Abstract
Recommendations for the measurement of brachial flow-mediated dilation (FMD) typically suggest images be obtained at identical times in the cardiac cycle, usually end diastole (QRS complex onset). This recommendation presumes that inter-individual differences in arterial compliance are minimized. However, published evidence is conflicting. Furthermore, ECG gating is not available on many ultrasound systems; it requires an expensive software upgrade or increased image processing time. We tested whether analysis of images acquired with QRS gating or with the more simplified method of image averaging would yield similar results. We analyzed FMD and nitroglycerin-mediated dilation (NMD) in 29 adults with type 2 diabetes mellitus and in 31 older adults and 12 young adults without diabetes, yielding a range of brachial artery distensibility. FMD and NMD were measured using recommended QRS-gated brachial artery diameter measurements and, alternatively, the average brachial diameters over the entire R-R interval. We found strong agreement between both methods for FMD and NMD (intraclass correlation coefficients = 0.88–0.99). Measuring FMD and NMD using average diameter measurements significantly reduced post-image-processing time (658.9 ± 71.6 vs. 1,024.1 ± 167.6 s for QRS-gated analysis, P < 0.001). FMD and NMD measurements based on average diameter measurements can be performed without reducing accuracy. This finding may allow for simplification of FMD measurement and aid in the development of FMD as a potentially useful clinical tool.
Keywords: endothelium, shear stress, flow-mediated dilation, method
endothelial dysfunction is characterized by a proinflammatory, prothrombotic, and vasoconstrictive phenotype (16). Maintenance of nitric oxide (NO) bioavailability through activation of endothelial NO synthase is of central importance in maintaining vascular homeostasis.
Substantial evidence supports the concept that detection of endothelial dysfunction in the brachial artery is an indicator of systemic endothelial dysfunction (32). Brachial artery reactivity [flow-mediated dilation (FMD)], as measured by high-resolution vascular ultrasound prior to and following a hyperemic flow stimulus, is highly dependent on NO bioavailability (9, 20). Furthermore, endothelial dysfunction as detected by FMD predicts future cardiovascular risk in those with and without diagnosed coronary artery disease (4, 5, 11, 15, 25, 26, 28, 30, 35). FMD's ability to stratify individuals into low-, intermediate-, and high-risk categories for future cardiovascular events (35, 36) not only yields potentially clinically relevant information but also identifies the efficacy of medical interventions that enhance endothelial function (23, 25).
The potential of brachial FMD to be used as a barometer of cardiovascular risk at a population level has led to efforts to standardize the technique with the hope of maximizing the validity and reproducibility of the measurement (7). Recent reviews suggest further refinements to these recommendations (8, 18, 29). These publications recommend measurement of brachial artery diameter at the same time in the cardiac cycle, most commonly gated to onset of the QRS complex at end diastole (7). This recommendation stems from concerns that the arterial distension occurring during systole may vary on the basis of non-endothelium-dependent factors affecting arterial compliance, reducing the measurement's validity as a measure of dynamic endothelial function. However, QRS-gated image capture is not available on all vascular ultrasound machines, particularly more economical and portable models. The lack of readily obtainable QRS-gated still images for analysis increases the complexity of postimaging processing and analysis and remains a barrier to any potential clinical application of FMD (32). Prior investigations into the necessity of QRS gating has yielded conflicting results (6, 13, 13). We hypothesized that deriving FMD using diameters from entire R-R intervals would be in strong agreement with traditional methods that select diameters at the onset of the QRS complex across a wide range of brachial artery distensibilities.
MATERIALS AND METHODS
Subjects
We randomly selected 1) healthy, physically active young adults (ages 18–35 yr), 2) healthy middle-aged adults (age 36–45 yr), 3) healthy older adults (age 46–70 yr), and 4) adults (age 36–70 yr) with type 2 diabetes mellitus (DM) from whom baseline FMD measurements were obtained for other study protocols for this analysis. This was done to ensure that our alternative method for FMD measurement remained robust across a wide range of baseline cardiovascular risk. Participants were recruited via flyers and internet advertisements. DM adults qualified for enrollment if they were between 35 and 70 yr of age and met the American Diabetes Association criteria for a diagnosis of type 2 DM (14). Potential healthy control subjects were excluded if they had a history of cardiovascular disease, showed evidence of active chronic liver, renal, or neoplastic disease, were pregnant at the time of screening, or used tobacco products within 1 yr of enrollment. Additionally, control subjects were excluded from enrollment during screening if they were found to have a fasting serum glucose ≥126 mg/dl, elevated blood pressure (systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or previous diagnosis of or current treatment for hypertension), or elevated triglycerides (>200 mg/dl) or if they qualified for the diagnosis of metabolic syndrome (10). Young adults were considered to be physically active if they ran ≥15 miles/wk, lifted weights at least three times per week, or both, for ≥6 mo prior to enrollment. Young adults not meeting any of these three criteria were excluded. All study protocols were approved by the Institutional Research Board of the Medical College of Wisconsin, and all subjects gave their verbal and written informed consent prior to all study procedures.
