Abstract
We investigated the role of somatosensory feedback on cardioventilatory responses to rhythmic exercise in five men. In a double-blind, placebo-controlled design, subjects performed the same leg cycling exercise (50/100/150/325 ± 19 W, 3 min each) under placebo conditions (interspinous saline, L3–L4) and with lumbar intrathecal fentanyl impairing central projection of spinal opioid receptor-sensitive muscle afferents. Quadriceps strength was similar before and after fentanyl administration. To evaluate whether a cephalad migration of fentanyl affected cardioventilatory control centers in the brain stem, we compared resting ventilatory responses to hypercapnia (HCVR) and cardioventilatory responses to arm vs. leg cycling exercise after each injection. Similar HCVR and minor effects of fentanyl on cardioventilatory responses to arm exercise excluded direct medullary effects of fentanyl. Central command during leg exercise was estimated via quadriceps electromyogram. No differences between conditions were found in resting heart rate (HR), ventilation [minute ventilation (V̇e)], or mean arterial pressure (MAP). Quadriceps electromyogram, O2 consumption (V̇o2), and plasma lactate were similar in both conditions at the four steady-state workloads. Compared with placebo, a substantial hypoventilation during fentanyl exercise was indicated by the 8–17% reduction in V̇e/CO2 production (V̇co2) secondary to a reduced breathing frequency, leading to average increases of 4–7 Torr in end-tidal Pco2 (P < 0.001) and a reduced hemoglobin saturation (−3 ± 1%; P < 0.05) at the heaviest workload (∼90% maximal V̇o2) with fentanyl. HR was reduced 2–8%, MAP 8–13%, and ratings of perceived exertion by 13% during fentanyl vs. placebo exercise (P < 0.05). These findings demonstrate the essential contribution of muscle afferent feedback to the ventilatory, cardiovascular, and perceptual responses to rhythmic exercise in humans, even in the presence of unaltered contributions from other major inputs to cardioventilatory control.
Keywords: hyperpnea, control of breathing, neural feedback
our aim was to assess the contribution of group III and IV afferent neurons innervating contracting locomotor muscles to circulatory and ventilatory control during rhythmic cycling exercise in healthy humans. There is substantial evidence supporting significant cardiovascular and ventilatory effects of this feedback pathway when studied in isolation. These findings include 1) increased impulse activity of group III and IV locomotor muscle afferents during walking in the decerebrate cat (2) and during and after fatiguing muscular contractions in anesthetized rabbits (21, 22); 2) increases in heart rate (HR) following electrical stimulation of hindlimb group III and IV muscle afferents in anesthetized vagotomized cats (64); 3) normal ventilatory responses to limb muscle electrical stimulation (which were subsequently prevented via spinal cord lesioning) in anesthetized dogs whose humoral influences on ventilatory control were controlled via cross-circulation techniques (41); 4) similar ventilatory responses during human voluntary limb exercise compared with those measured when the limb muscle contractions were evoked via electrical motor nerve stimulation sufficient to increase CO2 production (V̇co2) about twofold from resting level (9); 5) significant impact of local anesthetic blockade, or denervation of the dorsal roots, on the cardiovascular and ventilatory responses to isometric muscle contractions evoked by electrical spinal ventral root stimulation in anesthetized and decerebrate cats (18, 51) and to dynamic muscle contractions evoked by electrical nerve stimulation in anesthetized dogs (72); and 6) in vitro evidence in the isolated brain stem-spinal cord preparation showing that lumbar locomotor networks can rhythmically entrain medullary respiratory neurons (56).
Despite these important observations, which firmly established the existence of limb afferent activity in response to muscle contraction and their significant cardioventilatory effects, we are unsure of their relative importance in the intact exercising human largely because of the coexistence of strong feedforward influences from the higher central nervous system (CNS), i.e., so-called “central command” (25, 49). Furthermore, it is controversial as to whether afferent feedback from exercising muscle contributes significantly to cardiovascular responses to normal rhythmic exercise or whether the functional significance of this input is limited only to conditions of muscle ischemia as produced in sustained isometric muscle contractions with compromised blood flow (63).
To address the relative importance of the feedback influence from contracting muscles to cardioventilatory responses in the exercising human, local anesthetics (e.g., lidocaine) have been injected into the lumbar epidural space to block significant amounts of sensory feedback from the lower limbs (3, 27, 36, 55). Unfortunately, local anesthetics also reduce efferent nerve activity to the limbs, thereby “weakening” the locomotor musculature. As a consequence of the weakened musculature and in order to achieve the identical work rate, subjects are required to increase central motor command. In turn, augmented central command will exert powerful “feedforward” influences on cardiovascular and ventilatory regulation during exercise (25, 73) as demonstrated experimentally in humans via curare-induced (partial) muscle paralysis studies (8, 31, 57). Accordingly, epidural anesthesia creates a condition during constant-load exercise of reduced feedback in the face of increased feedforward influences and is therefore unsatisfactory to assess the potential contribution from limb feedback mechanisms.
We have chosen an approach using lumbar intrathecal injection of the selective μ-opioid receptor agonist fentanyl to attenuate the central projection of lower limb muscle afferent nerve fibers, most of which synapse on cells in the lumbar dorsal horn of the spinal cord (39, 53). These dorsal horn cells, in turn, relay to neural circuits in both the ventral lateral medulla and the nucleus tractus solitarii, where they influence breathing as well as both sympathetic and parasympathetic cardiovascular control (19). Stimulation of spinal opioid receptors, as found in the superficial dorsal horn (12, 13) and on primary afferent fibers (28), is known to inhibit transmission of nociceptive, mechanosensitive, and metabosensitive input from group III and IV afferents to the spinal cord (28, 40, 52, 78–80) without affecting the force-generating capacity of skeletal muscle (4, 33, 68). Intrathecal injection of opioid receptor agonists has been shown to reduce the group III/IV afferent-dependent HR, blood pressure, and ventilatory responses to isometric limb contractions in anesthetized cats (35, 52) and to ischemic muscle exercise in awake dogs (59). We recently reported (4) the effects of intrathecal fentanyl on central motor drive and locomotor muscle fatigue during the performance of a cycling time trial test. In this study we observed, serendipitously, a substantial fentanyl-induced hypoventilation during the time trial.
On the basis of these background findings, we have used lumbar intrathecal fentanyl in humans to achieve partial blockade of sensory afferents from contracting muscle in order to assess the contribution of these afferents to the cardioventilatory response to rhythmic lower limb cycling exercise over a wide range of intensities. To account for potential secondary actions of opioids on the central nervous control of ventilation (45) [e.g., via cephalad migration of fentanyl within the cerebrospinal fluid (CSF) to the medulla (70)] we also measured 1) the ventilatory response to hypercapnia at rest; 2) the cardioventilatory response to upper versus lower body exercise; and 3) circulating levels of fentanyl. Our findings support a significant contribution of muscle afferents to the control of ventilation, HR, and blood pressure during rhythmic steady-state exercise in the intact human.
METHODS
Subjects
Seven moderately trained male cyclists volunteered to participate in these studies [age 23.9 ± 3.6 yr, body mass 74.0 ± 8.3 kg, stature 1.80 ± 0.04 m, maximal O2 consumption (V̇o2max) 66.0 ± 2.4 ml·kg−1·min−1]. Written informed consent was obtained from each participant. The investigation consisted of a lower body and an upper body exercise component; the same set of subjects participated in both parts. All procedures were approved by the Institutional Review Board and conformed to the Declaration of Helsinki.
Protocol
Lower body exercise.
