Abstract
With increased interest in predictive medicine, development of a relatively noninvasive technique that can improve prediction of major clinical outcomes has gained considerable attention. Current tests that are the target of critical evaluation, such as flow-mediated vasodilation of the brachial artery and pulse-wave velocity, are specific to the larger conduit vessels. However, evidence is mounting that functional changes in the microcirculation may be an early sign of globalized microvascular dysfunction. Thus development of a test of microvascular reactivity that could be used to evaluate cardiovascular risk or response to treatment is an exciting area of innovation. This mini-review is focused on tests of microvascular reactivity to thermal stimuli in the cutaneous circulation. The skin may prove to be an ideal site for evaluation of microvascular dysfunction due to its ease of access and growing evidence that changes in skin vascular reactivity may precede overt clinical signs of disease. Evaluation of the skin blood flow response to locally applied heat has already demonstrated prognostic utility, and the response to local cooling holds promise in patients in whom cutaneous disorders are present. Whether either of these tests can be used to predict cardiovascular morbidity or mortality in a clinical setting requires further evaluation.
Keywords: endothelial, heat, hyperemia, cutaneous, cold
the need to measure vascular reactivity in the skin spans basic research on the nature of skin blood flow, identification of cardiovascular and metabolic disease risk in patients, measurement and evaluation of disease progression or treatment, and evaluation of microvascular or endothelial dysfunction in cutaneous disorders. The skin is an ideal site for evaluation of microvascular reactivity because it is easily accessible, and stimuli can be applied with non- or minimally invasive approaches. It has been suggested the cutaneous circulation can serve as a model for generalized microvascular dysfunction (35, 53, 89), and an increasing number of studies back this sentiment. For example, changes in skin vascular reactivity are often observed before clinical signs of microvascular dysfunction during the early stages of some diseases, suggesting skin vascular reactivity may serve as a harbinger of more globalized microvascular dysfunction (38, 39, 51–55, 65). However, clinical enthusiasm for the development of such tests must not precede careful assessment. Despite great leaps in our knowledge over the last decade, many of the complexities in the regulation of cutaneous vascular tone remain unclear. Furthermore, if tests of microvascular function are to be of any utility as a predictor of cardiovascular disease, they must provide independent and prognostic value beyond the Cardiorisk or Framingham Risk Score. The goal of this mini-review is to provide a general overview of cutaneous microvascular reactivity, with an emphasis on tests employing thermal provocation, to highlight what is known about the mechanisms underlying the tests and to review recent literature on the use of the tests to predict microvascular dysfunction and progression.
TESTS OF ENDOTHELIAL AND MICROVASCULAR FUNCTION
The term “endothelial dysfunction” was established in the mid-eighties following the major breakthrough by Furchgott and Zawadzki (27) that acetylcholine requires the presence of endothelial cells to relax the underlying vascular smooth muscle. Today, endothelial function typically refers to the ability of the endothelium to release any number of different compounds able to induce a direct relaxation of smooth muscle cells within the vascular wall. To determine whether endothelial “dysfunction” is present, this endothelium-dependent vasomotor response is typically compared with the effect of an exogenous vasoactive donor [such as nitric oxide (NO) via sodium nitroprusside], which acts directly on smooth muscle cells. Thus a comparison between the endothelium-dependent and endothelium-independent responses can be made.
Even before NO was identified as the factor released by acetylcholine, it had been observed in humans (and even earlier in animals) that endothelium-dependent relaxation of coronary arteries was impaired in atherosclerotic patients (60). It was subsequently suggested that this endothelial dysfunction could be an early marker of atherosclerosis (41). Since that time, there has been a profound interest in trying to develop a test of endothelial function in humans that could provide predictive value of cardiovascular risk. The most extensively used of the various tests currently available is flow-mediated vasodilation (FMD) of the brachial artery by Doppler ultrasound, which is a noninvasive, indirect method to evaluate endothelial dysfunction (18). Growing evidence demonstrates FMD responses are significantly correlated with coronary artery function (3, 82) and even provide independent prognostic value when added to traditional measures of cardiovascular risk (19, 75). That said, endothelial testing by FMD is not without controversy, is dependent on trained personnel, requires substantial equipment, and is specific to the larger conduit vessels (9, 73, 74).
This latter point is important, as most studies aimed at investigating endothelial dysfunction have focused on conductance arteries as a surrogate endpoint to coronary artery disease. However, a growing body of evidence suggests the microcirculation may be the initial site for endothelial damage in subjects at risk of cardiovascular disease (13). Furthermore, endothelial alterations may appear earlier in resistance arteries than in conduit arteries in some diseases (1, 42) and may precede large artery stiffening (40, 81). Generalized microvascular dysfunction has been identified as a crucial step in complications associated with pathophysiological conditions associated with diabetes, hypertension, coronary artery disease, peripheral artery disease, essential hypertension, renal failure, hypercholesterolemia, systemic sclerosis, and aging (42).
Taking the above into consideration, it is not surprising that brachial FMD does not correlate well with intrabrachial arterial infusions of acetylcholine or other tests of microvascular function in the resistance arteries, demonstrating they are measuring different aspects of vascular function (25, 42, 69, 72). An intra-arterial infusion of acetylcholine into the forearm requires a relatively high degree of invasiveness, limiting its utility as routine measurement for use in large-scale clinical trials. Thus a test of microvascular function in resistance arteries that does not require the same level of invasiveness as intra-arterial infusions of vasoactive agents is needed. The skin may prove to be the ideal site for these measurements.
