Abstract
Human skin blood flow responses to body heating and cooling are essential to the normal processes of physiological thermoregulation. Large increases in skin blood flow provide the necessary augmentation of convective heat loss during environmental heat exposure and/or exercise, just as reflex cutaneous vasoconstriction is key to preventing excessive heat dissipation during cold exposure. In humans, reflex sympathetic innervation of the cutaneous circulation has two branches: a sympathetic noradrenergic vasoconstrictor system, and a non-noradrenergic active vasodilator system. Noradrenergic vasoconstrictor nerves are tonically active in normothermic environments and increase their activity during cold exposure, releasing both norepinephrine and cotransmitters (including neuropeptide Y) to decrease skin blood flow. The active vasodilator system in human skin does not exhibit resting tone and is only activated during increases in body temperature, such as those brought about by heat exposure or exercise. Active cutaneous vasodilation occurs via cholinergic nerve cotransmission and has been shown to include potential roles for nitric oxide, vasoactive intestinal peptide, prostaglandins, and substance P (and/or neurokinin-1 receptors). It has proven both interesting and challenging that no one substance has been identified as the sole mediator of active cutaneous vasodilation. The processes of reflex cutaneous vasodilation and vasoconstriction are both modified by acute factors, such as exercise and hydration, and more long-term factors, such as aging, reproductive hormones, and disease. This review will highlight some of the recent findings in these areas, as well as interesting areas of ongoing and future work.
Keywords: temperature regulation, sympathetic nervous system, circulation, human
one of the most striking features of the human cutaneous circulation is the wide range of blood flow this circulation is capable of attaining. Human skin blood flow can range from almost zero (in conditions of whole body and/or local cooling) to up to 8 l/min (or ∼60% of cardiac output) in conditions of severe heat stress (38, 62). The skin circulation, therefore, has the complex capability of moving from very high to very low blood flows and controlling all levels in between to match the integrative requirements of human physiology. The level of blood flow in a given environmental or exercise condition is controlled by a complex interplay of reflex (whole body) and local control mechanisms, which influence both skin blood flow and each other. The focus of this review is on reflex control of skin blood flow in humans, with brief discussions of some short- and long-term modifiers of this control. The mechanisms involved specifically in local control of skin blood flow are the focus of another review in this Highlighted Topics series (35a). This mini-review series is designed to provide a brief, nonexhaustive overview of each topic area. For further information on topics related to reflex control of skin blood flow, the interested reader is referred to several excellent reviews (10, 21, 27, 33, 38, 83).
The human skin circulation is best known for its role in thermoregulation. The ability of skin blood flow to reach such high levels during body heating is necessary to increase convective heat transfer from the body core to the surface of the skin, and the resultant heat dissipation (in conjunction with sweating) is essential to the maintenance of normal body temperatures. Increased skin blood flow leads to increased skin blood volume, due to the arrangement of venous plexuses close to the skin surface (38, 62). Thus, with reflex cutaneous vasodilation, more blood is transferred from the core to the surface of the skin, where heat transfer can occur. Under optimal conditions, the skin is cooled by evaporation of sweat, and the thermal gradient at the skin allows heat to dissipate from the blood to the skin and to the environment. The cooler blood is then transferred back to the body core, where it minimizes increases in core temperature that occur during exercise and/or environmental heat exposure.
MECHANISMS OF REFLEX CUTANEOUS VASOCONSTRICTION AND VASODILATION
Reflex Cutaneous Vasoconstriction
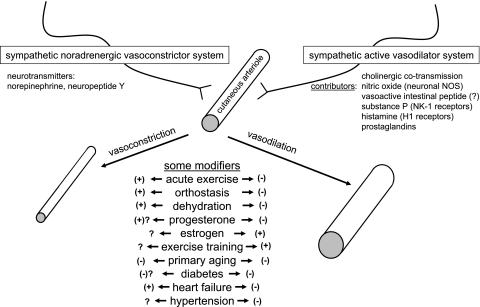
The reflex innervation of the human skin circulation occurs via two branches of the sympathetic nervous system (Fig. 1). Sympathetic noradrenergic vasoconstrictor nerves provide tonic innervation, contributing to a relatively low skin blood flow at rest in normothermic environments (∼250 ml/min) (3). Thus, in resting subjects in normothermic environments, interruption of sympathetic noradrenergic innervation of the skin [by various methods, including proximal nerve block and/or presynaptic inhibition with bretylium (described below)] usually causes a small, passive vasodilation, due to withdrawal of the tonic activity of vasoconstrictor nerves. The extent of the passive vasodilation due to vasoconstrictor withdrawal depends on the thermal environment of the “baseline” condition (which leads to the extent of vasoconstrictor tone). Thus, in warmer environments, there may be little to no passive vasodilation with interruption of vasoconstrictor innervation, whereas, in cooler environments, this vasodilation would be more pronounced. Even in cool environments, however, this passive vasodilation is severalfold smaller than the reflex vasodilation that occurs during whole body heating or exercise.
Fig. 1.
Major contributors to reflex neurogenic cutaneous vasoconstriction and vasodilation in humans, along with a summary of some potential modifiers of these processes. Many of these areas are incompletely understood, as indicated by “?”, and, therefore, are important topics for future research. NOS, nitric oxide synthase; NK-1, neurokinin-1.
Sympathetically mediated cutaneous vasoconstriction represents the “first line of defense” during exposure to cold environmental temperatures. Decreases in mean skin and/or internal temperatures cause reflex activation of sympathetic vasoconstrictor nerves, resulting in cutaneous vasoconstriction and decreases in skin blood flow (9, 75). Mechanisms involved in reflex cutaneous vasoconstriction are now recognized to be more complex than previously thought. Several studies have used prolonged ramp cooling protocols (15–45 min) in conjunction with pharmacological blockade to identify specific contributors and their roles in the vasoconstrictor response over a range of skin temperatures. In addition to norepinephrine-mediated vasoconstriction, Stephens et al. identified a role for noradrenergic cotransmitters in young men (75) by using combinations of local presynaptic and postsynaptic (combined α1 and α2 inhibition) pharmacological blockade. Presynaptic inhibition of noradrenergic nerves was accomplished by local iontophoresis of bretylium tosylate, which is taken up specifically into presynaptic noradrenergic nerve terminals, where it blocks all neurotransmission from those nerves (26, 43). Using these approaches, Stephens et al. (75) showed that complete postsynaptic blockade of norepinephrine-mediated vasoconstriction did not completely inhibit the reflex vasoconstrictor response to 15 min of progressive decreases in skin temperature, although presynaptic inhibition with bretylium abolished the response. This suggested the existence of cotransmitter-mediated vasoconstriction, which was later shown by the same group to be most likely mediated by neuropeptide Y, since antagonism of this peptide with BIBP-3226, along with double blockade of adrenergic receptors, abolished the reflex vasoconstrictor response (77). The authors also noted that the involvement of other vasoconstrictor cotransmitters, including ATP, should not be ruled out (77). Intracellular signaling for reflex cutaneous vasoconstriction includes activation of the Rho kinase pathway, the role of which becomes more prominent in older individuals (53).