During preliminary visits, all participants were thoroughly familiarized with hypercapnic ventilatory response (HCVR) measurements and bicycling exercise. Furthermore, each subject performed a maximal incremental exercise test [20 W + 25 W/min (6)] on a bicycle ergometer (Velotron, Elite Model, Racer Mate, Seattle, WA) for the determination of peak power output (Wpeak) and V̇o2max. On two separate days, double blind and in random order, all subjects performed constant-load bicycle exercise either under placebo conditions including a sham injection of neutral saline into the ligaments between the processus spinosi of the vertebrae at the L3–L4 vertebral interspace or under experimental conditions including intrathecal fentanyl applied through the same vertebral interspace. The bicycle ergometer exercise was identical on both days and consisted of 50, 100, and 150 W for 3 min each, followed by 4 min against 80% of Wpeak (325 ± 19 W). There was no break between each workload. At exercise onset subjects were instructed to slowly pick up their pace, and the recording period started after the individual target pedal cadence (101 ± 5 rpm), as determined during the practice session, was reached (<10 s). To determine whether fentanyl migrated within the CSF to reach the medulla, HCVR measurements were conducted in each subject before and again 10–25 min after the fentanyl/placebo injection. As previously described (4), we also tested the effects of intrathecal fentanyl on the resting maximal voluntary contraction force (MVC) of the quadriceps by comparing MVCs measured before to those measured 5–10 min after the injection of fentanyl/placebo (but before exercise).
Upper body exercise.
During preliminary visits, the same participants were thoroughly familiarized with upper body ergometry (arm cycling; Pro 2, SciFit, Tulsa, OK). On two separate days, double blind and in random order, all subjects performed constant-load exercise under either placebo or fentanyl conditions (see above). The exercise was identical on both days and consisted of 25, 50, and 75 W for 3 min each. There was no break between the first and the second workload, but subjects rested for ∼3 min before the final workload. The target cadence was set at 60 rpm, and the recording period started after this cranking frequency was reached (<10 s).
The exercise sessions in each component of the study were separated by at least 48 h and were completed at the same time of day. In both parts of the study, the duration from the fentanyl/placebo injection to the end of exercise was <60 min. Subjects were instructed to refrain from caffeine for 12 h and from stressful exercise for 48 h before each exercise trial. Ambient temperature and relative humidity were not different between conditions.
Exercise Responses
Ventilation and pulmonary gas exchange were measured breath by breath at rest and throughout exercise with an open-circuit system including two pneumotachographs (Hans Rudolph, model 3800) (inspiration, expiration) and two Perkin-Elmer mass spectrometers (model 1100) for the analysis of mixed expired and end-tidal gases. Arterial O2 saturation (SpO2) was estimated with a pulse oximeter (Nellcor N-595, Pleasanton, CA) with adhesive forehead sensors. HR was measured from the R-R interval of an electrocardiogram with a three-lead arrangement. Ratings of perceived exertion (RPE) were obtained at rest and during the final 30 s of each workload (leg exercise only) with Borg's modified CR10 scale (14). Arterialized (Finalgon, Boehringer Ingelheim, Germany) capillary blood samples were collected from an earlobe at rest and during the final 30 s of each workload for determination of whole blood lactate concentration ([La−]B) with an electrochemical analyzer (YSI 1500 Sport). During the lower body exercise part of the study, systolic and diastolic blood pressure were manually measured on the left arm at rest and during the last minute of each workload. Mean arterial pressure (MAP) was calculated as diastolic pressure + ⅓ × (systolic pressure − diastolic pressure). To determine a potential diffusion of fentanyl into the systemic circulation, venous blood samples were drawn ∼40 min after the placebo/fentanyl injection and again immediately after the exercise, i.e., ∼60 min after injection. The blood samples were stored and later analyzed for fentanyl content via liquid chromatography-tandem mass spectrometry (37).
Hypercapnic Ventilatory Response Measurement
CO2 rebreathing tests were performed before and 10–25 min after the fentanyl/placebo injection. Participants sat comfortably in a chair while breathing through a mouthpiece. Upon reaching a steady state of eupneic air breathing (<3 min), a valve close to the mouthpiece was turned to switch the subjects into a rebreathing circuit. The rebreathing circuit was initially filled with a gas mixture consisting of 3% CO2, 27% N2, and 70% O2, and the bag volume was set at 0.5 liters above the subject's vital capacity. Subjects rebreathed the gas mixture until end-tidal Pco2 (PetCO2) reached ∼55 Torr. The measurements were repeated three times, and the tests were separated by at least 5 min of exposure to room air to allow ventilatory variables to return to baseline levels. The HCVR response slope for each trial [change in minute ventilation (ΔV̇e)/ΔPetCO2] was determined by applying a best-fit regression line across the linear range of response of V̇e vs. PetCO2 throughout the rebreathing period.
Surface electromyography.
During the leg exercise high workload, quadriceps electromyogram (EMG) was recorded from the right vastus lateralis with electrodes with full-surface solid adhesive hydrogel (Kendall H59P, Mansfield, MA) and on-site amplification (5). Raw EMG signal from the vastus lateralis corresponding to each muscle contraction was recorded for later analysis. The EMG signal was amplified and filtered by a Butterworth band-pass filter (BMA-830, CWE, Ardmore, PA) with a low-pass cutoff frequency of 10 Hz and a high-pass cutoff frequency of 1 kHz. The slope of the filters was −6 dB/octave. The filtered EMG signals were sampled at 2 kHz by a 16-bit A/D converter (PCI-MIO-16XE-50, National Instruments, Austin, TX) with custom software (Labview 6.0, National Instruments). A computer algorithm identified the onset of activity where the rectified EMG signals deviated by >2 SD above the baselines for at least 100 ms. Each EMG burst was inspected to verify the timing identified by the computer. For data analysis, the integral of each burst [integrated EMG (iEMG)] was calculated with the formula iEMG [|m(t)|] = ∫0t|m(t)|dt, where m is the raw EMG signal. As an estimate of central neural drive during the high leg workload, mean values for iEMG during each muscle contraction (cycle revolution) were calculated, averaged over each 60-s period of the 4-min bout, and normalized to the first minute of exercise. However, there are potential limitations of surface EMG to reflect central motor drive (26, 42). For example, significant filtering effects and/or amplitude cancellation might attenuate increases in motor unit activity and thus increases in central motor command (42). The unfavorable signal-to-noise ratio associated with exercise at low intensities, i.e., relatively low motor unit activity, only allowed us to measure surface EMG during the high workload.
Intrathecal Fentanyl
A 20-gauge intravenous catheter was inserted, and a bolus of ∼500 ml of normal saline was infused as a precaution to correct any potential drop in blood pressure (66). The subjects were placed in the flexed sitting position, and the skin and subcutaneous tissue were anesthetized at the L3–L4 vertebral interspace with 2–4 ml of 1% (10 mg/ml) lidocaine. In the experimental trial (fentanyl) a 25-gauge, 3.5-in. Pecan needle was advanced to the subarachnoid space. Free-flowing CSF confirmed subarachnoid positioning of the needle tip. A small amount of CSF was aspirated and 1 ml of fentanyl (0.05 mg/ml) injected. The needle was then removed, and the subjects remained in an upright sitting position to minimize the potential risk of cephalad movement within the CSF. The participants were observed for 5 min with repeated sampling of vital signs. In the placebo trial the needle was advanced (L3–L4), but just before entering the subarachnoid space 1 ml of preservative-free normal saline was injected. The needle was then removed, and the subjects were observed for 5 min with repeated sampling of vital signs. To minimize the potential risk of a cephalad spread within the CSF, subjects remained in an upright sitting position during the remainder of the experiment. Cutaneous hypoaesthesia to pinprick and cold perception on the torso and upper limbs was examined before exercise.