ANATOMIC CONSIDERATIONS FOR TESTS OF VASCULAR REACTIVITY IN THE SKIN
The blood vessels in most areas of the skin are similar to those in other vascular beds, with blood vessels composed of vascular smooth muscle and an endothelium that releases a host of vasodilator and vasoconstrictor agents, such as NO, prostanoids, and endothelium-derived hyperpolarizing and constricting factors. Most of the body surface area is covered with “hairy” or nonacral (also called nonglabrous) skin, whereas the skin of the fingers, lips, ears, palms, and plantar aspects of the feet is acral or glabrous skin. Blood vessels in glabrous skin are primarily innervated by sympathetic adrenergic nerves and contain a high proportion of arteriovenous anastomoses, whereas blood vessels in the nonglabrous skin contain both adrenergic and sympathetic cholinergic nerves, both of which are involved in temperature regulation. Both skin types are richly populated by sensory nerves that respond to thermal, chemical, and mechanical stimuli. These nerves serve to provide feedback to the central nervous system, but also release local neuropeptides and other vasoactive agents that influence cutaneous vascular tone. Due to the structural and neurovascular regulatory differences in acral vs. nonacral skin, regulation of cutaneous vascular tone in these areas can differ, as well as the susceptibility of these areas to microvascular dysfunction. For example, in the finger pad, the reactive hyperemic response following vascular occlusion is ∼60% dependent on NO (71), whereas the skin on the forearm does not display a profound NO-dependent component to this same stimuli (95, 100). Recognizing the difference in types of skin is important, as tests of vascular reactivity are evaluating different aspects of a disease process, depending on the location where the tests are performed (11).
TECHNICAL CONSIDERATIONS FOR PERFORMING TESTS OF MICROVASCULAR REACTIVITY IN THE SKIN
Study of microvascular reactivity in the skin has most commonly been performed in humans using single-point laser Doppler flowmetry, with evaluation of the skin blood flow response as the outcome variable of interest. The use of laser Doppler to record skin blood flow is relatively straightforward, but has some inherent strengths and limitations, depending on the methods used, as discussed in greater detail elsewhere (17, 45, 98, 99). Pertinent to tests of microvascular reactivity in the skin, there is a relatively wide heterogeneity in the level of skin blood flow from one site to the next, even across the forearm (17, 45), resulting in a lack of standardization in the assessment of microvascular function reported in the literature (for reviews on this topic see Refs. 17, 89). It is, therefore, recommended to normalize the measurement at a given site to a characteristic for that site, such as baseline or maximal vasodilation. However, which characteristic to use in specific circumstances is the subject of some debate. In general, responses are normalized to maximal skin blood flow for studies in which a given perturbation will result in vasodilation. This can be achieved with local skin heating to at least 42°C or by administration of sodium nitroprusside, administered by iontophoresis or microdialysis. Alternatively, with local skin cooling, the practice is to normalize to baseline. However, this does present some documented challenges as well (17, 30, 76, 77). A suggestion in this case may be to perform baseline measurements at a specific clamped local temperature, such as 33°C.
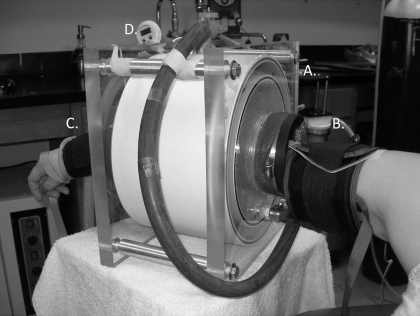
While the approach to scale responses to a maximal vasodilatation by heating the skin to 42–44°C is acceptable in healthy subjects and in mechanistically driven, carefully controlled studies, it should not be extrapolated in patient populations or in longitudinal studies without further consideration. For example, if the intent is to determine whether a decreased skin blood flow response to a specific stimuli is the result of a functional abnormality or a structural defect, it is not reasonable to compare maximal red blood cell flux values obtained with laser Doppler between subjects or across skin sites due to the high site-to-site and individual variability (17, 45). To determine whether maximal skin blood flow is actually reduced, one could measure absolute forearm vascular changes to whole arm heating in a water spray device using plethysmography, as pictured in Fig. 1 (44, 83, 84). Using this approach, the majority of blood flow above resting levels is directed to the skin, providing a measurement of absolute blood flow. This approach requires additional training and equipment and is unfortunately rarely used in studies investigating microvascular function in skin.
Fig. 1.
Photograph of the arm spray device in which maximal skin blood flow (SkBF) can be measured. A: the device is attached to a temperature-controlled water bath that circulates water into the cylindrical device. Water is applied to the forearm via numerous spray nozzles. B: the upper cuff for venous occlusion plethysmography. C: the distal cuff to occlude blood flow to the hand. D: temperature gauge within the water spray.
One approach to overcome the spatial heterogeneity problem is to use laser Doppler imaging, which applies the same physical principle as laser Doppler flowmetry. In imaging, the laser beam is reflected by a computer-driven mirror to progressively scan the area of interest. Although laser Doppler imaging provides an integrated index over a larger cutaneous surface than afforded with single-point laser Doppler flowmetry, thereby decreasing spatial variability, it is lacking in temporal aspects because it is not possible to record continuously from the area of interest. It is, therefore, possible to miss key time points in the pattern of responses in the skin to thermal provocation. This is important as the timing and pattern of responses provide insight into the potential mechanism of microvascular function or dysfunction with thermal provocation, as discussed below.
A relatively new approach to measure skin blood flow that holds promise for tests of microvascular reactivity is speckle contrast imaging (12, 21), which has been used to assess skin blood flow in a limited number of patients with microvascular disease (68). Laser speckle contrast imaging is an optical image technique that has been developed for imaging in vivo blood flow dynamics and vascular structure with high spatial and temporal resolution. Coherent light detected by a camera is time integrated over the exposure duration to yield an image with a speckle pattern of variable amplitude, dependent on motion and other parameters (88). The full-field spatiotemporal characteristics of microcirculation is then recorded in real time. Unfortunately, there are insufficient data at this time to know whether laser speckle contrast imaging will be an advantage over more traditional measures of skin blood flow with laser Doppler approaches.