Reflex Cutaneous Vasodilation
The question of what mediates the enormous increases in skin blood flow during heat stress has been an area of intense study (and some debate) over many decades. Dual sympathetic innervation of the skin (vasoconstrictor as well as vasodilator nerves) was first demonstrated in the 1930s (23, 56). Sympathetic active vasodilator nerves do not exhibit tonic activity in normothermia, but, once activated during hyperthermia, are responsible for most (up to ∼90%) of the substantial vasodilation that can occur (38, 62). The exact mechanisms for active vasodilation in human skin have proved elusive; however, several component mechanisms have been identified over the past ∼20 yr.
Because active vasodilation and sweating are both important mechanisms of heat dissipation during whole body hyperthermia, investigators have proposed mechanistic links between the two neural mechanisms (19, 20, 23). Earlier ideas were that cholinergic sudomotor nerves either caused active vasodilation themselves, or that they activated another substance (notably bradykinin), which then caused active vasodilation (19, 23). Several investigators subsequently demonstrated that, while postsynaptic muscarinic receptor blockade with atropine completely abolishes sweating, it causes only minor delays or decreases in cutaneous active vasodilation (46, 51, 61). Furthermore, direct intradermal blockade of bradykinin receptors does not alter skin blood flow responses during core hyperthermia, providing further evidence that bradykinin itself does not have a role (44).
A link to cholinergic innervation was demonstrated by Kellogg et al. (46), who used local administration of botulinum toxin to presynaptically block neurotransmission from cholinergic nerves. Botulinum toxin abolished active cutaneous vasodilation during body heating, whereas an attenuated vasodilator response occurred at sites pretreated with atropine (postsynaptic muscarinic receptor blockade), as in previous work (46). Taking together the evidence that presynaptic inhibition of cholinergic neurotransmission, but not postsynaptic muscarinic receptor blockade, blocked cutaneous active vasodilation, the authors concluded that an unidentified cholinergic cotransmitter was responsible for the vasodilator mechanism (46).
Whether the cholinergic nerves in question are the same as the sudomotor nerves causing sweating in a given area is unclear, but appears unlikely based on existing evidence. Although the two responses occur generally in the same time frame (i.e., humans begin to dissipate heat when core temperature has increased by a few tenths of a degree Celsius), the temporal relationship between the onset (threshold) for sweating and the threshold for active vasodilation is not constant: one may occur before, after, or concurrently with the other in a given subject. Evidence against a single neural source for sweating and active vasodilation also comes from studies in which thresholds for sweating and thresholds for active vasodilation can shift independently of each other due to acute perturbations. For example, acute exercise shifts the core temperature threshold for active cutaneous vasodilation to higher internal temperatures, but does not affect the threshold for sweating (42).
More recent attempts to elucidate specific substance(s) responsible for cutaneous active vasodilation point to multiple possible contributors, reminiscent of multiple redundant mechanisms of vasodilation in exercising skeletal muscle (39). There is a significant contribution of nitric oxide (NO) to active cutaneous vasodilation, which is variable among individuals and averages ∼30% in young healthy people (40, 69, 70). Recent data from Kellogg et al. (47) suggest that the NO component of reflex cutaneous vasodilation appears to originate primarily from neuronal NO synthase (NOS) rather than endothelial NOS. However, inhibition of NO synthesis does not abolish active cutaneous vasodilation, indicating the existence of other vasodilator pathways that work in synergy with and/or complement the NO vasodilation pathway.
An attractive candidate for “the” substance that causes active vasodilation in human skin has been vasoactive intestinal peptide (VIP), due to its known role as a vasodilator and its colocalization in cholinergic nerve terminals. A role for VIP in human active vasodilation was suggested by a report of diminished active vasodilation during intradermal microdialysis of the VIP antagonist VIP10–28 (1). In a subsequent study, Wilkins et al. (89) did not find a decrease in active vasodilation during administration of VIP10–28. It is relevant in this context that the role of VIP in human skin has proven challenging to investigate due to limited receptor affinity of the available antagonist (1) and due to technical challenges associated with administration of a peptide via microdialysis (89). Interestingly, in people with cystic fibrosis, in whom VIP levels in the skin are diminished, active cutaneous vasodilator responses appear to be normal (63, 88). However, since VIP is not completely absent in cystic fibrosis patients, this observation in and of itself does not rule out VIP as a potential contributor. Thus the role of VIP in active cutaneous vasodilation remains a mystery.
Another potential contributor to the mechanism of active cutaneous vasodilation is substance P (91). Substance P has been localized in human skin (28, 34), causes vasodilation, which includes a NO component, and binds with high affinity to neurokinin-1 (NK-1) receptors (91). Wong and Minson (91) studied the role of substance P and NK-1 receptors in cutaneous active vasodilation using the approach of desensitization of NK-1 receptors via prior administration of substance P. They found that desensitized sites showed a decrease of ∼35% in active vasodilation, suggesting that substance P (or another NK-1 agonist) is a significant contributor to active vasodilation (91). The authors did not use a NK-1 antagonist specifically, due to concerns regarding nonspecific effects of available NK-1 receptor antagonists. It remains unclear whether “cleaner” NK-1 antagonists would cause effects similar to those seen with desensitization of NK-1 receptors, and whether substance P or some other NK-1 agonist is the physiological vasodilator contributing to the results they observed (91).