Study Protocol
Subjects arrived at the Medical College of Wisconsin's Adult Translational Research Unit after an overnight (8–12 h) fast. Vital signs (heart rate and blood pressure) were measured in triplicate, and height, weight, and waist circumference at the umbilicus were measured and recorded. All studies were performed between 8 and 10 AM to limit the influence of circadian variations on endothelial function.
Participants were then asked to lie supine in a quiet, dimly lit, temperature-controlled (22–24°C) study room for 20 min prior to any measurements of brachial artery reactivity. B-mode ultrasound images (Logiq 500PRO, GE, Milwaukee, WI; or MicroMaxx, Sonosite, Seattle, WA) of the brachial artery in the dominant arm were obtained with the arm supinated and abducted ∼80°. Images were obtained at a standard depth of 4 cm. The linear-array ultrasound probe (11 MHz) was positioned longitudinally at a site 1–3 cm proximal to the cubital fossa. A three-lead ECG system was attached to the participant, and its input was sent to the ultrasound machines for simultaneous recording of the ECG representation of the cardiac cycle with our brachial artery images to facilitate determination of the timing of events in the brachial artery relative to the cardiac cycle.
Brachial Artery Measurements
Flow- and nitroglycerin-mediated dilation.
Brachial artery diameters were measured throughout the cardiac cycle at rest (baseline) and during reactive hyperemia produced by 5 min of forearm cuff inflation above suprasystolic levels (50 mmHg above systolic pressure or 200 mmHg, whichever was greater). Images of the brachial artery in longitudinal cross section were recorded continuously throughout the entire study onto a VHS tape. From the recorded images, 100 frames (10 frames/s for 10 s) were captured and digitized using edge-detection software (Brachial Analyzer version 4.2.2, Medical Imaging Applications) at baseline and 1, 2, and 3 min after cuff deflation.
We determined the set of post-cuff-release values to be used for measurements of brachial diameter for each subject as follows. The 10-s interval 1 min after cuff release (55–65 s), 2 min after cuff release (115–125 s), or 3 min after cuff release (175–185 s) with the highest mean percent FMD over the complete set of R-R intervals was selected for comparison with baseline. Postreactive hyperemia FMD average measures (FMD%Ave and FMDmmAve) were calculated for each subject by averaging the mean brachial diameter of each complete, individual R-R interval during the selected captured 10-s interval following cuff release and subtracting this value from the mean diameters for each complete R-R interval captured during the 10-s baseline brachial artery measurement. Images for QRS analyses were selected by the sonographer but were analyzed using the automated edge-detection software. FMD%Ave is this difference divided by the calculated mean baseline brachial diameter. QRS-gated FMD (FMD%QRS and FMDmmQRS) were calculated in an identical manner by substitution of manually selected diameters at the onset of the QRS complex for the average diameter across each R-R interval. From 10 subjects randomly selected from studies in our laboratory, intra- and interobserver correlation coefficients are as follows: FMD%QRS = 0.96 and 0.85, FMD%Ave = 0.99 and 0.87. The mean absolute differences measured between observers was as follows: FMD%QRS = 1.2 ± 0.8%, FMD%Ave = 1.0 ± 0.8%. Similar assessments of nitroglycerin-mediated dilation (NMD) measurements were made following sublingual administration of 0.4 mg of nitroglycerin. The 10-s span after nitroglycerin dilation with the greatest average brachial diameter (sampled at 2, 3, 4, and 5 min after nitroglycerin administration) was the time span chosen for analysis for each individual subject.
Flow velocity and shear stress in the brachial artery.
Baseline brachial flow velocity and peak hyperemic flow velocity (recorded immediately after cuff release) were measured using pulse-wave Doppler signals with the sample volume placed at the center of the lumen of the brachial artery signal with a 60° correction for the angle of insonation. We measured baseline and hyperemic brachial artery shear stress (dyn/cm2) using the following formulas: 2.8 * baseline flow velocity/baseline diameter and 2.8 * hyperemic flow velocity/baseline diameter, respectively. These equations assume a constant viscosity of 0.035 dyn·s·cm−2.
Brachial artery compliance.
Brachial artery compliance, as estimated by cross-sectional distensibility (10−3/mmHg), was measured using the following formula: (2 * ΔD * Dmin + ΔD2)/(ΔP * Dmin2) (12), where ΔD is the difference between the average minimum and maximum baseline brachial artery diameter for each complete R-R interval recorded at baseline, ΔP is the pulse pressure averaged from three baseline blood pressure measurements, and Dmin is the average minimum baseline brachial artery diameter. From 10 subjects randomly selected from studies in our laboratory, the intra- and interobserver correlation coefficients for brachial artery distensibility were 0.98 and 0.85, respectively. The mean absolute difference measured between observers was 0.4 ± 0.4 10−3/mmHg.