EVALUATING MICROVASCULAR REACTIVITY IN THE SKIN
Acetylcholine iontophoresis is the most commonly used approach to study microvascular function in the skin and has been expertly reviewed elsewhere (89). However, there are some limitations to the approach that need to be recognized, and since it is the most widely used test, a brief discussion here is germane to the thermal tests of microvascular reactivity that are the focus of this mini-review.
The use of acetylcholine delivered by iontophoresis as a specific test of endothelial function per se is debatable. This method drives acetylcholine through the interstitium surrounding the blood vessels, which is quite different compared with delivery by arterial infusion. When delivered through the lumen of a blood vessel, the drug is in contact with the endothelium first and foremost, whereas, when delivered outside the vessel, this is not the case. Acetylcholine is known to have a number of different effects in skin on blood vessels and the surrounding nerves, leading to the release of nonendothelium-derived substances, including neuropeptides (7, 20). For example, acetylcholine induces an axon reflex that participates in the increase of skin blood flow (7). With iontophoresis, the anodal current on its own is known to cause a current-induced hyperemia (7, 22, 23). Thus, when acetylcholine is delivered to the skin by iontophoresis, the vasodilator response is not specifically endothelium dependent. Furthermore, the specific mechanisms by which acetylcholine causes vasodilation in the skin is not fully understood. Inhibition of NO synthase has only a modest effect on the cutaneous vasodilation to acetylcholine, with a substantial prostanoid-dependent component and a component that is not sensitive to combined NO and COX inhibition (36, 50). An observed reduction in the vasodilator response to acetylcholine is not attributable to a specific mechanism. The thermal tests discussed below also have some inherent limitations, but may provide additional insight into specific pathologies or provide an alternative index of globalized microvascular dysfunction. For a more comprehensive treatment on the mechanisms of the local thermal control of the skin, the reader is referred to the mini-review by Johnson and Kellogg as part of this series (43).
LOCAL THERMAL HYPEREMIA AS A TEST OF VASCULAR REACTIVITY
The skin blood flow response to the standard local heating protocol most commonly used as a test of thermal hyperemia is mediated by at least two independent phases: an initial peak in cutaneous blood flow during the first 10 min, followed by a plateau after 20–30 min of warming (17, 66). The initial rapid phase is predominantly mediated by local sensory nerves and can be significantly attenuated in the presence of local anesthesia (Fig. 1) (4, 66), but not by proximal neural blockade or muscarinic receptors blockade (48, 66). In contrast, the 20- to 30-min plateau is mediated predominantly by NO (47, 66), with recent evidence suggesting the NO is generated from the endothelial NO synthase isoform (49), although this is not a universal finding (80). In either case, NO synthase inhibition does not fully suppress the plateau phase, suggesting other vasodilators may be involved. Neither prostanoids nor histamine seems to play a role (28, 29, 64, 96). Recent studies have demonstrated the complexity and integrated nature of the cutaneous response to local heating, and there is now evidence for an involvement of the sympathetic neurotransmitters norepinephrine and neuropeptide Y in both the initial peak and sustained plateau phases (32). How these sympathetic neurotransmitters may interact with the sensory neurotransmitters and NO is an area of exciting exploration. The challenge, of course, is that it is not possible to determine the underlying cause of the dysfunction in conditions in which the thermal hyperemic response is diminished without pharmacological intervention.
Recently, two different studies examined the reproducibility of the thermal hyperemic response, an important assessment for determining its potential as a clinical tool. In the first study, Agarwal and colleagues (2) compared day-to-day thermal hyperemia to other tests of vascular responsiveness (reactive hyperemia and acetylcholine iontophoresis) on the forearm and reported that the reactive hyperemic response was the most reproducible of the tests, with a coefficient of variation of 9.3% when expressed as a change in perfusion from baseline, but not when expressed as percent change from baseline values (2, 37). Unfortunately, they did not perform a maximal heating stimulus to scale responses to maximal blood flow. Roustit et al. (76) recently examined the reproducibility of local thermal hyperemia on both the finger pad and the forearm. These authors reported that reproducibility of the test on the finger pad was considered acceptable, but the forearm displayed greater interday variability, which the authors contributed to the greater spatial variability of capillary density in the skin of the forearm compared with the finger. Reproducibility of the initial peak response on the forearm, when expressed as a percentage of maximal blood flow, was also considered good and has been used by this group to measure skin neurovascular function in patients with systemic sclerosis (78). In this latter study, the authors suggest that thermal hyperemia could be monitored as a clinical test for neurovascular function.
Microvascular reactivity has been investigated using thermal hyperemia in a number of different pathological conditions in recent years. Consistent data show that thermal hyperemia is impaired in diabetes (16, 52, 93, 94), with advanced age (36, 62, 67), in smokers (24), and in renal failure patients (55, 79). Furthermore, thermal hyperemia has been used as a clinical surrogate marker in various diseases, such as Raynaud's phenomenon and systemic sclerosis (10, 68, 78). Patients with low-flow postural tachycardia syndrome exhibit exclusively a decreased plateau (65), while those with chronic spinal cord injury exhibited a decreased axon reflex (70, 91).