Additional vasodilators that have been shown to have roles in human active vasodilation are histamine (via H1 receptor activation) (92) and vasodilator prostanoids (58). Wong et al. (92) demonstrated that H1 receptor activation contributes to active vasodilation by showing a significant decrease in cutaneous vascular responses to whole body heating during blockade of H1 receptors with pyrilamine. Combined pyrilamine plus NG-nitro-l-arginine methyl ester (l-NAME) did not cause a further inhibition, suggesting that H1 activation contributes to a portion of the NO component of active vasodilation (92). H2 receptor blockade with cimetidine did not alter the reflex vasodilation. To evaluate a role for vasodilator prostaglandins in reflex cutaneous vasodilation, McCord et al. (58) used intradermal microdialysis of ketorolac (a nonselective cyclooxygenase inhibitor) and of l-NAME (NOS inhibitor). They noted that ketorolac and l-NAME each caused significant inhibition of the reflex vasodilator response, and that combined ketorolac + l-NAME resulted in further inhibition compared with either inhibitor alone. Interestingly, cyclooxygenase inhibition did not have any influence on the vasodilator response to local warming of the skin (58), emphasizing the distinct sets of mechanisms associated with the two cutaneous vasodilator responses (35a).
Thus a synthesis of the work of many laboratories over several decades shows a complex mechanism of control of skin blood flow during whole body (reflex) heating (Fig. 1, right). Sympathetic neurogenic vasodilation in human skin is an active process, and the mechanism involves cholinergic nerve cotransmission. Several specific vasodilators appear to be involved in the mechanism, which includes roles (or potential roles) for NO, VIP, substance P/NK-1 receptors, histamine, and prostaglandins. The implications of these multiple mechanisms, and interactions among them, remain exciting areas for future research.
MODIFIERS OF REFLEX CUTANEOUS VASODILATION AND VASOCONSTRICTION
Acute/Short-Term Modifiers
Acute exercise.
Performing muscular exercise is a common way to cause “endogenous” increases in core temperature and elicit reflex cutaneous vasodilation and sweating. When studying the skin circulation, however, it is important to recognize that the exercise itself causes reflex responses, which are separate from those of hyperthermia, and can modify vasodilator responses to heat stress. In normothermic or hyperthermic conditions, the onset of exercise causes a reflex vasoconstriction in the skin, as part of redistribution of blood from nonworking tissues to active skeletal muscle (35, 36). However, prolonged dynamic exercise usually causes sufficient core hyperthermia to elicit reflex neurogenic vasodilation in the skin. During exercise, therefore, the skin must “compete” with active skeletal muscle for blood flow, and thus the response is modified compared with similar levels of hyperthermia in resting humans (37, 42, 81). Specifically, acute dynamic exercise shifts the threshold for reflex vasodilation to higher internal temperatures (37, 42, 81). The extent of the shift is dependent on the intensity of the exercise, such that higher intensity exercise is associated with a more delayed onset of cutaneous vasodilation (81). Furthermore, the neural mechanism for the exercise-related shift in cutaneous vasodilation is a shift in active vasodilation (rather than augmented vasoconstrictor activity), since bretylium-treated sites were shown to have similar threshold shifts as untreated sites during acute dynamic exercise (42). Additionally, at core temperatures above 38°C during exercise, skin blood flow becomes less responsive to further increases in internal temperature, and the relation between the two variables plateaus or exhibits a significant decrease in slope (2).
Orthostasis.
During upright posture (orthostasis), activation of the arterial baroreflex results in an increase in heart rate and peripheral vasoconstriction to maintain perfusion pressure to the brain in the face of increased gravitational forces, which promote venous pooling in dependent limbs, and a decrease in venous return. The skin circulation participates in the systemic vasoconstrictor response, demonstrating reflex vasoconstriction (measured as a decrease in cutaneous vascular conductance) in both thermoneutral and warm environments when exposed to orthostatic stress [such as lower body negative pressure (LBNP)] (11, 41). Based on measurements of cutaneous vascular conductance with and without bretylium blockade of noradrenergic neurotransmission, the decrease in skin blood flow with LBNP during body heating appears to be due to a withdrawal of active vasodilator input to the skin (11, 41), whereas increased vasoconstrictor activity causes the vasoconstriction with LBNP in normothermia (41). It is relevant to note in this context that there is local heterogeneity in cutaneous vascular responses to LBNP. Mack (57) used laser Doppler scanning techniques and reported that some areas decreased and others actually increased vascular conductance during LBNP.
Despite several reports of cutaneous vasoconstriction during orthostatic stress, measurements of skin sympathetic nerve activity have produced mixed results, showing either no change (86) or a small decrease (15) in skin sympathetic nerve activity with various orthostatic challenges during mild heat stress, and no change during pronounced heat stress (13). This is likely due to the fact that integrated measurements of sympathetic outflow to the skin include four nerve types, sudomotor, vasodilator, vasoconstrictor, and pilomotor, and it is difficult to distinguish among these when conducting integrated recordings in intact humans (13).
Hydration/osmolality.
Endurance exercise is often associated with dehydration, particularly in prolonged events like distance running, where availability of fluids during the event or practice may be limited. Exposure to hot environments exacerbates this tendency and can lead to more rapid dehydration due to increased sweat loss. Dehydration and associated hyperosmolality cause decreases in heat dissipation responses, including both skin blood flow and sweating, leading to increased risk of hyperthermia and often to impaired exercise performance (59, 64, 65).
In a classic study, Nadel and colleagues (59) showed that the internal temperature threshold for cutaneous vasodilation was shifted to higher internal temperatures in individuals dehydrated by ∼3% body weight. Furthermore, the sensitivity (or responsiveness) of the vasodilation as a function of internal temperature was decreased in the dehydrated group. The delay in threshold and decrease in sensitivity of the skin blood flow response with dehydration have been seen in many subsequent studies of hypovolemia (16, 17). These changes result in lower skin blood flow during prolonged exercise in the heat compared with similar exercise in a euhydrated condition (22). Hyperosmolality per se appears to be an important component of the signal for delayed cutaneous vasodilation during dehydration, since hyperosmolality even in the absence of hypovolemia causes delayed cutaneous vasodilation (17, 71). Recent work from Shibasaki and colleagues (71) showed that hyperosmolality has the specific effect of shifting the threshold for onset of active cutaneous vasodilation to higher internal temperatures. Interestingly, hyperhydration does not appear to confer a particular advantage in thermoregulatory responses, resulting in skin blood flow and other thermoregulatory responses that are not different from a control euhydrated condition (54, 55, 59).