Statistical Analysis
A two-way analysis of variance (ANOVA) with repeated measures was performed to evaluate differences between the placebo and fentanyl trials. A least significant difference test identified the means that were significantly different with P < 0.05. Results are expressed as means ± SE.
RESULTS
Resting Ventilatory Responses to CO2, Cutaneous Hypoaesthesia, and Venous Fentanyl Concentrations
Table 1 shows individual effects of placebo and fentanyl on HCVR slopes measured before and 10–20 min after the injection. Because of the substantial impact of fentanyl on the HCVR slope of subjects 6 and 7, the exercise data sets of both individuals were excluded from our analyses of fentanyl effects on the group mean exercise responses (see data on these subjects at the end of results).
Table 1.
Individual effects of placebo and fentanyl on HCVR slopes measured before and 10–20 min after inection
| HCVR Slope (ΔV̇e/ΔPetCO2) |
||||||
|---|---|---|---|---|---|---|
| Subject | Preplacebo | Postplacebo | % Change | Prefentanyl | Postfentanyl | % Change |
| 1 | 2.59 | 2.44 | −6 | 1.75 | 1.64 | −6 |
| 2 | 1.78 | 1.71 | −4 | 1.71 | 1.72 | +1 |
| 3 | 2.17 | 1.91 | −12 | 1.60 | 1.52 | −5 |
| 4 | 1.32 | 1.70 | +29 | 1.93 | 2.02 | +5 |
| 5 | 3.71 | 3.91 | +6 | 5.28 | 4.79 | −9 |
| Mean ± SE* | 2.31 ± 0.41 | 2.33 ± 0.42 | 2.45 ± 0.71 | 2.35 ± 0.62 | ||
| 6 | 4.43 | 4.70 | +6 | 4.53 | 2.73 | −40 |
| 7 | 2.76 | 2.73 | −1 | 2.71 | 1.81 | −33 |
HCVR, hypercapnic ventilatory response; V̇e, minute ventilation; PetCO2, end-tidal Pco2.
P = 0.87 for preplacebo vs. postplacebo and P = 0.32 for prefentanyl vs. postfentanyl.
Neurological examinations just before the start of the exercise revealed cutaneous hypoaesthesia to pinprick and cold perception below T2–T3 in five of the seven subjects. This was evident by sensory changes on the torso at—or below—T2 and by the absence of sensory changes on the upper limbs (demarcating C8 and above). The two subjects who were excluded from the analysis reported sensory changes at C7. These findings confirm the intended reduction of afferent feedback from lower limb locomotor muscle but also suggest that lumbar intrathecal fentanyl might have affected opioid-mediated neural feedback from at least a portion of the torso.
No detectable levels of circulating fentanyl were found in systemic venous blood samples obtained in any of the subjects immediately before (40 min after injection) and after (∼60 min after injection) the exercise tests.
Effects of Fentanyl on Resting Quadriceps MVC
Quadriceps MVCs were similar before and after the administration of lumbar intrathecal fentanyl (566 ± 52 N and 570 ± 50 N, respectively; P = 0.65), suggesting that the force-generating capacity of the quadriceps was not affected by the drug.
Lower Body Cycling Exercise
Metabolic rate.
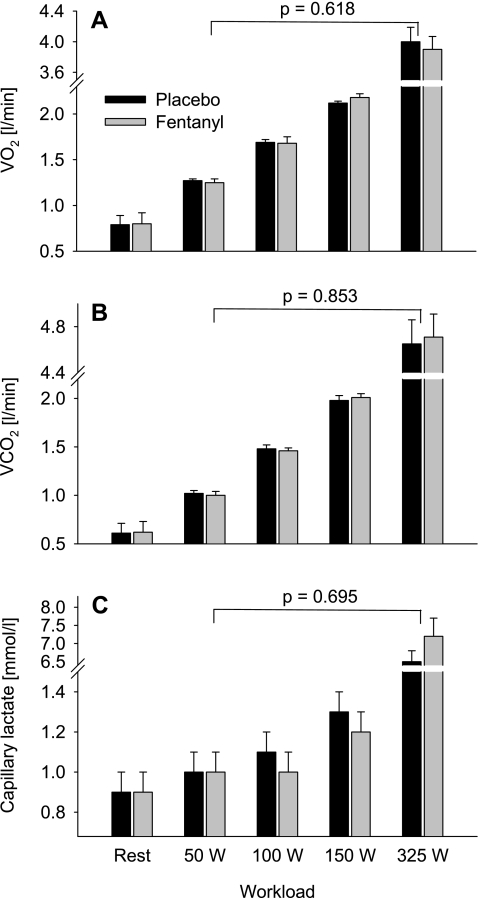
Fentanyl had no effect on resting V̇o2, V̇o2 at any of the work rates, or the capillary plasma lactate concentrations during leg cycling exercise (Fig. 1). Individual target pedal cadence (101 ± 5 rpm) was the same during all work rates in the placebo and fentanyl trials.
Fig. 1.
Metabolic responses during the final minute of leg cycling exercise at 4 different workloads with (fentanyl) and without (placebo) partially blocked somatosensory neural feedback from the working locomotor muscles. A: O2 consumption (V̇o2). B: CO2 production (V̇co2). C: capillary lactate concentration. The ANOVA P value indicates the overall main effect of fentanyl.
Ventilation.
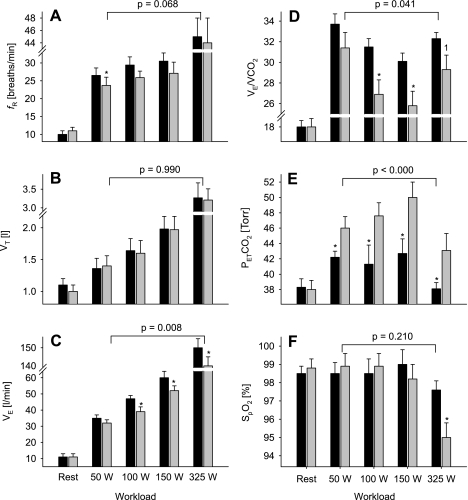
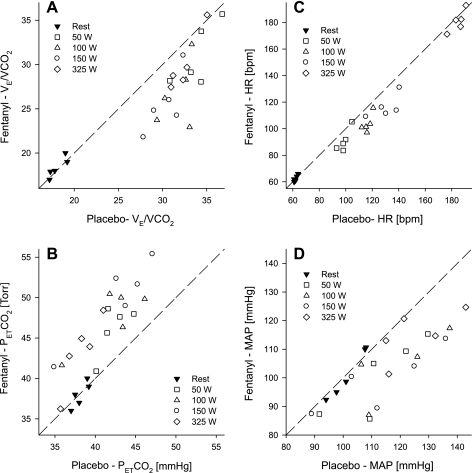
At rest breathing room air, fentanyl had no effect on V̇e, breathing pattern, PetCO2, or arterial hemoglobin saturation (Figs. 2 and 3 and Table 2). During all but the lowest workload with fentanyl, V̇e and V̇e/V̇co2 were attenuated (8–17%) compared with placebo exercise. This reduction in V̇e/V̇co2 resulted in 4- to 7-Torr average increases in PetCO2 across all workloads. The lower V̇e during the fentanyl exercise was mainly caused by a reduction in breathing frequency since tidal volumes at various workloads were nearly identical during both trials. Compared with placebo exercise, the reduction in V̇e during the fentanyl trial also resulted in a significant reduction in V̇e/V̇o2 and end-tidal Po2 (PetO2) at most work rates but had no significant impact on reducing hemoglobin O2 saturation until the highest workload. Figure 3, A and B, illustrate individual subject responses to fentanyl and placebo exercise at rest and at various work rates. Whereas there was no difference between placebo and fentanyl at rest, V̇e/V̇co2 at each work rate was lower in all five subjects during the fentanyl exercise. This consistent hypoventilation caused an increase in PetCO2 at all workloads and in all subjects. These fentanyl-induced lower ventilatory responses and CO2 retention obtained in the steady-state after 3 min of exercise were already present over the initial 15 s of exercise at each work rate (data not shown).