Statistical Analysis
SigmaStat 3.1 and SPSS 12.0 were used for the statistical analyses. Baseline characteristics for DM and non-DM adults were compared using ANOVA, χ2 tests, or Fisher's exact tests as appropriate. Data were logarithmically transformed or analyzed using appropriate nonparametric testing if found to have a skewed distribution. Post hoc testing using Student-Newman-Keuls test or Dunn's method was performed as applicable. Intraclass correlation coefficients were used to compare average-based and QRS-gated based measurements for FMD and NMD determinations using all participants in the analysis. The entire dataset was used in Bland-Altman plots that were generated by comparing the average of two sets of values with the difference between the same two sets of values for average FMD and NMD measurement protocols vs. QRS-gated FMD and NMD measurement protocols. P < 0.05 was considered to be significant.
RESULTS
Participant Demographics
A total of 31 DM, 17 middle-aged, 17 older, and 12 young, physically active adults were initially chosen at random for this study. Five subjects (2 DM and 3 older adults) were excluded because of technically inadequate scans, leaving 29 DM and 14 older adults. The baseline profiles of each group and comparisons between participant groups are shown in Table 1. As discussed in materials and methods, selection of our older adult population excluded participants with cardiovascular risk factors, including hypertension and hyperlipidemia, resulting in healthy middle-aged and older adult populations on few prescription medications. As expected, the DM cohort had a significantly larger waist circumference and higher serum triglyceride levels than both nondiabetic groups. The medications taken by the subjects are shown in Table 2.
Table 1.
Subect demographics
| Young Adults (n = 12) | Middle-Aged Adults (n = 17) | Older Adults (n = 14) | Type 2 DM Adults (n = 29) | |
|---|---|---|---|---|
| Age,a yr | 26 ± 3 | 41 ± 3 | 50 ± 4 | 54 ± 8 |
| Sex, %female | 42 | 59 | 71 | 63 |
| Body mass index,b kg/m2 | 24 ± 4 | 29 ± 6 | 28 ± 4 | 35 + 8 |
| Waist circumference,c cm | 79 ± 11 | 96 ± 17 | 95 ± 10 | 113 + 18 |
| Blood pressure, mmHg | ||||
| Systolicd | 115 ± 8 | 118 ± 18 | 130 ± 21 | 133 ± 20 |
| Diastolice | 55 ± 9 | 71 ± 11 | 78 ± 14 | 74 ± 10 |
| Resting heart rate,f beats/min | 54 ± 7 | 64 ± 11 | 65 ± 10 | 71 ± 12 |
| Cholesterol, mg/dl | ||||
| Totalg | 172 ± 25 | 205 ± 50 | 179 ± 32 | |
| LDLh | 102 ± 22 | 128 ± 37 | 100 ± 30 | |
| HDL | 52 ± 15 | 60 ± 19 | 50 ± 13 | |
| Triglycerides,i mg/dl | 97 ± 39 | 86 ± 34 | 147 ± 67 |
Values are means ± SD. Lipid profile data were not available for the young adult group. DM, diabetes mellitus.
P <0.001 overall; P < 0.05, young and middle-aged vs. older and type 2 DM adults.
P < 0.001 overall; P < 0.05, young vs. type 2 DM adults.
P < 0.001 overall; P < 0.05, young and middle-aged vs. type 2 DM adults.
P = 0.006 overall; P < 0.05, young and middle-aged vs. type 2 DM adults.
P < 0.001 overall; P < 0.05, young adults vs. all other groups.
P < 0.001 overall; P < 0.05, young vs. older and DM adults.
P = 0.034 overall; P < 0.05, middle-aged vs. older adults.
P = 0.02 overall; P < 0.05, older vs. middle-aged and DM adults.
P = 0.001 overall; P < 0.05, older and middle-aged vs. DM adults.
Table 2.
Medication profiles of participant populations
| Medications | Young Adults (n = 12) | Middle-Aged Adults (n = 17) | Older Adults (n = 14) | DM Adults (n =29) |
|---|---|---|---|---|
| β-Blockers | 0 | 0 | 0 | 7 |
| Ca2+ channel blockers | 0 | 0 | 0 | 3 |
| ACE inhibitors | 0 | 0 | 0 | 6 |
| ANG II receptor blockers | 0 | 0 | 0 | 8 |
| HMG-CoA reductase inhibitors | 0 | 0 | 0 | 12 |
| Aspirin | 0 | 0 | 0 | 13 |
| Metformin | 0 | 0 | 0 | 22 |
| Sulfonylureas | 0 | 0 | 0 | 11 |
| Thiazoladinediones | 0 | 0 | 0 | 3 |
| Multivitamin | 0 | 5 | 3 | 12 |
| Hormone replacement therapy | 0 | 1 | 2 | 2 |
ACE, angiotensin-converting enzyme. HMG-CoA, 3-hydroxy-3-methyl-glutaryl-CoA.