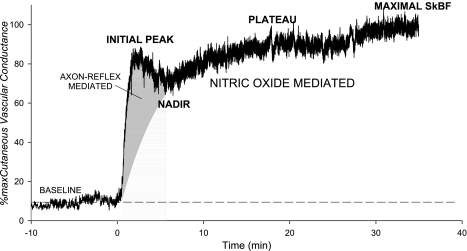
One of the most provocative studies to date using thermal hyperemia evaluated parameters of the thermal hyperemic response in end-stage renal patients (55) in both cross-sectional and longitudinal designs. The high-risk population was characterized by a markedly diminished local thermal hyperemic response, evident at all measured data points: the initial heat peak, nadir, and second heat peak (see Fig. 2). The most robust measurement of the thermal hyperemic response was the area under the curve of the 30-min heating. These authors reported that patients who had abnormal thermal responses showed increased cardiovascular mortality, despite similar Cardiorisk and Framingham Risk Assessments to those without impaired thermal responses. This suggests thermal hyperemia as a test of microvascular reactivity may improve cardiovascular risk prediction through incorporation with the risk assessments. Importantly, a more common test of vascular reactivity, reactive hyperemia following occlusive blood flow restriction, was not found to correlate with cardiovascular risk in this study, although it has been shown to be inversely correlated to the severity of cardiovascular risk exposure in a large female population (92) and to be enhanced by statins (8) in hypercholesterolemic patients. Like the thermal hyperemic response, reactive hyperemia represents a complex microvascular response, but, unlike thermal hyperemia, it is independent of NO (95, 100). Rather, there seems to be significant involvement of the local sensory nerves and an EDHF pathway via large-conductance Ca2+-activated K channels (59). Thus the reactive hyperemic and thermal hyperemic responses may be measuring different aspects of microvascular function and, when combined in studies or clinical trials, may provide insight into mechanism of dysfunction.
Fig. 2.
Representative tracing of the SkBF response to local heating to 42°C, followed by maximal heating to 43.5°C. The initial rapid rise relies predominantly on the local sensory nerves and nitric oxide. The second rise leads to a plateau that predominantly dependent on nitric oxide.
LOCAL COOLING AS A TEST OF VASCULAR REACTIVITY
Compared with thermal hyperemia, the cutaneous response to local cooling has received much less attention, with the majority of studies focused on cutaneous disorders in which a microvascular dysfunction is present. However, with recent advances in our understanding of this surprisingly complex response, local cooling may demonstrate utility as a tool to investigate different aspects of microvascular reactivity than are currently in use. Local cooling has been used to evaluate vascular responsiveness in familial hypertension (63), differences between men and women (14), with aging (56, 57, 86, 87), and in patients with Raynaud's phenomenon (26, 61).
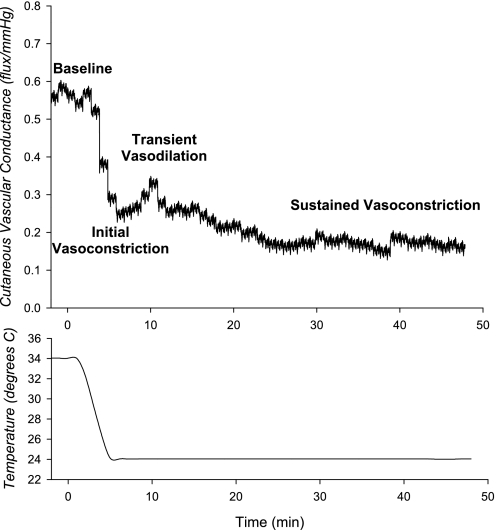
Investigating the mechanisms that underlie the cutaneous response to local cooling has been an area of much recent activity. A typical pattern of the response to local cooling is displayed in Fig. 3, but, like the response to local heating, the rate of change in temperature, and perhaps even the extent of temperature change, will impact the pattern and the underlying mechanisms involved (31, 46). In general, the response is characterized by an initial decrease in skin blood flow, followed by a transient vasodilation, and a secondary progressive vasoconstriction. There is a clear and well-established adrenergic nerve component involving norepinephrine-mediated vasoconstriction throughout the cooling stimulus (33, 46). This is thought to mediate the initial decrease in skin blood flow at the initiation of cooling. However, the complexity of the mechanisms that mediate this response was demonstrated by the Flavahan laboratory (5, 6, 15), in which they found that local cooling of skin blood vessels in vitro stimulates mitochondrial production of reactive oxygen species, which then acts on Rho kinase to cause the translocation of α2c-receptors to the plasma membrane. Norepinephrine released from adrenergic nerves then acts on the upregulated receptors to overcome an overall reduced neurotransmitter release associated with the local cooling stimulus, resulting in net cutaneous vasoconstriction. Thompson-Torgerson and colleagues (85) confirmed this finding in vivo by applying a Rho kinase inhibitor alone and in combination with postsynaptic blockade of α1,2-receptors. The early transient vasodilation often observed with local cooling is unexplained at the present time, but it is not obviously linked to sensory nerve function. However, sensory nerves typically associated with vasodilation appear to play a counterintuitive role in the response, in which blockade of these nerves unmasks an underlying vasodilator response (33, 46). A role for NO in the local cooling response has also been demonstrated, in which prolonged cooling inhibits NO synthase (34, 97).
Fig. 3.
Representative tracing of the SkBF response to local cooling to 24°C. Data are redrawn from Ref. 75. The initial decrease in SkBF with cooling is followed by a transient vasodilation. This is then followed by a progressive decrease in SkBF with sustained cooling.
As the microvascular responses to local cooling are studied in more patient populations, information regarding how various pathologies impact the pattern of the local cooling response may provide additional insight into the underlying mechanism of the response. For example, changes in the cutaneous microvascular response to local cooling with advanced age have been the focus of a number of studies from the Kenney laboratory (56, 57, 86, 87). This group has found impaired norepinephrine-mediated vasoconstriction (87) and blunted responses to local cooling (86) in the elderly. Importantly, older skin relies on Rho kinase-dependent pathways to a greater extent than the skin of younger subjects (58). It was suggested that this may be due to pathological changes in global microvascular function associated with increased oxidative stress with aging. Consistent with this idea, it has been demonstrated during whole body cooling that local administration of tetrahydrobiopterin, required in the prejunctional biosynthesis of catecholamines, corrects the age-related decline in cutaneous vasoconstriction (56, 57). Whether local cooling can be used as a biological test of oxidative stress or provide prognostic insight into microvascular dysfunction associated with cardiovascular or metabolic disease is not known at the present time.