Fig. 2.
Ventilatory response and hemoglobin saturation [arterial O2 saturation by pulse oximetry (SpO2)] during the final minute of leg cycling exercise at 4 different workloads with (fentanyl, gray bars) and without (placebo, black bars) partially blocked somatosensory neural feedback from the working locomotor muscles. A: respiratory frequency (fR). B: tidal volume (Vt). C: minute ventilation (V̇e). D: V̇e/V̇co2. E: end-tidal Pco2 (PetCO2). F: SpO2. The ANOVA P value indicates the overall main effect of fentanyl. *P < 0.05; 1P = 0.08.
Fig. 3.
Identity plots to illustrate individual subject response at rest and during exercise under conditions of placebo and fentanyl. A: V̇e/V̇co2. B: PetCO2. C: heart rate (HR). bpm, beats per minute. D: mean arterial blood pressure (MAP).
Table 2.
Physiological response to last minute of lower body leg cycling exercise at four different workloads
| Rest |
50 W |
100 W |
150 W |
325 ± 19 W |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Fentanyl | Placebo | Fentanyl | Placebo | Fentanyl | Placebo | Fentanyl | Placebo | Fentanyl | ANOVA P Value | |
| Exercise time, min | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 4.0 | 4.0 | |||
| % of Wpeak | 13 ± 1 | 13 ± 1 | 25 ± 1 | 25 ± 1 | 38 ± 1 | 38 ± 1 | 80 | 80 | |||
| % of V̇o2max | 27 ± 2 | 27 ± 2 | 37 ± 3 | 37 ± 3 | 48 ± 3 | 48 ± 3 | 87 ± 2 | 87 ± 2 | |||
| V̇e/V̇o2 | 14 ± 0.3 | 14 ± 0.3 | 27.1 ± 1.3 | 25.1 ± 1.6 | 27.7 ± 0.8 | 23.2 ± 0.9* | 28.1 ± 1.7 | 23.7 ± 1.0* | 37.4 ± 1.3 | 36.1 ± 1.5 | 0.006 |
| PetO2, Torr | 98.0 ± 2.4 | 97.9 ± 2.1 | 96.0 ± 1.5 | 90.1 ± 2.6* | 96.9 ± 1.8 | 89.7 ± 1.5* | 97.9 ± 1.5 | 89.5 ± 2.8* | 107.9 ± 1.2 | 104.9 ± 1.2 | 0.014 |
| RPE (dyspnea) | 0.5 ± 0.2 | 0.4 ± 0.1 | 0.9 ± 0.2 | 0.6 ± 0.1 | 1.2 ± 0.2 | 0.7 ± 0.1 | 6.6 ± 0.7 | 5.4 ± 0.8* | 0.005 | ||
| RPE (limb) | 0.2 ± 0.1 | 0.3 ± 0.2 | 0.4 ± 0.2 | 0.5 ± 0.2 | 0.7 ± 0.1 | 0.6 ± 0.1 | 7.3 ± 0.6 | 6.4 ± 0.5* | 0.003 | ||
Values are expressed as means ± SE. Wpeak, peak power output; V̇o2max, maximal O2 consumption (V̇o2); PetO2, end-tidal Po2; RPE, rating of perceived exertion. P value indicates the overall main effect of fentanyl.
P < 0.05 vs. placebo.
Heart rate and mean arterial blood pressure.
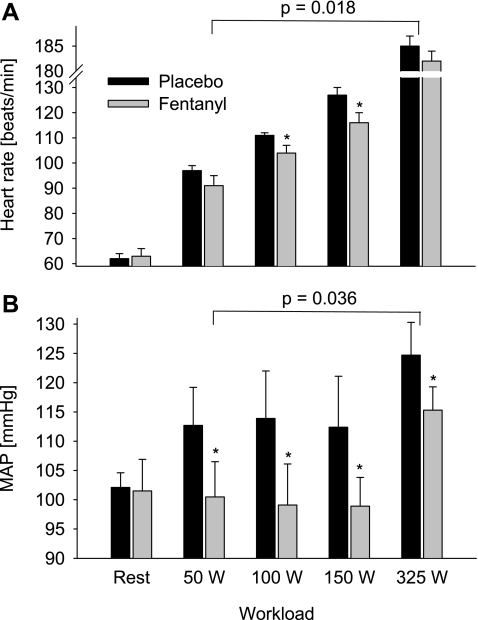
At rest, fentanyl had no effect on HR and MAP (Fig. 4). Throughout the exercise, fentanyl had a significant overall main effect on HR. The difference failed to reach significance at 50 W (−6 ± 3%, P = 0.11) and at 325 W (−2 ± 1%, P = 0.32), but HR was significantly lower during the fentanyl exercise at 100 W (−7 ± 2%) and 150 W (−8 ± 2%). MAP was significantly lower at each workload during the fentanyl trial (8–13%), and this difference was present in all subjects. Figure 3, C and D, illustrate individual subject responses for HR and MAP, respectively. MAP at each work rate was lower during the fentanyl exercise in all five subjects (Fig. 3D), and HR was similarly reduced, although there were a few individual exceptions where HR was unchanged.
Fig. 4.
HR (A) and MAP (B) response during the final minute of leg cycling exercise at 4 different workloads without (Placebo) and with (Fentanyl) partially blocked somatosensory neural feedback from locomotor muscles. The P value indicates the overall main effect of fentanyl. *P < 0.05.
Integrated EMG.
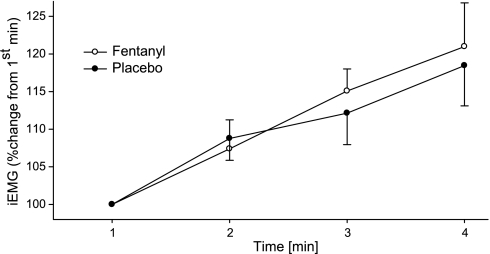
During the high constant-load leg cycling trial, iEMG of vastus lateralis rose significantly from the first to the final minute of exercise in both conditions (P < 0.05; Fig. 5). The change in vastus lateralis iEMG was similar in both trials; there was no overall main effect of fentanyl (P = 0.3).
Fig. 5.
Myoelectrical activity [integrated EMG (iEMG)] of vastus lateralis muscle used to illustrate the similarity in the feedforward component (central neural drive) during the high constant-workload (325 ± 19 W) leg cycling trial with intact (Placebo) and partially blocked (Fentanyl) somatosensory afferent feedback. iEMG during each muscle contraction (cycle revolution) was calculated, averaged over each 60-s period, and normalized to the 1st minute of exercise. Pedal cadence was held constant (101 ± 5 rpm) throughout the exercise.
Rating of perceived exertion.
There was a significant overall main effect of fentanyl on dyspnea RPE (P = 0.005), which was consistently rated between 20% and 40% lower during the fentanyl exercise (Table 2). Furthermore, there was also a significant overall main effect of fentanyl on limb RPE (P = 0.003). Although the difference in limb RPE was not significant between placebo and fentanyl at the three lower workloads (48 ± 3% V̇o2max), during the high workload (87 ± 2% V̇o2max) the rating for limb discomfort in the fentanyl trial was ∼13% lower.