Chronic/Long-Term Modifiers of Reflex Cutaneous Vasodilation and Vasoconstriction
Menstrual cycle/female reproductive hormones.
Resting body temperature changes during the course of the menstrual cycle, being ∼0.5°C higher in the midluteal phase (when progesterone and estrogen are elevated) compared with the early follicular phase when both hormones are low. Additionally, careful measurement can sometimes detect a small decrease in resting body temperature during the preovulatory phase, when estrogen is elevated unopposed by progesterone (78). These changes in core body temperature are mirrored by shifts in the reflex thermoregulatory control of skin blood flow, which appear to support the shifts in body temperature. For example, the threshold for the onset of cutaneous vasodilation and sweating is shifted to higher internal temperatures in the midluteal phase of the menstrual cycle (79). This shift also occurs with the exogenous progestins and estrogens found in oral contraceptives (8, 25, 74) and persists in the presence of local noradrenergic inhibition with bretylium, demonstrating a shift in control of the active vasodilator system to higher internal temperatures with these hormones (8). Furthermore, the increase in body temperature and the associated shifts in control of skin blood flow and sweating responses are not a classical “fever” in the sense that neither temperature nor skin blood flow responses were altered by cyclooxygenase inhibition with ibuprofen (6). Another interesting influence of luteal phase hormones is that they appear to increase the plateau level of skin blood flow during exercise in a warm environment (52). This may offset the effect of the increased threshold for vasodilation seen during the luteal phase in terms of overall heat dissipation during prolonged exercise.
Also of interest are influences of hormone replacement therapy on skin blood flow responses in women after the menopause. Estrogen replacement therapy (without concomitant progesterone) causes a decrease in the threshold for cutaneous vasodilation during body heating (4, 80), whereas including a progesterone component appeared to reverse this effect (4). In general, with regard to specific effects of each hormone, estrogen appears to have the effect of decreasing body temperature and thermoregulatory thresholds, whereas progesterone appears to oppose these influences and promote an increase in body temperature (7, 74, 78).
With regard to vasoconstrictor responses, the progressive cutaneous vasoconstriction during a 15-min ramp cooling protocol (as a function of skin temperature) was similar in low- and high-hormone phases of oral contraceptive use, but was shifted such that a higher internal temperature was maintained throughout cooling in the high-hormone phase (9). In a later study, Stephens et al. (76) showed that the contribution of noradrenergic cotransmitters to reflex cutaneous vasoconstriction was altered as a function of reproductive hormone status in women. The contribution of cotransmitters demonstrated in men (75) was shown to occur in women during the high-hormone phase of oral contraceptive use, but did not occur during the low-hormone phase, suggesting a role of reproductive hormones to alter cotransmitter-mediated vasoconstriction in young women.
Exercise training and heat acclimation.
Exercise training and heat acclimation improve thermoregulation in the sense that people who are exercise trained and physically active have earlier and more responsive skin blood flow responses (relative to body temperature) compared with individuals who are untrained and/or sedentary. Classic studies from the 1960s and 1970s showed that exercise training and heat acclimation both cause a leftward shift in the skin blood flow-internal temperature relationship, such that the onset of vasodilation occurs at lower internal temperatures and exhibits greater sensitivity (18, 60). The result is greater levels of skin blood flow and sweating (and thus more efficient heat dissipation) for any given level of core hyperthermia following training (60). Heat acclimation increases the thermoregulatory benefits of exercise training even further, with larger threshold shifts and greater increase in skin blood flow and sweating for a given level of core temperature (18, 60). The beneficial effects of exercise training on reflex cutaneous vasodilator responses have been shown in both young and older people (82). Furthermore, noradrenergic blockade with bretylium does not prevent the shift in threshold to lower body temperatures with training, showing that this shift is mostly or entirely mediated by an augmentation in active vasodilation, rather than by modifications in vasoconstrictor system function (82).
Aging.
Older adults can exhibit impaired thermoregulation and are often advised to use caution and/or avoid exposure to environmental extremes, including heat waves or excessively cold temperatures. Alterations in skin blood flow responses with aging contribute importantly to these impairments in thermoregulation (33). Although noted briefly here, this topic is covered in detail by Holowatz and Kenney in a separate review in this Highlighted Topics series (32a). Reflex cutaneous vasodilator responses are decreased in healthy older individuals during body heating or exercise (50). Changes in skin blood flow control in older adults are associated with altered roles for NO and prostaglandins compared with younger individuals (29, 30).
Aging is also associated with decreased reflex vasoconstriction in the skin during cold exposure (48) and greater decreases in body temperature, even when exposed to relatively mild body cooling (14). Thompson and Kenney (85) demonstrated that older subjects have minimal contribution of noradrenergic cotransmitters and thus rely entirely on vasoconstriction to norepinephrine during body cooling. Moreover, the vasoconstrictor response to norepinephrine is decreased in older humans (84, 90). Findings regarding potential therapeutic interventions for these impairments [as discussed by Holowatz and Kenney in the accompanying review (32a)] may ultimately have important clinical implications, particularly in individuals who may have additional risk factors for cold susceptibility.
Diabetes.
Diabetes is associated with increased risk for heat illness (66, 67), indicating a potential for thermoregulatory dysfunction. Skin blood flow responses measured in individuals with diabetes support the idea that changes in this circulation can contribute to this impaired body temperature regulation. During whole body heating, individuals with type 2 diabetes had elevated (delayed) thresholds for the onset of cutaneous vasodilation compared with control subjects of similar age and body size (87). Using bretylium iontophoresis to isolate the active vasodilator system in forearm skin, Wick et al. (87) identified that the threshold shift was due to a shift in control of active vasodilation to higher internal temperatures. Furthermore, overall cutaneous vasodilator responses (measured as forearm vasodilation by venous occlusion plethysmography) were lower at any increment in internal temperature (up to an increase of 1.0°C) during body heating in individuals with type 2 diabetes (73). This impaired cutaneous vasodilation appears to include a decrease in vasodilator responsiveness to NO and/or a decrease in maximal vasodilator responsiveness (to sodium nitroprusside) in this group (72). It is currently unknown whether the decreased vasodilation to sodium nitroprusside is due to an inability of the cGMP system to respond, or due to vessel structural remodeling causing a reduced maximum, or some combination of these.