Before a test of vascular reactivity to local cooling can be used as a clinical tool, studies addressing the reproducibility of the test are needed. Roustit and colleagues (77) recently provided such a study as they evaluated the short-term (same day) and long-term (1 wk) reproducibility of 5- and 30-min local cooling tests from a clamped baseline temperature (33°C) to either 24 or 15°C. These authors found much higher variability in the response to 24°C cooling than to 15°C and recommend the 30-min test as providing the lowest variability. In this study, a cold-induced vasodilation was observed in some subjects at the onset of cooling, as described by others (34, 97). This was not surprising, as they used a high rate of cooling (−16°C/min) known to stimulate this response, a choice based on their interest in developing a test for use in Raynaud's phenomenon patients, in whom the cold-induced vasodilation may prove to have clinical utility. The rate of cooling and final temperature achieved that would best work for evaluation in other patient groups has not been studied.
CONCLUSION AND PERSPECTIVES
With increased interest in predictive medicine, development of a relatively noninvasive technique that can improve prediction of major clinical outcomes above and beyond the Framingham Risk Score has gained considerable attention. The Framingham Risk Score was developed to offer prospective risk assessment for coronary heart risk in men and women who do not have overt coronary disease. In a recent review of studies claiming additional predictive value beyond the Framingham Risk Score, most studies were reported to contain flaws in their design, analyses, and/or reporting (90). If we are to determine whether thermal stimuli tests in the skin can provide prognostic value, more research is needed, and closer scrutiny needs to be applied. At a minimum, a good test of microvascular reactivity should 1) have a sound physiological and mechanistic basis that is well characterized; 2) be reproducible, observer independent, and easily standardized; and 3) provide a demonstrated ability to predict morbidity, mortality, and/or improvement in risk with treatment. If a given test falls short of being able to provide an overall risk evaluation for global microvessel dysfunction, but still provides important insight into microvascular function of the skin as a clinical endpoint, then the test may be useful. Preliminary findings suggest that the local hyperemic response to skin heating may provide prognostic value, but whether or not the test can be reliably used in a clinical setting with the intent to treat has not been demonstrated. Local cooling as a clinical test is still in the very early stages of development and evaluation. Although preliminary findings hold promise, more work needs to be done before thermal tests of microvascular reactivity are ready for clinical primetime.
GRANTS
Dr. Minson gratefully acknowledges the funding of his work in this review (National Heart, Lung, and Blood Institute Grants HL070928 and HL081671).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
I express my appreciation to colleagues and graduate students who have contributed to our understanding of cutaneous vascular regulation.
REFERENCES
- 1. Abularrage CJ, Sidawy AN, Aidinian G, Singh N, Weiswasser JM, Arora S. Evaluation of the microcirculation in vascular disease. J Vasc Surg 42: 574–581, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Agarwal SC, Allen J, Murray A, Purcell IF. Comparative reproducibility of dermal microvascular blood flow changes in response to acetylcholine iontophoresis, hyperthermia and reactive hyperaemia. Physiol Meas 31: 1–11, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 26: 1235–1241, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Arildsson M, Asker CL, Salerud EG, Stromberg T. Skin capillary appearance and skin microvascular perfusion due to topical application of analgesia cream. Microvasc Res 59: 14–23, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Bailey SR, Eid AH, Mitra S, Flavahan S, Flavahan NA. Rho kinase mediates cold-induced constriction of cutaneous arteries: role of alpha2C-adrenoceptor translocation. Circ Res 94: 1367–1374, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Bailey SR, Mitra S, Flavahan S, Flavahan NA. Reactive oxygen species from smooth muscle mitochondria initiate cold-induced constriction of cutaneous arteries. Am J Physiol Heart Circ Physiol 289: H243–H250, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Berghoff M, Kathpal M, Kilo S, Hilz MJ, Freeman R. Vascular and neural mechanisms of ACh-mediated vasodilation in the forearm cutaneous microcirculation. J Appl Physiol 92: 780–788, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Binggeli C, Spieker LE, Corti R, Sudano I, Stojanovic V, Hayoz D, Luscher TF, Noll G. Statins enhance postischemic hyperemia in the skin circulation of hypercholesterolemic patients: a monitoring test of endothelial dysfunction for clinical practice? J Am Coll Cardiol 42: 71–77, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Black MA, Cable NT, Thijssen DHJ, Green DJ. Importance of measuring the time-course of flow-mediated dilation (FMD) in humans. Hypertension 51: 203–210, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Boignard A, Salvat-Melis M, Carpentier PH, Minson CT, Grange L, Duc C, Sarrot-Reynauld F, Cracowski JL. Local hyperemia to heating is impaired in secondary Raynaud's phenomenon. Arthriris Res Ther 7: R1103–R1112, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Braverman IM. The cutaneous microcirculation. J Invest Dermatol 5: 3–9, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Briers JD. Laser Doppler, speckle and related techniques for blood perfusion mapping and imaging. Physiol Meas 22: R35–R66, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Brodsky SV, Gealekman O, Chen J, Zhang F, Togashi N, Crabtree M, Gross SS, Nasjletti A, Goligorsky