Upper Body Exercise
Metabolic rate.
Fentanyl had no effect on resting or exercise V̇o2 or capillary lactate concentrations during arm cycling exercise (Table 3).
Table 3.
Physiological response to last minute of upper body arm exercise at three different workloads
| Rest |
25 W |
50 W |
75 W |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo | Fentanyl | Placebo | Fentanyl | Placebo | Fentanyl | Placebo | Fentanyl | ANOVA P Value | |
| Exercise time, min | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | |||
| HR, beats/min | 65 ± 3 | 66 ± 4 | 88 ± 4 | 89 ± 4 | 105 ± 6 | 104 ± 5 | 130 ± 7 | 132 ± 7 | 0.907 |
| V̇e, l/min | 13 ± 2 | 14 ± 1 | 33 ± 2 | 31 ± 3 | 45 ± 2 | 43 ± 2 | 64 ± 4 | 62 ± 3 | 0.321 |
| fR, breaths/min | 15 ± 1 | 15 ± 1 | 25 ± 2 | 23 ± 2* | 28 ± 3 | 26 ± 3* | 32 ± 5 | 33 ± 5 | 0.051 |
| Vt, liters | 0.9 ± 0.3 | 1.0 ± 0.1 | 1.3 ± 0.2 | 1.4 ± 0.2 | 1.6 ± 0.1 | 1.6 ± 0.2 | 2.0 ± 0.3 | 1.9 ± 0.3 | 0.136 |
| V̇o2, l/min | 0.69 ± 0.03 | 0.68 ± 0.03 | 0.92 ± 0.04 | 0.93 ± 0.05 | 1.26 ± 0.05 | 1.24 ± 0.05 | 1.70 ± 0.05 | 1.71 ± 0.05 | 0.431 |
| V̇co2, l/min | 0.49 ± 0.05 | 0.48 ± 0.04 | 0.83 ± 0.03 | 0.85 ± 0.04 | 1.30 ± 0.06 | 1.28 ± 0.04 | 1.95 ± 0.05 | 1.92 ± 0.04 | 0.488 |
| V̇e/V̇o2 | 19.1 ± 0.3 | 20.1 ± 0.2 | 35.7 ± 1.4 | 33.6 ± 1.1* | 35.6 ± 1.7 | 34.8 ± 1.5 | 37.6 ± 1.6 | 36.4 ± 1.8 | 0.182 |
| V̇e/V̇co2 | 26.7 ± 0.3 | 27.9 ± 0.8 | 38.7 ± 1.8 | 36.5 ± 1.0* | 33.7 ± 1.5 | 32.9 ± 0.8 | 32.8 ± 1.6 | 32.4 ± 1.4 | 0.187 |
| PetO2, Torr | 102.8 ± 2.1 | 102.3 ± 1.7 | 104.1 ± 2.7 | 99.7 ± 2.3 | 103.9 ± 1.5 | 101.3 ± 1.6* | 108.4 ± 2.2 | 106.9 ± 2.1* | 0.033 |
| PetCO2, Torr | 36.4 ± 1.0 | 36.7 ± 1.2 | 36.2 ± 1.7 | 37.4 ± 1.4 | 37.5 ± 1.2 | 39.2 ± 1.1* | 36.4 ± 1.7 | 38.1 ± 1.5* | 0.014 |
| SpO2, % | 98.3 ± 0.2 | 98.7 ± 0.2 | 98.7 ± 0.3 | 98.4 ± 0.3 | 99.1 ± 0.2 | 99.0 ± 0.3 | 97.2 ± 0.3 | 97.1 ± 0.3 | 0.901 |
| [La−]B, mmol/l | 0.7 ± 0.1 | 0.7 ± 0.1 | 1.5 ± 0.2 | 1.5 ± 0.1 | 2.8 ± 0.3 | 2.7 ± 0.2 | 4.8 ± 0.5 | 4.8 ± 0.5 | 0.935 |
Values are expressed as means ± SE. HR, heart rate; fR, respiratory frequency; Vt, tidal volume; V̇co2, CO2 production; SpO2, arterial O2 saturation by pulse oximetry; [La−]B, whole blood lactate concentration. ANOVA P value indicates the overall main effect of fentanyl.
P < 0.05 vs. placebo.
Ventilation.
At rest, fentanyl had no effect on V̇e, V̇e/V̇co2, PetCO2, breathing pattern, and arterial hemoglobin saturation (Table 3). Throughout the exercise, there was no significant overall main effect of fentanyl on V̇e and V̇e/V̇co2. V̇e/V̇co2 during exercise with fentanyl was between 1% and 5% lower than placebo during each workload, and this hypoventilation caused significant 1.7 ± 0.4 Torr (range 1–3 Torr) and 1.9 ± 0.4 Torr (range 1–3 Torr) increases in PetCO2 at 50 and 75 W, respectively. In both cases, four of the subjects had a <2-Torr and one subject had a 3-Torr increase in PetCO2 with fentanyl. The slightly lower V̇e during fentanyl exercise was caused by a reduction in breathing frequency.
Heart rate.
Mean HR at rest and during each workload was identical in the placebo and fentanyl trials (all P > 0.6; Table 2). The HR in one of the five subjects was consistently between 5 and 8 beats/min lower during the fentanyl exercise.
It should be noted that the two subjects with a fentanyl-induced reduction in resting ventilatory response to hypercapnia (Table 1) showed the greatest CO2 retention of any of the remaining five subjects during leg cycling (+10 to +12 Torr ΔPetCO2, fentanyl vs. placebo) and also during upper body exercise (+5 to +11 Torr ΔPetCO2) (data not shown).
DISCUSSION
The goal of this investigation was to evaluate the relative importance of muscle afferent feedback to cardioventilatory control in rhythmically exercising humans. Lumbar intrathecal fentanyl was used to block the cortical/medullary projection of μ-opioid receptor-sensitive lower limb muscle afferents at rest and during bicycling exercise of various intensities ranging from ∼25% to 90% of V̇o2max. The results from additional experiments conducted to test for a cephalad spread and thus a potential direct effect of the drug on medullary opioid receptors of the cardiovascular and respiratory control centers in the brain stem provide evidence in favor of a segmental effect of the drug at or below the cervical level. Furthermore, in light of the identical MVC force of the quadriceps muscles, the similar levels of plasma lactate and V̇o2 at each exercise load under both fentanyl and placebo conditions, and the similar output from the locomotor muscle motoneuron pool (as estimated via quadriceps EMG) during exercise, we concluded that feedforward influences on cardioventilatory regulation were similar during placebo and fentanyl exercise. Although having no effect at rest, breathing room air or CO2, the blockade of μ-opioid receptor-sensitive lower limb muscle afferents resulted in a substantial respiratory frequency-related reduction in exercise hyperpnea during bicycling exercise, which caused average increases in PetCO2 of 4–7 Torr at the various work rates. Furthermore, HR and blood pressure were also not affected at rest but were reduced 10–15% when the identical bicycle exercises were performed with attenuated neural feedback from the lower limbs. We interpret this evidence to mean that opioid receptor-sensitive afferent feedback from rhythmically contracting limb muscles is required for normal cardiovascular and ventilatory control during steady-state rhythmic exercise in healthy humans.