With regard to vasoconstrictor function, individuals with type 2 diabetes had higher cutaneous vascular conductance at rest compared with controls, and this difference was abolished by bretylium, suggesting less noradrenergic vasoconstrictor tone in the diabetics (87). However, vasoconstrictor responses to 3 min of whole body cooling were similar to those of controls (87); therefore, the details of potential differences in reflex cutaneous vasoconstrictor function in diabetes remain to be further explored.
An important consideration in the foregoing observations is that the individuals studied were relatively healthy: they were living with diabetes, but had relatively well-controlled glucose levels and no clinically significant comorbid conditions (painful neuropathy, cardiovascular disease, etc.). It is likely that less healthy individuals with diabetes may have more extensive impairments in reflex control of skin blood flow.
Hypertension.
Individuals with essential hypertension exhibited decreased cutaneous vasodilator responsiveness compared with healthy controls during exercise (49). During resting heat stress, Kellogg et al. (45) observed similar forearm vasodilator responses (using venous occlusion plethysmography) in hypertensive and control subjects. In contrast, Holowatz and Kenney (31) observed decreased cutaneous vasodilator responses in hypertensive subjects during body heating when responses were measured using laser Doppler flowmetry. In addition to different methodology between the two sets of studies, differences in results could also be due to the fact that hypertensive patients as a population are heterogenous, and one subgroup studied may have more or less vascular dysfunction compared with another.
With regard to potential changes in mechanisms of reflex vasodilation in hypertensive subjects, Holowatz and Kenney showed that inhibition of arginase (which may compete with NOS) augmented reflex vasodilation in hypertensive subjects, but not in the normotensive group (32), and that local antioxidant administration also improved vasodilator responses in hypertensive subjects (31). In addition to these mechanisms, maximal vasodilation in the skin of hypertensive subjects was shown to be decreased (5, 32), suggesting that chronic structural changes may also contribute to impaired cutaneous vasodilator responses in this group.
Heart failure.
Chronic heart failure is associated with profound impairments in the ability of the circulatory system to respond appropriately to challenges to homeostasis, and the skin circulation is no exception. Cui et al. (12) showed that skin blood flow responses (measured as forearm blood flow) were almost 50% lower in chronic heart failure patients relative to controls during increases in internal temperature of ∼0.85°C. Green and colleagues (24) also showed decreased skin blood flow responses in heart failure during mild environmental heating (∼0.3°C increase in rectal temperature). As with hypertensive subjects, heart failure patients showed decreased maximal vasodilation in the skin (12), suggesting that vascular remodeling may contribute to the impaired response. Additionally, heart failure patients had a smaller contribution of NO to reflex cutaneous vasodilation, which may have contributed to the impaired response (24). With regard to cutaneous vasoconstrictor mechanisms, a classic study from Zelis and colleagues (93) showed augmented vasoconstriction in the skin at the onset of and throughout exercise in heart failure patients (when control subjects showed vasodilation), suggesting augmented cutaneous vasoconstrictor activity. Along with vascular mechanisms, a limited ability to increase cardiac output in these patients likely limits increases in skin blood flow as well. Epidemiologically, individuals with heart disease have an increased risk of heat illness (68), and these reports suggest that impaired cutaneous vasodilation contributes to thermoregulatory dysfunction in this population.
SUMMARY AND CONCLUSIONS
In summary, the reflex control of skin blood flow in humans includes sympathetic neurogenic vasodilation during body heating and noradrenergic vasoconstriction during body cooling. Figure 1 summarizes the contributors to reflex cutaneous vasoconstriction and vasodilation and some of the modifiers of these processes. Each of these physiological processes has proven to be more complex than was thought even a few years ago. Both cutaneous vasoconstriction and cutaneous vasodilation are modified by factors, including exercise, reproductive hormones, aging, and disease. To the extent possible, studies of the skin circulation and its reflex control should take into account and control for these various modifiers. Importantly, recent gains in understanding of specific and complex mechanisms of reflex cutaneous vasodilation and vasoconstriction may provide clinically relevant therapeutic possibilities for populations in whom (or conditions in which) one or more of these pathways are functioning at less than optimal levels.