MS. Prevention and reversal of premature endothelial cell senescence and vasculopathy in obesity-induced diabetes by ebselen. Circ Res 94: 377–384, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Cankar K, Finderle Z. Gender differences in cutaneous vascular and autonomic nervous response to local cooling. Clin Auton Res 13: 214–220, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Chotani MA, Flavahan S, Mitra S, Daunt D, Flavahan NA. Silent alpha(2C)-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries. Am J Physiol Heart Circ Physiol 278: H1075–H1083, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Colberg SR, Parson HK, Nunnold T, Holton DR, Swain DP, Vinik AI. Change in cutaneous perfusion following 10 weeks of aerobic training in Type 2 diabetes. J Diabetes Complications 19: 276–283, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci 27: 503–508, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Deanfield J, Donald A, Ferri C, Giannattasio C, Halcox J, Halligan S, Lerman A, Mancia G, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Schiffrin EL, Taddei S, Webb DJ. Endothelial function and dysfunction. I. Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens 23: 7–17, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction. Testing and clinical relevance. Circulation 115: 1285–1295, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Douglas WW, Ritchie JM. The excitatory action of acetylcholine on cutaneous non-myelinated fibres. J Physiol 150: 501–514, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Draijer M, Hondebrink E, van Leeuwen T, Steenbergen W. Review of laser speckle contrast techniques for visualizing tissue perfusion. Lasers Med Sci 24: 639–651, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Durand S, Fromy B, Bouye P, Saumet JL, Abraham P. Current-induced vasodilation during water iontophoresis (5 min, 0.10 mA) is delayed from current onset and involves aspirin sensitive mechanisms. J Vasc Res 39: 59–71, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Durand S, Fromy B, Bouye P, Saumet JL, Abraham P. Vasodilatation in response to repeated anodal current application in the human skin relies on aspirin-sensitive mechanisms. J Physiol 540: 261–269, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Edvinsson ML, Andersson SE, Xu CB, Edvinsson L. Cigarette smoking leads to reduced relaxant responses of the cutaneous microcirculation. Vasc Health Risk Manag 4: 699–704, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eskurza I, Seals DR, DeSouza CA, Tanaka H. Pharmacologic versus flow-mediated assessments of peripheral vascular endothelial vasodilatory function in humans. Am J Cardiol 88: 1067–1069, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Foerster J, Kuerth A, Niederstrasser E, Krautwald E, Pauli R, Paulat R, Eweleit M, Riemekasten G, Worm M. A cold-response index for the assessment of Raynaud's phenomenon. J Dermatol Sci 45: 113–120, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980 [DOI] [PubMed] [Google Scholar]
- 28. Golay S, Haeberli C, Delachaux A, Liaudet L, Kucera P, Waeber B, Feihl F. Local heating of human skin causes hyperemia without mediation by muscarinic cholinergic receptors or prostanoids. J Appl Physiol 97: 1781–1786, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Gooding KM, Hannemann MM, Tooke JE, Clough GF, Shore AC. Maximum skin hyperaemia induced by local heating: possible mechanisms. J Vasc Res 43: 270–277, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Hodges GJ, Kosiba WA, Zhao K, Alvarez GE, Johnson JM. The role of baseline in the cutaneous vasoconstrictor responses during combined local and whole body cooling in humans. Am J Physiol Heart Circ Physiol 293: H3187–H3192, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Hodges GJ, Kosiba WA, Zhao K, Johnson JM. The involvement of heating rate and vasoconstrictor nerves in the cutaneous vasodilator response to skin warming. Am J Physiol Heart Circ Physiol 296: H51–H56, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hodges GJ, Kosiba WA, Zhao K, Johnson JM. The involvement of norepinephrine, neuropeptide Y, and nitric oxide in the cutaneous vasodilator response to local heating in humans. J Appl Physiol 105: 233–240, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hodges GJ, Traeger JA, 3rd, Tang T, Kosiba WA, Zhao K, Johnson JM. Role of sensory nerves in the cutaneous vasoconstrictor response to local cooling in humans. Am J Physiol Heart Circ Physiol 293: H784–H789, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Hodges GJ, Zhao K, Kosiba WA, Johnson JM. The involvement of nitric oxide in the cutaneous vasoconstrictor response to local cooling in humans. J Physiol 574: 849–857, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol 105: 370–372, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol 563: 965–973, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Houghton BL, Meendering JR, Wong BJ, Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol 572: 811–820, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ijzerman RG, de Jongh RT, Beijk MA, van Weissenbruch MM, Delemarre-van de Waal HA, Serne EH, Stehouwer CD. Individuals at increased coronary heart disease risk are characterized by an impaired microvascular function in skin. Eur J Clin Invest 33: 536–542, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Ijzerman RG, Serne EH, van Weissenbruch MM, de Jongh RT, Stehouwer CD. Cigarette smoking is associated with an acute impairment of microvascular function in humans. Clin Sci (Lond) 104: 247–252, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Isaksson H, Cederholm T, Jansson E, Nygren A, Ostergren J. Therapy-resistant hypertension associated with central obesity, insulin resistance, and large muscle fibre area. Blood Press 2: 46–52, 1993 [DOI] [PubMed] [Google Scholar]