Evaluation of Potential Medullary Effects of Lumbar Intrathecal Fentanyl
A cephalad movement of fentanyl within the CSF to the brain stem, or passage of fentanyl to the systemic circulation and then across the blood-brain barrier (65), would negate the implications of our findings for exercise hyperpnea since a direct effect of the drug on medullary opioid receptors is known to impact medullary neurons involved in cardiovascular (16, 61, 69) and ventilatory (44, 45) control. These central effects of opioids are manifested in a reduced rate and depth of resting ventilation and an attenuated tidal volume and ventilatory response to imposed hypercapnia in humans (10, 45, 76).
Limited evidence on cephalad migration of fentanyl following lumbar injections has shown: 1) in sheep an appearance of trace amounts of fentanyl in cisternal CSF (70); 2) in recumbent humans significant movement of fentanyl from lower to higher lumbar segments (24); and 3) after epidural application of fentanyl detectable amounts appearing in the cervical CSF in recumbent humans (32). In these animal and human studies there was a striking degree of between-subject variability in the degree of cephalad migration of fentanyl, and the investigators speculated that this might be explained, at least in part, by variations in axial spinal length (24, 32, 70).
We have several lines of evidence to suggest that the effects of lumbar intrathecal fentanyl we observed on the cardioventilatory response to exercise were attributable to blockade of spinal μ-opioid receptor-sensitive afferents rather than to any effects of the drug at the medullary level. First, neither resting eupneic ventilation and PetCO2 nor ventilatory responsiveness to hypercapnia at rest was affected by intrathecal fentanyl, suggesting that the drug remained below medullary neuronal sites concerned with the chemical control of breathing. We have confirmed these results, using a steady-state CO2 response test in nine subjects before and after lumbar intrathecal fentanyl while maintaining an upright posture (unpublished findings). In two of the initial group of our seven subjects the tidal volume and ventilatory response to CO2 were substantially attenuated after the drug injection (Table 1)—a result that is consistent with the individual variability in fentanyl migration reported within the spinal column. These two subjects were excluded from any further analysis of their exercise response. Second, no detectable level of fentanyl was found in systemic venous blood samples obtained before and after exercise. We also note that the reduced exercise ventilation with lumbar intrathecal fentanyl was due to a reduced breathing frequency, whereas the reduced CO2 response reported after intravenous fentanyl was due exclusively to a reduced tidal volume response (10). Third, neurological examinations immediately before exercise revealed cutaneous hypoaesthesia to pinprick and cold perception only below T2–T3. Finally, compared with placebo exercise, both HR and ventilation were attenuated during leg fentanyl exercise (Fig. 2), whereas during arm exercise HR was unchanged and differences in V̇e/V̇o2 were relatively small for the placebo versus fentanyl trials. For the arm exercise comparisons, despite the absence of a significant difference in absolute ventilation, a small degree of alveolar hypoventilation during fentanyl exercise at 50 and 75 W was suggested by the 1–2 Torr higher average PetCO2 and reduced breathing frequency (Table 3); however, these relatively small differences might be explained by the attenuated afferent feedback at the thoracic or cervical spinal level from various trunk muscles involved in upper body exercise.
In summary, we did not directly address the question of how cephalad fentanyl migrated after lumbar injection. However, we can state with certainty that the cardioventilatory responses to rhythmic leg exercise were significantly attenuated while ventilation, blood pressure, and HR at rest—during room air breathing or in response to hypercapnia—were not. These contrasts between the effects of fentanyl at rest and during exercise imply that the exercise effects were not likely to have been mediated by the same CNS influences that are purported to depress ventilation in the resting subject while breathing room air or CO2.
Relative Contribution of Muscle Afferents to Exercise Hyperpnea
Our data allow us to approximate the relative amount of the exercise hyperpnea attributable to muscle afferents. First, we observed at each of 100, 150, and 325 W that absolute V̇e was reduced 8–10 l/min with the μ-opioid receptor agonist. Coincidentally, PetCO2 also rose an average of 4–7 Torr at each of these work rates, and these levels of systemic hypercapnia—presumably acting via feedback chemoreceptor stimulation—would be expected to have masked a greater fall in V̇e (11). On the basis of measured CO2 responsiveness of our subjects at rest (on average a 2.4 l/min increase in V̇e per 1 Torr increase in PetCO2; ΔV̇e/ΔPetCO2, Table 1) we approximated that the rising PetCO2 prevented a further 15, 18, and 12 l/min reduction in V̇e during the 100-, 150-, and 325-W exercise, respectively. Thus, in total, we estimate that the blockade of afferent input resulted in reductions in V̇e at 100, 150, and 325 W that averaged 49% (23 in 47 l/min), 43% (26 in 60 l/min), and 15% (22 in 150 l/min), respectively. Similarly, in our previous study employing 5-km cycling time trial tests (4), we observed a fentanyl-induced hypoventilation and CO2 retention throughout the race that averaged +3–8 Torr PetCO2, but the relative reductions in V̇e/V̇co2 and levels of CO2 retention were greatest at the beginning of the trial and subsided as peripheral fatigue, metabolic acidosis, and arterial hypoxemia progressed over the latter half of the time trial. Therefore, both the present and our previous findings with μ-opioid receptor agonists suggest that afferent feedback from contracting muscle may have its greatest influence on exercise hyperpnea during moderate exercise intensities when other potentially overriding humoral and feedforward neural inputs are minimal.
Our estimates are only approximations (and likely minimal estimates of the actual contribution) because we do not know the completeness of our afferent blockade; nor can we be certain that our CO2 responses (as measured at rest) remain unaltered during all exercise intensities (7, 17, 54) or that our afferent blockade did not compromise any “interactive” effects of peripheral feedback with ongoing central command (74). We have also not accounted for the ventilatory equivalent of the mild hypoxemia (plus hypercapnia) accompanying the hypoventilation during the heavy exercise intensity. Furthermore, the magnitude of the reduced HR response to exercise with fentanyl is likely underestimated in the face of baroreceptor compensation for the lower MAP. More conservatively, we believe our results most clearly permit the interpretation that muscle afferent feedback is essential to the normal cardioventilatory response to exercise in the healthy human and that the relative strength of this contribution is likely exercise intensity (and/or duration) dependent.
Epidural Anesthesia vs. Intrathecal Opioids
Local anesthetics applied to the lumbar epidural space have been used to investigate the influence of lower limb muscle afferents on the cardioventilatory response in exercising humans. These studies, including our own, found reduced, similar, or increased cardioventilatory responses during exercise with partially blocked spinal neurotransmission to and from the working muscle (3, 27, 29, 30, 36, 38, 55, 67). However, epidural lidocaine attenuates efferent as well as afferent nerve activity (see introduction), and this drug-induced “muscle weakening” inevitably requires an increase in central motor command (i.e., feedforward) in order to achieve a given external workload. Thus partial afferent blockade with local anesthetics creates a condition of reduced feedback in the face of increased feedforward, and this increase in central command, by itself, augments the cardioventilatory response to exercise as demonstrated in curarization experiments (8, 31, 57). With this in mind, the resultant, net effect on ventilatory and/or circulatory responses during exercise with partially blocked feedback via local anesthetics depends upon the degree to which the increase in central motor command/feedforward response balances the reduced feedback from the working limb muscle.
To circumvent these confounding effects of local epidural anesthetics we used fentanyl, a μ-opioid receptor agonist that has no effect on the force-generating capacity of the lower limb muscles (4, 33, 68), suggesting that efferent motor activity was not compromised after the intrathecal injection of the drug. Unchanged plasma lactate levels during exercise also suggest that relative exercise intensities were similar in fentanyl versus placebo trials at all work rates. These similarities combined with similar quadriceps muscle EMG at the highest workload form the basis for our presumption that any influence from feedforward central motor command was also similar during placebo and fentanyl exercise (Fig. 5). Accordingly, we believe the use of lumbar intrathecal fentanyl provided a suitable means of evaluating the influence of reduced locomotor muscle afferent feedback, per se, on the cardioventilatory response in exercising humans.
