PCV. They also found significant improvements in blood gases after an initiation of mechanical ventilation (14). In both papers, the authors speculated that improvements in blood gases with PCV were due to the improvement in ventilation distribution expected from the enhanced pressure equilibration between alveolar units with heterogeneous time constants. As we discussed above, in a severely constricted network, pressure equilibration alone resulted in changes in ventilation heterogeneity much smaller than those seen when we incorporated the dynamic properties of active ASM in the network model. Switching from VCV to PCV with airway dimensions unaffected by dynamic forces only minimally reduced the heterogeneity in ventilation. Therefore, we believe that clinical improvements seen in PCV may have resulted from the dilating effects of tidal breathing on the ASM, rather than from just pressure equilibration.
Increasing level of bronchoconstriction.
Inspiratory flow is generated by the difference between airway opening and pleural pressure, referred to as transpulmonary pressure. During mechanical ventilation with PCV, this is accomplished by specifying the ventilator's driving pressure, Pi. Our model predicts that, in a severe asthma attack, if Pi is insufficient for maintaining Vt, catastrophic airway closure may follow (Fig. 3). In a spontaneously breathing person, the increase in transpulmonary pressure is generated by a reduction in pleural pressure. Therefore, during severe airway constriction, a compensatory reduction in inspiratory pleural pressure by respiratory muscles is required to maintain ventilation. In our model, this is equivalent to the compensatory increase in driving pressure inherent to VCV, which leads to a redistribution of Vt to regions of the lung with relatively less constriction. This redistribution can be thought of as a long-range negative feedback that causes airway dilatation of relatively well-ventilated airways. This long-range negative feedback combined with the short-range positive feedback results in a patchiness of ventilation distribution similar to that observed in several complex systems in nature (13). It should be noted that, despite the patchiness if the respiratory muscles generate enough pressure, Vt can be maintained and total closure of all airways can be prevented. However, during increased airway obstruction if respiratory muscles become fatigued and are unable to maintain ventilation, airflow will not be redistributed to ventilating regions. This will eliminate the long-range negative feedback and thus the compensatory airway dilation, eventually leading to catastrophic closure of all airways.
Ventilation heterogeneity and ASM dynamic behavior.
From our results, we conclude that the dynamic behavior of active ASM may play an important role in reducing heterogeneity of ventilation and magnifying the Vt sensitivity to Pi during PCV. The significant reduction in ventilation heterogeneity when active ASM behavior was included in our model reaffirmed our hypothesis that positive feedback might contribute to nonlinearity in airway response under PCV. The data also suggest that the sensitivity to Pi in PCV could be advantageously exploited to improve ventilation and its uniformity by judicious adjustments in the ventilator pressure settings.
Model assumptions.
The network model is structurally symmetric. Tr could vary breath by breath but was set to be uniform throughout the airway tree. Symmetry breaking was achieved by adding a small random variation in wall thickness (1% coefficient of variation) to all airways. The purpose of this approach was to evaluate the effects caused purely by the dynamic behavior of the ASM independent of other preexisting heterogeneity. Variations of smooth muscle activation within the airway tree, regional differences in pleural pressure within the thorax, and the asymmetry of the airway tree were not included in our network model. We expect that these features, if incorporated into the model, would precipitate symmetry breaking and the formation of ventilation defects at lower values of Tr. Furthermore, they would promote the systematic localization of ventilation defects and magnify the patchiness in ventilation during VCV. As a result, to reduce ventilation heterogeneity during PCV, the setting of Pi may have to be increased relative to that predicted by this model. Also, the presence of structural or functional parameter heterogeneity could be expected to reduce the overall Vt sensitivity of the system, which in the limit would behave as one with fixed obstructions and minimal dynamic effects of ASM.
Additional reasons that Vt sensitivity to Pi in a real lung could be different from that predicted by this symmetric network model are discussed here. Although the model incorporates structural and functional features that are consistent with a human lung, it can be expected to exhibit general behaviors of a complex branching network in conditions within the physiological range. However, the effects of some assumptions on the model's complex behavior are difficult to predict. For example, the model assumes linear chest wall and parenchyma compliances and ignores the passive characteristics of the airway wall. Nonlinear mechanical properties of these elements could affect the magnitude of the predicted sensitivity in different ways. On the one hand, it is well known that the passive properties of the respiratory system become stiffer at low and high lung volumes. This could result in an even greater sensitivity of Vt to the Pi setting than those predicted for a constant compliant chest wall under physiological conditions. Likewise, nonlinear changes in parenchymal tethering forces with lung volume could result in higher than predicted Vt sensitivity. Also, the potential formation of liquid bridging (6), not included in this model, could result in rapid airway closure without luminal cross-sectional area being reduced to zero. This phenomenon, ignored in our model, could further increase the Vt sensitivity to changes in Pi by precipitating airway closure as Pi is reduced.
On the other hand, the passive nonlinear characteristics of the airway wall could tend to reduce the magnitude of the Vt sensitivity by acting to stabilize drastic changes in airway lumen. This effect would be more likely prominent in central airways, which are protected by cartilaginous rings or plates, and less important in peripheral airways, which are simulated in this model (generations 4 to 16 of the human airway tree). Given the opposing effects of the different assumptions on the model, it is difficult to speculate whether the Vt sensitivity to Pi, during PCV predicted for our bronchoconstricted model, could be an over- or an underestimation of the sensitivity in a real lung. Thus these predictions have to await experimental validations.