GRANTS
The author's work in these areas has been supported by National Heart, Lung, and Blood Institute Grant HL73884, American Heart Association Grant 0750036Z, and by Center for Translational Science Activities Grant UL1 RR024150 (to the Mayo Clinic).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Bennett LA, Johnson JM, Stephens DP, Saad AR, Kellogg DL., Jr Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature of humans. J Physiol 552: 223–232, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brengelmann GL, Johnson JM, Hermansen L, Rowell LB. Altered control of skin blood flow during exercise at high internal temperatures. J Appl Physiol 43: 790–794, 1977 [DOI] [PubMed] [Google Scholar]
- 3. Brengelmann GL, Savage MV. Temperature regulation in the neutral zone. Ann N Y Acad Sci 813: 39–50, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Brooks EM, Morgan AL, Pierzga JM, Wladkowski SL, O'Gorman JT, Derr JA, Kenney WL. Chronic hormone replacement therapy alters thermoregulatory and vasomotor function in postmenopausal women. J Appl Physiol 83: 477–484, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Carberry PA, Shepherd AM, Johnson JM. Resting and maximal forearm skin blood flows are reduced in hypertension. Hypertension 20: 349–355, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Charkoudian N, Johnson JM. Altered reflex control of cutaneous circulation by female sex steroids is independent of prostaglandins. Am J Physiol Heart Circ Physiol 276: H1634–H1640, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Charkoudian N, Johnson JM. Female reproductive hormones and thermoregulatory control of skin blood flow. Exerc Sport Sci Rev 28: 108–112, 2000 [PubMed] [Google Scholar]
- 8. Charkoudian N, Johnson JM. Modification of active cutaneous vasodilation by oral contraceptive hormones. J Appl Physiol 83: 2012–2018, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Charkoudian N, Johnson JM. Reflex control of cutaneous vasoconstrictor system is reset by exogenous female reproductive hormones. J Appl Physiol 87: 381–385, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Crandall CG. Heat stress and baroreflex regulation of blood pressure. Med Sci Sports Exerc 40: 2063–2070, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crandall CG, Johnson JM, Kosiba WA, Kellogg DL., Jr Baroreceptor control of the cutaneous active vasodilator system. J Appl Physiol 81: 2192–2198, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Cui J, Arbab-Zadeh A, Prasad A, Durand S, Levine BD, Crandall CG. Effects of heat stress on thermoregulatory responses in congestive heart failure patients. Circulation 112: 2286–2292, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Cui J, Wilson TE, Crandall CG. Orthostatic challenge does not alter skin sympathetic nerve activity in heat-stressed humans. Auton Neurosci 116: 54–61, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Degroot DW, Kenney WL. Impaired defense of core temperature in aged humans during mild cold stress. Am J Physiol Regul Integr Comp Physiol 292: R103–R108, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Dodt C, Gunnarsson T, Elam M, Karlsson T, Wallin BG. Central blood volume influences sympathetic sudomotor nerve traffic in warm humans. Acta Physiol Scand 155: 41–51, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Fortney SM, Nadel ER, Wenger CB, Bove JR. Effect of acute alterations of blood volume on circulatory performance in humans. J Appl Physiol 50: 292–298, 1981 [DOI] [PubMed] [Google Scholar]
- 17. Fortney SM, Wenger CB, Bove JR, Nadel ER. Effect of hyperosmolality on control of blood flow and sweating. J Appl Physiol 57: 1688–1695, 1984 [DOI] [PubMed] [Google Scholar]
- 18. Fox RH, Goldsmith R, Kidd DJ, Lewis HE. Blood flow and other thermoregulatory changes with acclimatization to heat. J Physiol 166: 548–562, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fox RH, Hilton SM. Bradykinin formation in human skin as a factor in heat vasodilatation. J Physiol 142: 219–232, 1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fox RH, Hilton SM. Sweat gland activity as a contributory factor to heat vasodilation in the human skin. J Physiol 133: 68–69, 1956. 13346635 [Google Scholar]
- 21. Gonzalez-Alonso J, Crandall CG, Johnson JM. The cardiovascular challenge of exercising in the heat. J Physiol 586: 45–53, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gonzalez-Alonso J, Mora-Rodriguez R, Below PR, Coyle EF. Dehydration reduces cardiac output and increases systemic and cutaneous vascular resistance during exercise. J Appl Physiol 79: 1487–1496, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Grant RT, Holling HE. Further observations on the vascular responses of the human limb to body warming: evidence for sympathetic vasodilator nerves in the normal subject. Clin Sci 3: 273–285, 1938 [Google Scholar]
- 24. Green DJ, Maiorana AJ, Siong JH, Burke V, Erickson M, Minson CT, Bilsborough W, O'Driscoll G. Impaired skin blood flow response to environmental heating in chronic heart failure. Eur Heart J 27: 338–343, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Grucza R, Pekkarinen H, Titov EK, Kononoff A, Hanninen O. Influence of the menstrual cycle and oral contraceptives on thermoregulatory responses to exercise in young women. Eur J Appl Physiol Occup Physiol 67: 279–285, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Haeusler G, Haefely W, Huerlimann A. On the mechanism of the adrenergic nerve blocking action of bretylium. Naunyn Schmiedebergs Arch Pharmakol 265: 260–277, 1969 [DOI] [PubMed] [Google Scholar]
- 27. Hodges GJ, Johnson JM. Adrenergic control of the human cutaneous circulation. Appl Physiol Nutr Metab 34: 829–839, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Hokfelt T, Lundberg JM, Schultzberg M, Johansson O, Skirboll L, Anggard A, Fredholm B, Hamberger B, Pernow B, Rehfeld J, Goldstein M. Cellular localization of peptides in neural structures. Proc R Soc Lond B Biol Sci 210: 63–77, 1980 [DOI] [PubMed] [Google Scholar]