- 41. Jayakody L, Kappagoda T, Senaratne MP, Thomson AB. Impairment of endothelium-dependent relaxation: an early marker for atherosclerosis in the rabbit. Br J Pharmacol 94: 335–346, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Joannides R, Bellien J, Thuillez C. Clinical methods for the evaluation of endothelial function–a focus on resistance arteries. Fundam Clin Pharmacol 20: 311–320, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Johnson JM, Kellogg DL. Local thermal control of the human cutaneous circulation. J Appl Physiol (June 3, 2010). doi:10.1152/japplphysiol.00407.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Johnson JM, O'Leary DS, Taylor WF, Kosiba W. Effect of local warming on forearm reactive hyperaemia. Clin Physiol 6: 337–346, 1986 [DOI] [PubMed] [Google Scholar]
- 45. Johnson JM, Taylor WF, Shepherd AP, Park MK. Laser-doppler measurement of skin blood flow: comparison with plethymography. J Appl Physiol 56: 798–803, 1984 [DOI] [PubMed] [Google Scholar]
- 46. Johnson JM, Yen TC, Zhao K, Kosiba WA. Sympathetic, sensory, and nonneuronal contributions to the cutaneous vasoconstrictor response to local cooling. Am J Physiol Heart Circ Physiol 288: H1573–H1579, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Kellogg DL, Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol 86: 1185–1190, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Kellogg DL, Jr, Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res 77: 1222–1228, 1995 [DOI] [PubMed] [Google Scholar]
- 49. Kellogg DL, Jr, Zhao JL, Wu Y. Endothelial nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. Am J Physiol Heart Circ Physiol 295: H123–H129, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kellogg DL, Jr, Zhao JL, Coey U, Green JV. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol 98: 629–632, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Khan F, Belch JJ, MacLeod M, Mires G. Changes in endothelial function precede the clinical disease in women in whom preeclampsia develops. Hypertension 46: 1123–1128, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Khan F, Elhadd TA, Greene SA, Belch JJ. Impaired skin microvascular function in children, adolescents, and young adults with type 1 diabetes. Diabetes Care 23: 215–220, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Khan F, Patterson D, Belch JJ, Hirata K, Lang CC. Relationship between peripheral and coronary function using laser Doppler imaging and transthoracic echocardiography. Clin Sci (Lond) 115: 295–300, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Kraemer-Aguiar LG, Laflor CM, Bouskela E. Skin microcirculatory dysfunction is already present in normoglycemic subjects with metabolic syndrome. Metabolism 57: 1740–1746, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Kruger A, Stewart J, Sahityani R, O'Riordan E, Thompson C, Adler S, Garrick R, Vallance P, Goligorsky MS. Laser Doppler flowmetry detection of endothelial dysfunction in end-stage renal disease patients: correlation with cardiovascular risk. Kidney Int 70: 157–164, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Lang JA, Holowatz LA, Kenney WL. Local tetrahydrobiopterin administration augments cutaneous vasoconstriction in aged humans. J Physiol 587: 3967–3974, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lang JA, Holowatz LA, Kenney WL. Localized tyrosine or tetrahydrobiopterin supplementation corrects the age-related decline in cutaneous vasoconstriction. J Physiol 588: 1361–1368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lang JA, Jennings JD, Holowatz LA, Kenney WL. Reflex vasoconstriction in aged human skin increasingly relies on Rho kinase-dependent mechanisms during whole body cooling. Am J Physiol Heart Circ Physiol 297: H1792–H1797, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lorenzo S, Minson CT. Human cutaneous reactive hyperaemia: role of BKCa channels and sensory nerves. J Physiol 585: 295–303, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med 315: 1046–1051, 1986 [DOI] [PubMed] [Google Scholar]
- 61. Lutolf O, Chen D, Zehnder T, Mahler F. Influence of local finger cooling on laser Doppler flux and nailfold capillary blood flow velocity in normal subjects and in patients with Raynaud's phenomenon. Microvasc Res 46: 374–382, 1993 [DOI] [PubMed] [Google Scholar]
- 62. Martin HL, Loomis JL, Kenney WL. Maximal skin vascular conductance in subjects aged 5–85 yr. J Appl Physiol 79: 297–301, 1995 [DOI] [PubMed] [Google Scholar]
- 63. Maver J, Strucl M. Microvascular reactivity in normotensive subjects with a familial predisposition to hypertension. Microvasc Res 60: 241–248, 2000 [DOI] [PubMed] [Google Scholar]
- 64. McCord GR, Cracowski JL, Minson CT. Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Integr Comp Physiol 291: R596–R602, 2006 [DOI] [PubMed] [Google Scholar]
- 65. Medow MS, Minson CT, Stewart JM. Decreased microvascular nitric oxide-dependent vasodilation in postural tachycardia syndrome. Circulation 112: 2611–2618, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91: 1619–1626, 2001 [DOI] [PubMed] [Google Scholar]
- 67. Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol 93: 1644–1649, 2002 [DOI] [PubMed] [Google Scholar]
- 68. Murray AK, Moore TL, Manning JB, Taylor C, Griffiths CE, Herrick AL. Noninvasive imaging techniques in the assessment of scleroderma spectrum disorders. Arthritis Rheum 61: 1103–1111, 2009 [DOI] [PubMed] [Google Scholar]
- 69. Neunteufl T, Heher S, Katzenschlager R, Wolfl G, Kostner K, Maurer G, Weidinger F. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol 86: 207–210, 2000 [DOI] [PubMed] [Google Scholar]
- 70. Nicotra A, Asahina M, Mathias CJ. Skin vasodilator response to local heating in human chronic spinal cord injury. Eur J Neurol 11: 835–837, 2004 [DOI] [PubMed] [Google Scholar]
- 71. Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplification in humans. J Appl Physiol 101: 545–548, 2006 [DOI] [PubMed] [Google Scholar]
- 72. Park JB, Schiffrin EL. Small artery remodeling is the most prevalent (earliest?) form of target organ damage in mild essential hypertension. J Hypertens 19: 921–930, 2001 [DOI] [PubMed] [Google Scholar]