In summary, our simulations with an integrative network model of the lung provide new insights into complex interactions among airways and explore the impact of ventilation mode, or breathing pattern, on the stability of ventilation and its spatial distribution within the asthmatic lung. In contrast to VCV, which ensures the total ventilation but results in a patchy distribution of regional ventilation during bronchoconstriction, PCV results in a more uniform distribution, but the total ventilation could be highly sensitive to minor changes in driving pressure. Although the simplifications inherent to a theoretical model limit its direct clinical application, the insights derived from these simulations may provide a theoretical foundation to guide the selection of ventilation mode, the adjustment of ventilator settings for patients with asthma, and the interpretation of clinical observations.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grants HL-068011 and HL-087281.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Anafi RC, Wilson TA. Airway stability and heterogeneity in the constricted lung. J Appl Physiol 91: 1185–1192, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Brown RH, Mitzner W. Airway response to deep inspiration: role of nitric oxide. Eur Respir J 22: 57–61, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Fredberg JJ, Inouye D, Miller B, Nathan M, Jafari S, Raboudi SH, Butler JP, Shore SA. Airway smooth muscle, tidal stretches, and dynamically determined contractile states. Am J Respir Crit Care Med 156: 1752–1759, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Gillis HL, Lutchen KR. How heterogeneous bronchoconstriction affects ventilation distribution in human lungs: a morphometric model. Ann Biomed Eng 27: 14–22, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Gunst SJ. Contractile force of canine airway smooth muscle during cyclical length changes. J Appl Physiol 55: 759–769, 1983 [DOI] [PubMed] [Google Scholar]
- 6. Halpern D, Grotberg JB. Surfactant effects on fluid-elastic instabilities of liquid-lined flexible tubes: a model of airway closure. J Biomech Eng 115: 271–277, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Lopez-Herce J, Gari M, Bustinza A, de Lucas N, Carrillo A. On pressure-controlled ventilation in severe asthma. Pediatr Pulmonol 21: 401–403, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Lutchen KR, Gillis H. Relationship between heterogeneous changes in airway morphometry and lung resistance and elastance. J Appl Physiol 83: 1192–1201, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Macklem PT. The physiology of small airways. Am J Respir Crit Care Med 157: S181–S183, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Musch G, Layfield JD, Harris RS, Melo MF, Winkler T, Callahan RJ, Fischman AJ, Venegas JG. Topographical distribution of pulmonary perfusion and ventilation, assessed by PET in supine and prone humans. J Appl Physiol 93: 1841–1851, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Nucci G, Suki B, Lutchen K. Modeling airflow-related shear stress during heterogeneous constriction and mechanical ventilation. J Appl Physiol 95: 348–356, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Peslin R, Fredburg J. Oscillation mechanics of the respiratory system. In: Handbook of Physiology. The Respiratory System. Mechanics of Breathing. Bethesda, MD: Am. Physiol. Soc., 1986, sect. 3, vol. III, pt. 1, chapt. 11, p. 145–178 [Google Scholar]
- 13. Rietkerk M, Dekker SC, de Ruiter PC, van de Koppel J. Self-organized patchiness and catastrophic shifts in ecosystems. Science 305: 1926–1929, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Sarnaik AP, Daphtary KM, Meert KL, Lieh-Lai MW, Heidemann SM. Pressure-controlled ventilation in children with severe status asthmaticus. Pediatr Crit Care Med 5: 133–138, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Shen X, Wu MF, Tepper RS, Gunst SJ. Mechanisms for the mechanical response of airway smooth muscle to length oscillation. J Appl Physiol 83: 731–738, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Tobin MJ. Principles and Practice of Mechanical Ventilation. New York: McGraw-Hill, 1994 [Google Scholar]
- 17. Venegas JG. Viscoelastic properties of the contracting detrusor. I. Theoretical basis. Am J Physiol Cell Physiol 261: C355–C363, 1991 [DOI] [PubMed] [Google Scholar]
- 18. Venegas JG, Schroeder T, Harris S, Winkler RT, Melo MF. The distribution of ventilation during bronchoconstriction is patchy and bimodal: a PET imaging study. Respir Physiol Neurobiol 148: 57–64, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Venegas JG, Winkler T, Musch G, Vidal Melo MF, Layfield D, Tgavalekos N, Fischman AJ, Callahan RJ, Bellani G, Harris RS. Self-organized patchiness in asthma as a prelude to catastrophic shifts. Nature 434: 777–782, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Venegas JG, Woll JP, Woolfson SB, Cravalho EG, Resnick N, Yalla SV. Viscoelastic properties of the contracting detrusor. II. Experimental approach. Am J Physiol Cell Physiol 261: C364–C375, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Weibel ER. Morphometry of the human lung: the state of the art after two decades. Bull Eur Physiopath Respir 15: 999–1013, 1979 [PubMed] [Google Scholar]
- 22. Winkler T, Venegas JG. Complex airway behavior and paradoxical responses to bronchoprovocation. J Appl Physiol 103: 655–663, 2007 [DOI] [PubMed] [Google Scholar]