Comparisons of Measurements of FMD, Shear, and Vessel Compliance
Tables 3 and 4 demonstrate the brachial diameters, absolute FMD (FMDmm) and percent FMD (FMD%), and absolute NMD (NMDmm) and percent NMD (NMD%) for each method of measurement by cohort. Within subject groups, there were no significant differences between QRS-gated and averaged measurements for any of these parameters. Between groups, brachial artery diameter was larger in the young adult group than all other groups (P < 0.05), and FMD% and FMDmm were significantly lower in the DM group than all other groups. NMDmm was significantly greater in the young compared with older and DM adults (P < 0.05) and was also greater in middle-aged than DM adults (P < 0.05). The between-subjects findings were identical, regardless of the diameter measurement method employed.
Table 3.
Brachial artery diameter and FMD measurements
| FMD |
||||
|---|---|---|---|---|
| n | Baseline Diameter, mm | mm | % | |
| Young adults | 12 | |||
| QRS wave-gated | 5.29 ± 0.84 | 0.35 ± 0.12 | 6.6 ± 1.9 | |
| Average | 5.33 ± 0.82 | 0.35 ± 0.13 | 6.5 ± 1.7 | |
| Middle-aged adults | 17 | |||
| QRS wave-gated | 3.79 ± 0.72 | 0.25 ± 0.06 | 6.4 ± 0.7 | |
| Average | 3.86 ± 0.72 | 0.25 ± 0.06 | 6.4 ± 0.8 | |
| Older adults | 14 | |||
| QRS wave-gated | 3.51 ± 0.61 | 0.22 ± 0.05 | 6.6 ± 1.3 | |
| Average | 3.46 ± 0.56 | 0.23 ± 0.04 | 6.6 ± 1.0 | |
| DM adults | 29 | |||
| QRS wave-gated | 3.63 ± 0.71 | 0.13 ± 0.06 | 3.9 ± 2.0 | |
| Average | 3.64 ± 0.70 | 0.13 ± 0.06 | 3.8 ± 1.8 | |
Values are means ± SD. FMD, flow-mediated dilation. There were no significant differences in baseline diameter, FMDmm, and FMD% between measurement methods within each of the 3 study groups (P > 0.05 for all comparisons). For between-group comparisons, baseline diameter (QRS-gated and average): P < 0.05, young adults vs. all other groups. For FMDmm ECG and average-gated: P < 0.05, all groups vs. DM adults. For FMD% ECG and average-gated, P < 0.05, all groups vs. DM adults. Findings are identical for FMD ECG and FMD%.
Table 4.
Brachial artery diameter and NMD measurements
| NMD |
||||
|---|---|---|---|---|
| n | Baseline Diameter | mm | % | |
| Young adults | 12 | |||
| QRS wave-gated | 5.36 ± 0.81 | 1.21 ± 0.22 | 22.9 ± 4.8 | |
| Average | 5.42 ± 0.82 | 1.18 ± 0.21 | 24.9 ± 5.6 | |
| Middle-aged adults | 14 | |||
| QRS-gated | 4.00 ± 0.70 | 1.00 ± 0.21 | 25.6 ± 6.1 | |
| Average | 4.04 ± 0.70 | 0.98 ± 0.23 | 25.2 ± 6.0 | |
| Older adults | 11 | |||
| QRS wave-gated | 3.44 ± 0.63 | 0.86 ± 0.22 | 25.4 ± 6.0 | |
| Average | 3.48 ± 0.62 | 0.85 ± 0.22 | 24.7 ± 5.3 | |
| DM adults | 26 | |||
| QRS wave-gated | 3.63 ± 0.67 | 0.76 ± 0.20 | 21.3 ± 5.5 | |
| Average | 3.65 ± 0.67 | 0.75 ± 0.19 | 20.9 ± 5.4 | |
Values are means ± SD. NMD, nitroglycerin-mediated dilation. There were no significant differences in NMDmm and NMD% between measurement methods within each of the 3 study groups (P > 0.05 for all comparisons). Between-group differences for QRS-gated and average measurements: P < 0.001 overall, P < 0.05, young adults vs. all other groups (baseline diameter); P < 0.001 overall, P < 0.05, young vs. older and DM adults; P < 0.05, middle-aged vs. DM adults (NMDmm); no significant differences detected (NMD%). No differences between methods were detected.