Role of Muscle Afferents in Cardiovascular and Ventilatory Responses and Effort Perceptions: Past vs. Present Findings
What is the significant advance provided by our findings concerning the relative contribution of muscle afferents to exercise hyperpnea in the rhythmically exercising human and in the presence of all other known major influences on ventilatory control? First, we recognize that the century-long examination of the exercise hyperpnea question has revealed that it likely requires multiple major mechanisms—including neural and humoral components as well as memory-like poststimulus potentiation effects and perhaps even a “learned” feedforward effect linked to past experience (25, 34, 50, 60, 71). Our study has examined only one of these important inputs, demonstrating that the afferent feedback mechanism was essential to the production of a normal exercise hyperpnea as well as for normal pressor and tachycardic responses to rhythmic exercise. This demonstration is consistent with the well-established importance of group III and IV muscle afferents in ventilatory control when studied in isolation, but it uniquely extends previous findings by showing that partial blockade of the afferent input from rhythmically contracting muscle of varying intensities prevented the normal cardiovascular and ventilatory responses even in the presence of other known and presumably unaltered major inputs to circulatory and ventilatory control.
Our conclusion is not in agreement with most studies using epidural local anesthetics (see above), or some studies using local muscle ischemia (to enhance local metabolic stimuli), which reported no effect on ventilatory recovery following exercise (23, 62). Furthermore, our findings in humans are not consistent with those in anesthetized animals in which the rapid hyperpnea evoked via hindlimb electrical stimulation was unaffected by spinal cord transection (20, 46, 47, 75) or with the normal ventilatory response to electrical stimulation of limb muscles and relatively small increments in V̇o2 and V̇co2 in paraplegic humans (1, 15). On the basis of findings from many of these studies that have reported no or little effect of eliminating a single sensory input on exercise hyperpnea, some have postulated the emergence of redundant control mechanisms that are able to compensate for—and thereby mask the effects of—the absent sensory input (81). Perhaps, then, our findings with partial muscle afferent blockade and no accompanying muscle weakness may represent a minimal—if any—upregulation of the remaining controllers of the cardiovascular and ventilatory responses to exercise.
There is also skepticism concerning any role for muscle afferents on circulatory and ventilatory control during rhythmic exercise—as opposed to static ischemic exercise—based primarily on negative findings obtained during very light rhythmic exercise in the presence of “complete” sensory blockade (via epidural lidocaine) sufficient to prevent the pressor response to muscle ischemia in humans (29, 43) and with the use of opioid agonists in dogs undergoing mild-intensity exercise employing a small muscle mass (59). In contrast, our present results obtained during rhythmic leg cycling showed a substantial effect of partial blockade of muscle afferents across the range of moderate to heavy exercise intensities (requiring 3- to 8-fold increases in resting metabolic rate and 4- to 15-fold increases in resting V̇e) and over the 3-min time course at each work rate. These findings are in agreement with the significant activity of group III and IV muscle afferents observed in the rhythmically exercising cat with normal limb blood flow (2), with the significant impact of cold blockade of group III/IV afferents on the cardiovascular response to rhythmic electrical stimulation of limb muscle in the anesthetized dog (72), and with recent evidence in humans showing that muscle afferent blockade during rhythmic exercise significantly attenuated “baroreceptor resetting” (67). Pan et al. (58) also reported that bilateral spinal ablation of the dorsolateral sulcus and funiculus at L2 in the pony altered the transient changes in arterial Pco2 at exercise onset—but had no effect on arterial Pco2 in the steady state of exercise. Furthermore, Galbo et al. (31) implicated an important role for afferent feedback in heavy rhythmic cycling exercise in humans with their observation of a markedly attenuated ventilatory response following partial curarization when comparisons with controls were made at maximal exercise intensity and presumably at comparable levels of central command. As previously suggested (63), the relative importance of muscle afferents on cardioventilatory control during exercise is likely to be critically dependent upon the mass of muscle involved as well as the intensity of the exercise and perhaps even the magnitude of the intact cardiorespiratory response.
Finally, it has been argued that “perception of effort” is independent of afferent feedback from skeletal muscles (48). Certainly, the awareness of central motor command plays an important role in determining the perception of effort (31, 77); however, a critical role of peripheral muscle afferents is also likely to be important in this process (3, 77). Data from the present study confirm this latter notion. In the face of similar central command and blocked spinal opioid receptor-sensitive afferent feedback from the locomotor muscles, the RPE was lower compared with the placebo trial performed with an intact somatosensory feedback system (Table 2).
Summary
Our findings show that although only a portion of the locomotor muscle afferents, namely, the μ-opioid receptor-sensitive fibers, were blocked via lumbar intrathecal fentanyl, ventilation, HR, and blood pressure were significantly reduced compared with placebo exercise. This evidence supports the necessity of muscle afferents in regulating the normal steady-state circulatory and ventilatory responses in rhythmically exercising humans across a wide range of exercise intensities—even in the presence of apparently normal contributions from other major effectors of the cardioventilatory response.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute R01 Grant HL-15469.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank our subjects for their considerable time and patience during this study. We acknowledge Prof. Barbara Morgan, Prof. Urs Boutellier, Dr. C. Joel Hess, and Kathy S. Henderson for valuable assistance during the investigation. We highly appreciate Anthony J. Jacques' efforts in creating and modifying our data processing software. We thank Prof. Gordon Mitchell for his valuable feedback on the manuscript, Marc Kaufman for his advice, and Jackie Cuda for providing the upper body ergometer. J. A. Dempsey is grateful for long-term support from Mud Myers and Turk Gordon.