- 73. Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol 102: 1510–1519, 2007 [DOI] [PubMed] [Google Scholar]
- 74. Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol 568: 357–369, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol 51: 997–1002, 2008 [DOI] [PubMed] [Google Scholar]
- 76. Roustit M, Blaise S, Millet C, Cracowski JL. Reproducibility and methodological issues of skin post-occlusive and thermal hyperemia assessed by single-point laser Doppler flowmetry. Microvasc Res 79: 102–108, 2010 [DOI] [PubMed] [Google Scholar]
- 77. Roustit M, Maggi F, Isnard S, Hellmann M, Bakken B, Cracowski JL. Reproducibility of a local cooling test to assess microvascular function in human skin. Microvasc Res 79: 34–39, 2010 [DOI] [PubMed] [Google Scholar]
- 78. Roustit M, Simmons GH, Baguet JP, Carpentier P, Cracowski JL. Discrepancy between simultaneous digital skin microvascular and brachial artery macrovascular post-occlusive hyperemia in systemic sclerosis. J Rheumatol 35: 1576–1583, 2008 [PMC free article] [PubMed] [Google Scholar]
- 79. Stewart J, Kohen A, Brouder D, Rahim F, Adler S, Garrick R, Goligorsky MS. Noninvasive interrogation of microvasculature for signs of endothelial dysfunction in patients with chronic renal failure. Am J Physiol Heart Circ Physiol 287: H2687–H2696, 2004 [DOI] [PubMed] [Google Scholar]
- 80. Stewart JM, Medow MS, Minson CT, Taneja I. Cutaneous neuronal nitric oxide is specifically decreased in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 293: H2161–H2167, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sullivan JM, Prewitt RL, Josephs JA. Attenuation of the microcirculation in young patients with high-output borderline hypertension. Hypertension 5: 844–851, 1983 [DOI] [PubMed] [Google Scholar]
- 82. Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, Satomura K, Ohsuzu F, Kurita A. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol 82: 1535–1539, 1998 [DOI] [PubMed] [Google Scholar]
- 83. Taylor WF, Johnson JM, O'Leary D, Park MK. Effect of high local temperature on reflex cutaneous vasodilation. J Appl Physiol 57: 191–196, 1984 [DOI] [PubMed] [Google Scholar]
- 84. Taylor WF, Johnson JM, O'Leary DS, Park MK. Modification of the cutaneous vascular response to exercise by local skin temperature. J Appl Physiol 57: 1878–1884, 1984 [DOI] [PubMed] [Google Scholar]
- 85. Thompson-Torgerson CS, Holowatz LA, Flavahan NA, Kenney WL. Cold-induced cutaneous vasoconstriction is mediated by Rho kinase in vivo in human skin. Am J Physiol Heart Circ Physiol 292: H1700–H1705, 2007 [DOI] [PubMed] [Google Scholar]
- 86. Thompson CS, Holowatz LA, Kenney WL. Attenuated noradrenergic sensitivity during local cooling in aged human skin. J Physiol 564: 313–319, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Thompson CS, Holowatz LA, Kenney WL. Cutaneous vasoconstrictor responses to norepinephrine are attenuated in older humans. Am J Physiol Regul Integr Comp Physiol 288: R1108–R1113, 2005 [DOI] [PubMed] [Google Scholar]
- 88. Tom WJ, Ponticorvo A, Dunn AK. Efficienct processing of laser speckle contrast images. IEEE Trans Med Imaging 27: 1728–1738, 2008 [DOI] [PubMed] [Google Scholar]
- 89. Turner J, Belch JJ, Khan F. Current concepts in assessment of microvascular endothelial function using laser Doppler imaging and iontophoresis. Trends Cardiovasc Med 18: 109–116, 2008 [DOI] [PubMed] [Google Scholar]
- 90. Tzoulaki I, Liberopoulos G, Ioannidis JP. Assessment of claims of improved prediction beyond the Framingham risk score. JAMA 302: 2345–2352, 2009 [DOI] [PubMed] [Google Scholar]
- 91. Van Duijnhoven NT, Janssen TW, Green DJ, Minson CT, Hopman MT, Thijssen DH. Effect of functional electrostimulation on impaired skin vasodilator responses to local heating in spinal cord injury. J Appl Physiol 106: 1065–1071, 2009 [DOI] [PubMed] [Google Scholar]
- 92. Vuilleumier P, Decosterd D, Maillard M, Burnier M, Hayoz D. Postischemic forearm skin reactive hyperemia is related to cardovascular risk factors in a healthy female population. J Hypertens 20: 1753–1757, 2002 [DOI] [PubMed] [Google Scholar]
- 93. Wick DE, Roberts SK, Basu A, Sandroni P, Fealey RD, Sletten D, Charkoudian N. Delayed threshold for active cutaneous vasodilation in patients with Type 2 diabetes mellitus. J Appl Physiol 100: 637–641, 2006 [DOI] [PubMed] [Google Scholar]
- 94. Wigington G, Ngo B, Rendell M. Skin blood flow in diabetic dermopathy. Arch Dermatol 140: 1248–1250, 2004 [DOI] [PubMed] [Google Scholar]
- 95. Wong BJ, Wilkins BW, Holowatz LA, Minson CT. Nitric oxide synthase inhibition does not alter the reactive hyperemic response in the cutaneous circulation. J Appl Physiol 95: 504–510, 2003 [DOI] [PubMed] [Google Scholar]
- 96. Wong BJ, Williams SJ, Minson CT. Minimal role for H1 and H2 histamine receptors in cutaneous thermal hyperemia to local heating in humans. J Appl Physiol 100: 535–540, 2006 [DOI] [PubMed] [Google Scholar]
- 97. Yamazaki F, Sone R, Zhao K, Alvarez GE, Kosiba WA, Johnson JM. Rate dependency and role of nitric oxide in the vascular response to direct cooling in human skin. J Appl Physiol 100: 42–50, 2006 [DOI] [PubMed] [Google Scholar]
- 98. Yvonne-Tee GB, Rasool AH, Halim AS, Rahman AR. Noninvasive assessment of cutaneous vascular function in vivo using capillaroscopy, plethysmography and laser-Doppler instruments: its strengths and weaknesses. Clin Hemorheol Microcirc 34: 457–473, 2006 [PubMed] [Google Scholar]
- 99. Yvonne-Tee GB, Rasool AH, Halim AS, Rahman AR. Reproducibility of different laser Doppler fluximetry parameters of postocclusive reactive hyperemia in human forearm skin. J Pharmacol Toxicol Methods 52: 286–292, 2005 [DOI] [PubMed] [Google Scholar]
- 100. Zhao JL, Pergola PE, Roman LJ, Kellogg DL., Jr Bioactive nitric oxide concentration does not increase during reactive hyperemia in human skin. J Appl Physiol 96: 628–632, 2004 [DOI] [PubMed] [Google Scholar]