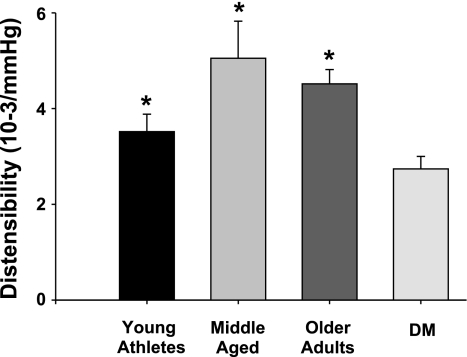
Brachial artery distensibility was significantly lower in the DM group than all other groups (Fig. 1). Brachial distensibility in young athletes trended lower than in older healthy control groups, but these differences did not reach statistical significance. Baseline shear was significantly lower in young athletes than older adults, but there was no significant difference in the peak hyperemic shear response [baseline shear: 28 ± 10, 33 ± 10, 45 ± 17, and 38 ± 14 dyn/cm2 (P = 0.008, overall P = 0.007, young athletes vs. older adults); peak hyperemic shear: 59 ± 14, 65 ± 26, 79 ± 32, and 68 ± 28 dyn/cm2 for young, middle-aged, older, and DM adults, respectively (P = 0.32 overall)].
Fig. 1.
Brachial artery distensibility between groups. Brachial distensibility was significantly lower in adults with type 2 diabetes mellitus (DM) than all other groups: 3.5 ± 1.2, 5.1 ± 3.2, 4.5 ± 1.1, and 2.7 ± 1.4 10−3/mmHg for young, middle-aged, older, and DM, respectively (P < 0.001 overall; P < 0.001, DM vs. middle-aged and older controls; P = 0.025 vs. young athletes).
To determine whether average and QRS-gated measurements yielded comparable results along a range of brachial artery distensibilities, we combined the populations and calculated the intraclass correlation coefficients between the QRS-gated and average measurements. Measurements based on average diameters showed a very high degree of similarity to QRS-gated FMD measurements (0.98, 0.88, 0.97, and 0.99 FMD%, FMDmm, NMD%, and NMDmm, respectively, P < 0.001 for all comparisons).
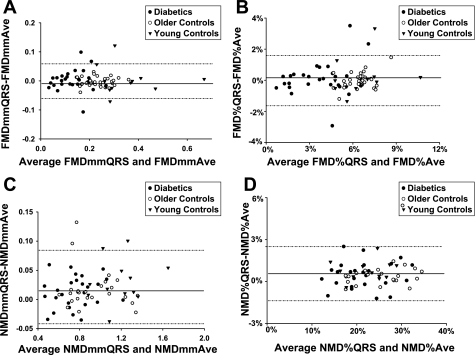
Furthermore, we generated Bland-Altman plots to find evidence of significant measurement bias or measurement discrepancies between QRS-gated and average FMD and NMD measurements (Fig. 2). The average bias approached zero for average FMD and NMD measurements compared with the QRS-gated measurements in all cases. The percent agreement of the average-based measurements within the 95% confidence interval of the average bias relative to the QRS-gated FMD and NMD were as follows: 94.4% (FMDmm), 94.4% (FMD%), 93.6% (NMDmm), and 98.4% (NMD%).
Fig. 2.
Bland-Altman plots for absolute and percent flow-mediated dilation (FMDmm and FMD%) and absolute and percent nitroglycerin-mediated dilation (NMDmm and NMD%) comparing average (Ave) with QRS-gated method of measurement. Overall, average biases were −0.0003, 0.06%, 0.017, and 0.57% for FMDmmAve (A), FMD%Ave (B), NMDmmAve (C), and NMD%Ave (D), respectively. At least 93% of all mean differences fell within the 95% confidence interval for the reported average biases.
The time saved by using average FMD and NMD measurements, rather than QRS-gated methods, was calculated (Table 5). In the hands of our technician with 3 years of experience with this technique, diameter measurements using the average diameter, compared with QRS-gated diameters, saved an average of 3.2 min for FMD measurement alone (P < 0.001) and an average of 6.1 min (P < 0.001) for studies including assessment of NMD.
Table 5.
Time required for QRS-gated vs. average FMD and NMD measurements
| FMD |
NMD |
Total |
|||
|---|---|---|---|---|---|
| Ave (sec) | QRS (sec) | Ave (sec) | QRS (sec) | Ave (sec) | QRS (sec) |
| 301 ± 33 | 495 ± 88* | 358 ± 45 | 530 ± 94† | 659 ± 72 | 1,024 ± 168‡ |
Values are means ± SD; n =10 for all measurements.
P < 0.001 vs. FMD-Ave.
P < 0.001 vs. NMD-Ave.
P < 0.001 vs. total Ave.