- 29. Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol 284: H1662–H1667, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Holowatz LA, Jennings JD, Lang JA, Kenney WL. Ketorolac alters blood flow during normothermia but not during hyperthermia in middle-aged human skin. J Appl Physiol 107: 1121–1127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holowatz LA, Kenney WL. Local ascorbate administration augments NO- and non-NO-dependent reflex cutaneous vasodilation in hypertensive humans. Am J Physiol Heart Circ Physiol 293: H1090–H1096, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Holowatz LA, Kenney WL. Up-regulation of arginase activity contributes to attenuated reflex cutaneous vasodilatation in hypertensive humans. J Physiol 581: 863–872, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a. Holowatz LA, Kenney WL. Peripheral mechanisms of thermoregulatory control of skin blood flow in aged humans. J Appl Physiol (April 22, 2010). doi:10.1152/japplphysiol.00338.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Holowatz LA, Thompson-Torgerson C, Kenney WL. Aging and the control of human skin blood flow. Front Biosci 15: 718–739, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol 30: 5–11, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Johnson JM. Nonthermoregulatory control of human skin blood flow. J Appl Physiol 61: 1613–1622, 1986 [DOI] [PubMed] [Google Scholar]
- 35a. Johnson JM, Kellogg DL., Jr Local thermal control of the human cutaneous circulation. J Appl Physiol (June 3, 2010). doi:10.1152/japplphysiol.00407.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnson JM, Park MK. Effect of heat stress on cutaneous vascular responses to the initiation of exercise. J Appl Physiol 53: 744–749, 1982 [DOI] [PubMed] [Google Scholar]
- 37. Johnson JM, Park MK. Effect of upright exercise on threshold for cutaneous vasodilation and sweating. J Appl Physiol 50: 814–818, 1981 [DOI] [PubMed] [Google Scholar]
- 38. Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Handbook of Physiology Environmental Physiology. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 4, vol. II, chapt. 11, p. 215–244 [Google Scholar]
- 39. Joyner MJ, Wilkins BW. Exercise hyperaemia: is anything obligatory but the hyperaemia? J Physiol 583: 855–860, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kellogg DL, Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol 85: 824–829, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Kellogg DL, Jr, Johnson JM, Kosiba WA. Baroreflex control of the cutaneous active vasodilator system in humans. Circ Res 66: 1420–1426, 1990 [DOI] [PubMed] [Google Scholar]
- 42. Kellogg DL, Jr, Johnson JM, Kosiba WA. Control of internal temperature threshold for active cutaneous vasodilation by dynamic exercise. J Appl Physiol 71: 2476–2482, 1991 [DOI] [PubMed] [Google Scholar]
- 43. Kellogg DL, Jr, Johnson JM, Kosiba WA. Selective abolition of adrenergic vasoconstrictor responses in skin by local iontophoresis of bretylium. Am J Physiol Heart Circ Physiol 257: H1599–H1606, 1989 [DOI] [PubMed] [Google Scholar]
- 44. Kellogg DL, Jr, Liu Y, McAllister K, Friel C, Pergola PE. Bradykinin does not mediate cutaneous active vasodilation during heat stress in humans. J Appl Physiol 93: 1215–1221, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Kellogg DL, Jr, Morris SR, Rodriguez SB, Liu Y, Grossmann M, Stagni G, Shepherd AM. Thermoregulatory reflexes and cutaneous active vasodilation during heat stress in hypertensive humans. J Appl Physiol 85: 175–180, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Kellogg DL, Jr, Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res 77: 1222–1228, 1995 [DOI] [PubMed] [Google Scholar]
- 47. Kellogg DL, Jr, Zhao JL, Wu Y. Neuronal nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. J Physiol 586: 847–857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kenney WL, Armstrong CG. Reflex peripheral vasoconstriction is diminished in older men. J Appl Physiol 80: 512–515, 1996 [DOI] [PubMed] [Google Scholar]
- 49. Kenney WL, Kamon E, Buskirk ER. Effect of mild essential hypertension on control of forearm blood flow during exercise in the heat. J Appl Physiol 56: 930–935, 1984 [DOI] [PubMed] [Google Scholar]
- 50. Kenney WL, Morgan AL, Farquhar WB, Brooks EM, Pierzga JM, Derr JA. Decreased active vasodilator sensitivity in aged skin. Am J Physiol Heart Circ Physiol 272: H1609–H1614, 1997 [DOI] [PubMed] [Google Scholar]
- 51. Kolka MA, Stephenson LA. Cutaneous blood flow and local sweating after systemic atropine administration. Pflügers Arch 410: 524–529, 1987 [DOI] [PubMed] [Google Scholar]
- 52. Kolka MA, Stephenson LA. Effect of luteal phase elevation in core temperature on forearm blood flow during exercise. J Appl Physiol 82: 1079–1083, 1997 [DOI] [PubMed] [Google Scholar]
- 53. Lang JA, Jennings JD, Holowatz LA, Kenney WL. Reflex vasoconstriction in aged human skin increasingly relies on Rho kinase-dependent mechanisms during whole body cooling. Am J Physiol Heart Circ Physiol 297: H1792–H1797, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Latzka WA, Sawka MN. Hyperhydration and glycerol: thermoregulatory effects during exercise in hot climates. Can J Appl Physiol 25: 536–545, 2000 [DOI] [PubMed] [Google Scholar]
- 55. Latzka WA, Sawka MN, Montain SJ, Skrinar GS, Fielding RA, Matott RP, Pandolf KB. Hyperhydration: thermoregulatory effects during compensable exercise-heat stress. J Appl Physiol 83: 860–866, 1997 [DOI] [PubMed] [Google Scholar]
- 56. Lewis T, Pickering GW. Vasodilation in the limbs in response to warming the body; with evidence for sympathetic vasodilator nerves in man. Heart 16: 33–51, 1931 [Google Scholar]
- 57. Mack GW. Assessment of cutaneous blood flow by using topographical perfusion mapping techniques. J Appl Physiol 85: 353–359, 1998 [DOI] [PubMed] [Google Scholar]
- 58. McCord GR, Cracowski JL, Minson CT. Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Integr Comp Physiol 291: R596–R602, 2006 [DOI] [PubMed] [Google Scholar]
- 59. Nadel ER, Fortney SM, Wenger CB. Effect of hydration state of circulatory and thermal regulations. J Appl Physiol 49: 715–721, 1980 [DOI] [PubMed] [Google Scholar]
- 60. Roberts MF, Wenger CB, Stolwijk JA, Nadel ER. Skin blood flow and sweating changes following exercise training and heat acclimation. J Appl Physiol 43: 133–137, 1977 [DOI] [PubMed] [Google Scholar]
- 61. Roddie IC, Shepherd JT, Whelan RF. The contribution of constrictor and dilator nerves to the skin vasodilatation during body heating. J Physiol 136: 489–497, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rowell LB. Cardiovascular adjustments to thermal stress. In: Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Organ Blood Flow. Bethesda: Am. Physiol. Soc., 1983, sect. 2, vol. III, pt. 2, chapt. 27, p. 967–1023 [Google Scholar]