DISCUSSION
This study demonstrates that measuring brachial artery diameter as an average of all diameter measurements taken throughout the cardiac cycle yields calculated FMD and NMD values that are in strong agreement with FMD and NMD values derived from traditional brachial diameter measurements at the onset of the QRS complex. This agreement is seen across the spectrum of brachial artery compliance represented in our population. These data suggest that FMD and NMD calculations using nongated protocols and automated edge-detection software are valid approximations of manual QRS-gated measurements. These data assuage concerns that nongated measurements would yield invalid results due to integration of structural characteristics of the vessel into FMD calculations (6, 7). Furthermore, these data suggest a valid method to simplify image capture requirements and reduce offline analysis time for brachial artery FMD studies, important steps in moving toward wider application of this technique in cardiovascular risk stratification (32).
The 2002 recommendations for the ultrasonographic assessment of FMD in the brachial artery represent a thorough review of proper techniques for the acquisition, analysis, and reporting of brachial FMD, along with discussion of appropriate training and quality assurance protocols (7). Since the writing of these guidelines, further refinements have been suggested to increase the validity and reproducibility of the results, including more specific suggestions for cuff location, justification for the use of a 5-min occlusion interval, and analysis of the shear stimulus (9, 18, 29). With respect to the timing of diameter measurements, there have been longstanding concerns that FMD measurements that incorporate non-end-diastolic diameters may introduce measurement errors due to fixed structural issues (7) and a very recent FMD review advocated using the QRS-gated approach (18). However, only three prior studies have investigated whether non-QRS-gated brachial measurements show significant differences from FMD measurements using only end-diastolic measurements. One study of 24 subjects at increased cardiovascular risk demonstrated wide variation in FMD% when the cardiac cycle was ignored and FMD% was calculated using the single largest and smallest brachial diameters measured at baseline and following reactive hyperemia (6). Our method of measuring FMD and NMD differs significantly from that reported in this prior work, and we find much greater agreement with QRS-gated FMD measurements.
Another prior report compared measurement of FMD using a novel filter to remove higher-frequency oscillations in the brachial diameter waveform that occur because of the influence of the cardiac cycle (13). The resultant waveform contains diameters theoretically under the influence of only intrinsic vasodilatory and vasoconstrictive influences. Using this method, the group found significant agreement between their method of brachial artery measurement and ECG-gated measurements. While promising, our results demonstrate that the added expense and technical complication posed by the addition of a filter may not be necessary to obtain valid measurements of FMD and NMD.
A recent observation by Padilla et al. (27) supports our findings. Internal data from their laboratory showed that continuous assessment of diameter at 5 frames/s yields the same FMD results as R-wave-gated images (n = 10, FMD = 7.58 ± 0.9 vs. 7.62 ± 0.9%, P = 0.655, intraclass correlation = 0.998). Our data confirm these findings in a larger dataset and demonstrate, for the first time, that utilization of the proposed approach is acceptable in populations with a wide range of compliance.
Fixed structural factors influencing vessel compliance, particularly those related to brachial artery remodeling in the setting of risk factors, have been reported to affect FMD responses. Structural remodeling involves complex interactions between mechanical forces, metabolic factors, and paracrine influences on vascular wall composition, leading to fibrotic changes, increased collagen cross-linking, smooth muscle hyperplasia, and, ultimately, reductions in overall arterial compliance. While earlier studies suggest that local reductions in brachial artery compliance are modest with aging and hypertension (19, 22), other work suggests that brachial compliance modifies the relationship between smoking, age, and FMD measured using QRS-gated diameters (33). However, our data demonstrate that use of average brachial artery diameters to measure FMD and NMD yields strong agreement with QRS-gated FMD and NMD measurements across a spectrum of individual brachial compliances. The use of average diameters may limit the effects of differences in brachial artery compliance on FMD and NMD by “averaging out” the extremes of the brachial diameter spectrum during the measurement period. A deeper understanding of the relative contributions of dynamic production of endothelium-derived NO and vascular stiffness to FMD measurements requires further study.
Viable clinical screening tools must be easily accessible, valid, reproducible, safe, and appropriately stratify risk. Furthermore, in order for a clinical screening tool to reach a stage where it can be applied at a population level, it must also be used in a rapid and cost-effective manner. We found that post-image analysis time required to measure FMD and NMD was significantly shorter when average brachial diameter measurements were used than when manually selected images on the QRS complex were used. For an individual or a small study the size of this report, a 3- to 6-min difference in the time required for data analysis has relatively little impact on resource utilization or costs. However, the importance of a small reduction in analysis time for an individual study is realized when FMD is projected for use as a potential population screening tool to assess cardiovascular risk. For example, using our measurement method to screen a population of 5,000 would save 250–500 h of analysis time over a QRS-gated protocol. Protocol simplification, reductions in resource utilization, and increased accessibility, all of which can be realized by application of our measurement protocol, are three of the many important factors to consider when FMD is contemplated as a population screening tool to detect individuals at greatest risk for adverse cardiovascular events (32). Software designs that allow for selection of ECG-gated images without additional hardware for ECG detection will also aid in the promulgation of FMD measurements in cardiovascular screening (34).
While not the primary purpose of this study, the vascular structure of our young adult athlete group merits consideration. Our young adults had a significantly larger baseline diameter than older and DM adults. This finding is consistent with prior exercise training, which is known to lead to vascular remodeling that includes increased luminal size (17, 21). We found no significant difference in FMD% in young athletic subjects relative to the other two, older control groups, a finding also consistent with prior work suggesting that, in healthy populations, increases in FMD with exercise are seen only within the first several weeks of training (31). The inverse relationship between brachial artery diameter and FMD% may also, in part, explain this finding (1). Interestingly, we also found that baseline shear was lower in young athletes than older adults, and we identified an unexpected trend toward reduced brachial distensibility relative to the healthy, older populations in this study (P = 0.09 vs. middle-aged adults and P = 0.07 vs. older adults). Taken together with differences in FMD% and brachial artery size, these data suggest significant vascular structural and dynamic adaptations to chronic exercise training, although our data are limited by a lack of a healthy young sedentary control group. These adaptations may complicate comparisons of vascular health between groups with disparate exercise training backgrounds that use FMD as the metric for estimating cardiovascular risk. Further work is necessary to delineate these alterations and how best to account for them when assessing the vascular health of athletically trained individuals.
Our study has several limitations. 1) Our findings can be generalized only to FMD protocols that place the blood pressure cuff below the antecubital fossa and image the artery proximal to cuff placement. FMD protocols that image distal to the cuff placement may expose the vessel to local ischemic effects and anatomic distortions that could potentially reduce the agreement between these measurements (29). However, on the basis of a recent meta-analysis, in >80% of reports of FMD measurements, the brachial artery was imaged proximal to the location of cuff occlusion. This allows our data to be generalized to the majority of laboratories (3). 2) Our formula for the shear stress calculation is based on an assumed and constant blood viscosity, applies only to straight vessels without branches, and does not account for pulsatile flow. However, on the basis of prior work in a large cohort (24), these assumptions appear to be reasonable in the brachial artery. 3) We chose to analyze a total of 30 s after cuff release for measurement of FMD. Other groups have shown that measurement of brachial diameters by continuous measurement of the posthyperemic diameter for ≥3 min after cuff release and use of the median baseline and peak diameters identified to calculate FMD more reliably identify true maximal FMD (2). This more accurate identification of the true maximal hyperemic diameter likely reduces variability due to measurement error, thus increasing study power. Similarly, increased variability secondary to incomplete sampling of the brachial diameter after cuff release may lead to an inability to discern differences between groups (2). We have analyzed our data using median, rather than mean, diameters, and our findings are similar (data not shown). Furthermore, our use of non-QRS-gated measurement of diameters would likely reap significantly greater reductions in analysis time for a continuous capture protocol of 180 s vs. the 30 s analyzed in our present study, particularly for those using automatic edge-detection software similar to that used in this study. Finally, our data arise from a small dataset, and independent validation of this finding would further support our hypothesis.
In conclusion, we found that use of average brachial artery diameters and automated edge-detection software to calculate FMD and NMD shows a high level of agreement with the currently accepted standard QRS-gated brachial artery measurement protocol over a range of brachial compliances. Use of the average brachial diameter over the cardiac cycles measured at baseline and peak reduces the technical complexity of the procedure and offline analysis time. To be sure, multiple issues, including standardization of protocols and normative values, as well as technical improvements to improve reproducibility and image quality and reduce the technical demands of the procedure, need to be addressed before the measurement of FMD is advocated as a valid clinical prediction tool. Nevertheless, our data support a methodology of FMD and NMD assessment that could allow for wider application of this methodology.