- 63. Savage MV, Brengelmann GL, Buchan AM, Freund PR. Cystic fibrosis, vasoactive intestinal polypeptide, and active cutaneous vasodilation. J Appl Physiol 69: 2149–2154, 1990 [DOI] [PubMed] [Google Scholar]
- 64. Sawka MN, Coyle EF. Influence of body water and blood volume on thermoregulation and exercise performance in the heat. Exerc Sport Sci Rev 27: 167–218, 1999 [PubMed] [Google Scholar]
- 65. Sawka MN, Francesconi RP, Young AJ, Pandolf KB. Influence of hydration level and body fluids on exercise performance in the heat. JAMA 252: 1165–1169, 1984 [PubMed] [Google Scholar]
- 66. Schuman SH. Patterns of urban heat-wave deaths and implications for prevention: data from New York and St. Louis during July, 1966. Environ Res 5: 59–75, 1972 [DOI] [PubMed] [Google Scholar]
- 67. Semenza JC, McCullough JE, Flanders WD, McGeehin MA, Lumpkin JR. Excess hospital admissions during the July 1995 heat wave in Chicago. Am J Prev Med 16: 269–277, 1999 [DOI] [PubMed] [Google Scholar]
- 68. Semenza JC, Rubin CH, Falter KH, Selanikio JD, Flanders WD, Howe HL, Wilhelm JL. Heat-related deaths during the July 1995 heat wave in Chicago. N Engl J Med 335: 84–90, 1996 [DOI] [PubMed] [Google Scholar]
- 69. Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol 85: 830–834, 1998 [DOI] [PubMed] [Google Scholar]
- 70. Shastry S, Minson CT, Wilson SA, Dietz NM, Joyner MJ. Effects of atropine and l-NAME on cutaneous blood flow during body heating in humans. J Appl Physiol 88: 467–472, 2000 [DOI] [PubMed] [Google Scholar]
- 71. Shibasaki M, Aoki K, Morimoto K, Johnson JM, Takamata A. Plasma hyperosmolality elevates the internal temperature threshold for active thermoregulatory vasodilation during heat stress in humans. Am J Physiol Regul Integr Comp Physiol 297: R1706–R1712, 2009 [DOI] [PubMed] [Google Scholar]
- 72. Sokolnicki LA, Roberts SK, Wilkins BW, Basu A, Charkoudian N. Contribution of nitric oxide to cutaneous microvascular dilation in individuals with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 292: E314–E318, 2007 [DOI] [PubMed] [Google Scholar]
- 73. Sokolnicki LA, Strom NA, Roberts SK, Kingsley-Berg SA, Basu A, Charkoudian N. Skin blood flow and nitric oxide during body heating in type 2 diabetes mellitus. J Appl Physiol 106: 566–570, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stachenfeld NS, Silva C, Keefe DL. Estrogen modifies the temperature effects of progesterone. J Appl Physiol 88: 1643–1649, 2000 [DOI] [PubMed] [Google Scholar]
- 75. Stephens DP, Aoki K, Kosiba WA, Johnson JM. Nonnoradrenergic mechanism of reflex cutaneous vasoconstriction in men. Am J Physiol Heart Circ Physiol 280: H1496–H1504, 2001 [DOI] [PubMed] [Google Scholar]
- 76. Stephens DP, Bennett LA, Aoki K, Kosiba WA, Charkoudian N, Johnson JM. Sympathetic nonnoradrenergic cutaneous vasoconstriction in women is associated with reproductive hormone status. Am J Physiol Heart Circ Physiol 282: H264–H272, 2002 [DOI] [PubMed] [Google Scholar]
- 77. Stephens DP, Saad AR, Bennett LA, Kosiba WA, Johnson JM. Neuropeptide Y antagonism reduces reflex cutaneous vasoconstriction in humans. Am J Physiol Heart Circ Physiol 287: H1404–H1409, 2004 [DOI] [PubMed] [Google Scholar]
- 78. Stephenson LA, Kolka MA. Esophageal temperature threshold for sweating decreases before ovulation in premenopausal women. J Appl Physiol 86: 22–28, 1999 [DOI] [PubMed] [Google Scholar]
- 79. Stephenson LA, Kolka MA. Menstrual cycle phase and time of day alter reference signal controlling arm blood flow and sweating. Am J Physiol Regul Integr Comp Physiol 249: R186–R191, 1985 [DOI] [PubMed] [Google Scholar]
- 80. Tankersley CG, Nicholas WC, Deaver DR, Mikita D, Kenney WL. Estrogen replacement in middle-aged women: thermoregulatory responses to exercise in the heat. J Appl Physiol 73: 1238–1245, 1992 [DOI] [PubMed] [Google Scholar]
- 81. Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Graded cutaneous vascular responses to dynamic leg exercise. J Appl Physiol 64: 1803–1809, 1988 [DOI] [PubMed] [Google Scholar]
- 82. Thomas CM, Pierzga JM, Kenney WL. Aerobic training and cutaneous vasodilation in young and older men. J Appl Physiol 86: 1676–1686, 1999 [DOI] [PubMed] [Google Scholar]
- 83. Thompson-Torgerson CS, Holowatz LA, Kenney WL. Altered mechanisms of thermoregulatory vasoconstriction in aged human skin. Exerc Sport Sci Rev 36: 122–127, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Thompson CS, Holowatz LA, Kenney WL. Cutaneous vasoconstrictor responses to norepinephrine are attenuated in older humans. Am J Physiol Regul Integr Comp Physiol 288: R1108–R1113, 2005 [DOI] [PubMed] [Google Scholar]
- 85. Thompson CS, Kenney WL. Altered neurotransmitter control of reflex vasoconstriction in aged human skin. J Physiol 558: 697–704, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Vissing SF, Scherrer U, Victor RG. Increase of sympathetic discharge to skeletal muscle but not to skin during mild lower body negative pressure in humans. J Physiol 481: 233–241, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wick DE, Roberts SK, Basu A, Sandroni P, Fealey RD, Sletten D, Charkoudian N. Delayed threshold for active cutaneous vasodilation in patients with Type 2 diabetes mellitus. J Appl Physiol 100: 637–641, 2006 [DOI] [PubMed] [Google Scholar]
- 88. Wilkins BW, Martin EA, Roberts SK, Joyner MJ. Preserved reflex cutaneous vasodilation in cystic fibrosis does not include an enhanced nitric oxide-dependent mechanism. J Appl Physiol 102: 2301–2306, 2007 [DOI] [PubMed] [Google Scholar]
- 89. Wilkins BW, Wong BJ, Tublitz NJ, McCord GR, Minson CT. Vasoactive intestinal peptide fragment VIP10–28 and active vasodilation in human skin. J Appl Physiol 99: 2294–2301, 2005 [DOI] [PubMed] [Google Scholar]
- 90. Wilson TE, Monahan KD, Short DS, Ray CA. Effect of age on cutaneous vasoconstrictor responses to norepinephrine in humans. Am J Physiol Regul Integr Comp Physiol 287: R1230–R1234, 2004 [DOI] [PubMed] [Google Scholar]
- 91. Wong BJ, Minson CT. Neurokinin-1 receptor desensitization attenuates cutaneous active vasodilatation in humans. J Physiol 577: 1043–1051, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol 560: 941–948, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zelis R, Mason DT, Braunwald E. Partition of blood flow to the cutaneous and muscular beds of the forearm at rest and during leg exercise in normal subjects and in patients with heart failure. Circ Res 24: 799–806, 1969 [DOI] [PubMed] [Google Scholar]