GRANTS
T. J. Kizhakekuttu is supported by National Heart, Lung, and Blood Institute (NHLBI) Ruth L. Kirschstein Training Grant T32 HL-007792-15, D. D. Gutterman is supported by NHLBI Grants HL-094971 and HL-080704, S. A. Phillips is supported by NHLBI Grant K23 HL-085614, and M. E. Widlansky is supported by NHLBI Grant K23 HL-089326 and Advancing a Healthier Wisconsin Grant 5520119-9520094. This work was supported by the Clinical Translational Research Institute at the Medical College of Wisconsin and NHLBI Grant HL-081587.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF, Jr, Lehman BT, Fan S, Osypiuk E, Vita JA. Clinical correlates and heritability of flow-mediated dilation in the community: The Framingham Heart Study. Circulation 109: 613–619, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 51: 203–210, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Bots ML, Westerink J, Rabelink TJ, de Koning EJ. Assessment of flow-mediated vasodilatation (FMD) of the brachial artery: effects of technical aspects of the FMD measurement on the FMD response. Eur Heart J 26: 363–368, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation 108: 2093–2098, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Chan SY, Mancini GB, Kuramoto L, Schulzer M, Frohlich J, Ignaszewski A. The prognostic importance of endothelial dysfunction and carotid atheroma burden in patients with coronary artery disease. J Am Coll Cardiol 42: 1037–1043, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Chuang ML, Douglas PS, Bisinov EA, Stein JH. Effect of cardiac cycle on ultrasound assessment of endothelial function. Vasc Med 7: 103–108, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Donald AE, Halcox JP, Charakida M, Storry C, Wallace SM, Cole TJ, Friberg P, Deanfield JE. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. J Am Coll Cardiol 51: 1959–1964, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond) 101: 629–635, 2001 [PubMed] [Google Scholar]
- 10. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 365: 1415–1428, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Fathi R, Haluska B, Isbel N, Short L, Marwick TH. The relative importance of vascular structure and function in predicting cardiovascular events. J Am Coll Cardiol 43: 616–623, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Frick M, Schwarzacher SP, Alber HF, Rinner A, Ulmer H, Pachinger O, Weidinger F. Morphologic rather than functional or mechanical sonographic parameters of the brachial artery are related to angiographically evident coronary atherosclerosis. J Am Coll Cardiol 40: 1825–1830, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Gemignani V, Bianchini E, Faita F, Giannarelli C, Plantinga Y, Ghiadoni L, Demi M. Ultrasound measurement of the brachial artery flow-mediated dilation without ECG gating. Ultrasound Med Biol 34: 385–391, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 26: 3160–3167, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of non-invasively-determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol 41: 1769–1775, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Gokce N, Keaney JF, Jr, Vita JA. Endotheliopathies: clinical manifestations of endothelial dysfunction. In: Thrombosis and Hemorrhage, edited by Loscalzo J, Shafer AI. Baltimore, MD: Williams & Wilkins, 1998, p. 901–924 [Google Scholar]
- 17. Green DJ. Exercise training as vascular medicine: direct impacts on the vasculature in humans. Exerc Sport Sci Rev 37: 196–202, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hayoz D, Rutschmann B, Perret F, Niederberger M, Tardy Y, Mooser V, Nussberger J, Waeber B, Brunner HR. Conduit artery compliance and distensibility are not necessarily reduced in hypertension. Hypertension 20: 1–6, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91: 1314–1319, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Jurva JW, Phillips SA, Syed AQ, Syed AY, Pitt S, Weaver A, Gutterman DD. The effect of exertional hypertension evoked by weight lifting on vascular endothelial function. J Am Coll Cardiol 48: 588–589, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Kawasaki T, Sasayama S, Yagi S, Asakawa T, Hirai T. Non-invasive assessment of the age related changes in stiffness of major branches of the human arteries. Cardiovasc Res 21: 678–687, 1987 [DOI] [PubMed] [Google Scholar]
- 23. Kitta Y, Obata JE, Nakamura T, Hirano M, Kodama Y, Fujioka D, Saito Y, Kawabata K, Sano K, Kobayashi T, Yano T, Nakamura K, Kugiyama K. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol 53: 323–330, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: The Framingham Heart Study. Hypertension 44: 134–139, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol 40: 505–510, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Neunteufl T, Heher S, Katzenschlager R, Wolfl G, Kostner K, Maurer G, Weidinger F. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol 86: 207–210, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Padilla J, Johnson BD, Newcomer SC, Wilhite DP, Mickleborough TD, Fly AD, Mather KJ, Wallace JP. Adjusting flow-mediated dilation for shear stress stimulus allows demonstration of endothelial dysfunction in a population with moderate cardiovascular risk. J Vasc Res 46: 592–600, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 104: 191–196, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Pyke KE, Tschakovsky ME. The shear stress stimulus FMD relationship: implications for FMD as an assessment of endothelial function. J Physiol 568: 357–369, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shimbo D, Grahame-Clarke C, Miyake Y, Rodriguez C, Sciacca R, Di TM, Boden-Albala B, Sacco R, Homma S. The association between endothelial dysfunction and cardiovascular outcomes in a population-based multi-ethnic cohort. Atherosclerosis 192: 197–203, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Tinken TM, Thijssen DH, Black MA, Cable NT, Green DJ. Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol 586: 5003–5012, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol 42: 1149–1160, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Witte DR, van der Graaf Y, Grobbee DE, Bots ML. Measurement of flow-mediated dilatation of the brachial artery is affected by local elastic vessel wall properties in high-risk patients. Atherosclerosis 182: 323–330, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol 91: 929–937, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation 115: 2390–2397, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120: 502–509, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]