REFERENCES
- 1. Adams L, Frankel H, Garlick J, Guz A, Murphy K, Semple SJ. The role of spinal cord transmission in the ventilatory response to exercise in man. J Physiol 355: 85–97, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol 82: 1811–1817, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Amann M, Proctor LT, Sebranek JJ, Eldridge MW, Pegelow DF, Dempsey JA. Somatosensory feedback from the limbs exerts inhibitory influences on central neural drive during whole body endurance exercise. J Appl Physiol 105: 1714–1724, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587: 271–283, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amann M, Romer LM, Subudhi AW, Pegelow DF, Dempsey JA. Severity of arterial hypoxaemia affects the relative contributions of peripheral muscle fatigue to exercise performance in healthy humans. J Physiol 581: 389–403, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amann M, Subudhi A, Foster C. Influence of testing protocol on ventilatory thresholds and cycling performance. Med Sci Sports Exerc 36: 613–622, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Asmussen E. Exercise and the regulation of ventilation. Circ Res 20: 132–145, 1967 [Google Scholar]
- 8. Asmussen E, Johansen SH, Jorgensen M, Nielsen M. On the nervous factors controlling respiration and circulation during exercise. Experiments with curarization. Acta Physiol Scand 63: 343–350, 1965 [DOI] [PubMed] [Google Scholar]
- 9. Asmussen E, Nielsen M, Wieth-Pedersen G. Cortical or reflex control of respiration during muscular work. Acta Physiol Scand 6: 168–175, 1943 [Google Scholar]
- 10. Babenco HD, Conard PF, Gross JB. The pharmacodynamic effect of a remifentanil bolus on ventilatory control. Anesthesiology 92: 393–398, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Bennett FM, Fordyce WE. Gain of the ventilatory exercise stimulus: definition and meaning. J Appl Physiol 65: 2011–2017, 1988 [DOI] [PubMed] [Google Scholar]
- 12. Besse D, Lombard MC, Besson JM. Autoradiographic distribution of mu, delta and kappa opioid binding sites in the superficial dorsal horn, over the rostrocaudal axis of the rat spinal cord. Brain Res 548: 287–291, 1991 [DOI] [PubMed] [Google Scholar]
- 13. Besse D, Lombard MC, Zajac JM, Roques BP, Besson JM. Pre- and postsynaptic distribution of mu, delta and kappa opioid receptors in the superficial layers of the cervical dorsal horn of the rat spinal cord. Brain Res 521: 15–22, 1990 [DOI] [PubMed] [Google Scholar]
- 14. Borg G. Borg's Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics, 1998 [Google Scholar]
- 15. Brown DR, Forster HV, Pan LG, Brice AG, Murphy CL, Lowry TF, Gutting SM, Funahashi A, Hoffman M, Powers S. Ventilatory response of spinal cord-lesioned subjects to electrically induced exercise. J Appl Physiol 68: 2312–2321, 1990 [DOI] [PubMed] [Google Scholar]
- 16. Caringi D, Mokler DJ, Koester DM, Ally A. Rostral ventrolateral medullary opioid receptor activation modulates pressor response to muscle contraction. Am J Physiol Heart Circ Physiol 274: H139–H146, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Clark JM, Sinclair RD, Lenox JB. Chemical and nonchemical components of ventilation during hypercapnic exercise in man. J Appl Physiol 48: 1065–1076, 1980 [DOI] [PubMed] [Google Scholar]
- 18. Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Craig AD. Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J Comp Neurol 361: 225–248, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Cross BA, Davey A, Guz A, Katona PG, MacLean M, Murphy K, Semple SJ, Stidwill R. The role of spinal cord transmission in the ventilatory response to electrically induced exercise in the anaesthetized dog. J Physiol 329: 37–55, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Darques JL, Decherchi P, Jammes Y. Mechanisms of fatigue-induced activation of group IV muscle afferents: the roles played by lactic acid and inflammatory mediators. Neurosci Lett 257: 109–112, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Darques JL, Jammes Y. Fatigue-induced changes in group IV muscle afferent activity: differences between high- and low-frequency electrically induced fatigues. Brain Res 750: 147–154, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Eiken O. Responses to dynamic leg exercise in man as influenced by changes in muscle perfusion pressure. Acta Physiol Scand Suppl 566: 1–37, 1987 [PubMed] [Google Scholar]
- 24. Eisenach JC, Hood DD, Curry R, Shafer SL. Cephalad movement of morphine and fentanyl in humans after intrathecal injection. Anesthesiology 99: 166–173, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Eldridge FL, Millhorn DE, Kiley JP, Waldrop TG. Stimulation by central command of locomotion, respiration and circulation during exercise. Respir Physiol 59: 313–337, 1985 [DOI] [PubMed] [Google Scholar]
- 26. Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol 96: 1486–1495, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Fernandes A, Galbo H, Kjaer M, Mitchell JH, Secher NH, Thomas SN. Cardiovascular and ventilatory responses to dynamic exercise during epidural anaesthesia in man. J Physiol 420: 281–293, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fields HL, Emson PC, Leigh BK, Gilbert RF, Iversen LL. Multiple opiate receptor sites on primary afferent fibres. Nature 284: 351–353, 1980 [DOI] [PubMed] [Google Scholar]
- 29. Freund PR, Rowell LB, Murphy TM, Hobbs SF, Butler SH. Blockade of the pressor response to muscle ischemia by sensory nerve block in man. Am J Physiol Heart Circ Physiol 237: H433–H439, 1979 [DOI] [PubMed] [Google Scholar]
- 30. Friedman DB, Brennum J, Sztuk F, Hansen OB, Clifford PS, Bach FW, Arendt-Nielsen L, Mitchell JH, Secher NH. The effect of epidural anaesthesia with 1% lidocaine on the pressor response to dynamic exercise in man. J Physiol 470: 681–691, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Galbo H, Kjaer M, Secher NH. Cardiovascular, ventilatory and catecholamine responses to maximal dynamic exercise in partially curarized man. J Physiol 389: 557–568, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gourlay GK, Murphy TM, Plummer JL, Kowalski SR, Cherry DA, Cousins MJ. Pharmacokinetics of fentanyl in lumbar and cervical CSF following lumbar epidural and intravenous administration. Pain 38: 253–259, 1989 [DOI] [PubMed] [Google Scholar]
- 33. Grant GJ, Susser L, Cascio M, Moses M, Zakowski MI. Hemodynamic effects of intrathecal fentanyl in nonlaboring term parturients. J Clin Anesth 8: 99–103, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Guz A. Brain, breathing and breathlessness. Respir Physiol 109: 197–204, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Hill JM, Kaufman MP. Attenuation of reflex pressor and ventilatory responses to static muscular contraction by intrathecal opioids. J Appl Physiol 68: 2466–2472, 1990 [DOI] [PubMed] [Google Scholar]
- 36. Hornbein TF, Sorensen SC, Parks CR. Role of muscle spindles in lower extremities in breathing during bicycle exercise. J Appl Physiol 27: 476–479, 1969 [DOI] [PubMed] [Google Scholar]
- 37. Huynh NH, Tyrefors N, Ekman L, Johansson M. Determination of fentanyl in human plasma and fentanyl and norfentanyl in human urine using LC-MS/MS. J Pharm Biomed Anal 37: 1095–1100, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Innes JA, De Cort SC, Evans PJ, Guz A. Central command influences cardiorespiratory response to dynamic exercise in humans with unilateral weakness. J Physiol 448: 551–563, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kalia M, Mei SS, Kao FF. Central projections from ergoreceptors (C fibers) in muscle involved in cardiopulmonary responses to static exercise. Circ Res 48: I48–I62, 1981 [PubMed] [Google Scholar]
- 40. Kalliomaki J, Luo XL, Yu YB, Schouenborg J. Intrathecally applied morphine inhibits nociceptive C fiber input to the primary somatosensory cortex (SI) of the rat. Pain 77: 323–329, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Kao FF. An experimental study of the pathway involved in exercise hyperpnea employing cross-circulation technique. In: The Regulation of Human Respiration, edited by Cunningham DJC, Lloyd BB. Oxford, UK: Blackwell, 1963, p. 461–502 [Google Scholar]
- 42. Keenan KG, Farina D, Maluf KS, Merletti R, Enoka RM. Influence of amplitude cancellation on the simulated surface electromyogram. J Appl Physiol 98: 120–131, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Kozelka JW, Christy GW, Wurster RD. Ascending pathways mediating somatoautonomic reflexes in exercising dogs. J Appl Physiol 62: 1186–1191, 1987 [DOI] [PubMed] [Google Scholar]
- 44. Lalley PM. mu-Opioid receptor agonist effects on medullary respiratory neurons in the cat: evidence for involvement in certain types of ventilatory disturbances. Am J Physiol Regul Integr Comp Physiol 285: R1287–R1304, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Lalley PM. Opioidergic and dopaminergic modulation of respiration. Respir Physiol Neurobiol 164: 160–167, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lamb TW. Ventilatory responses to hind limb exercise in anesthetized cats and dogs. Respir Physiol 6: 88–104, 1968 [DOI] [PubMed] [Google Scholar]
- 47. Levine S. Ventilatory response to muscular exercise: observations regarding a humoral pathway. J Appl Physiol 47: 126–137, 1979 [DOI] [PubMed] [Google Scholar]
- 48. Marcora S. Perception of effort during exercise is independent of afferent feedback from skeletal muscles, heart, and lungs. J Appl Physiol 106: 2060–2062, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Mateika JH, Duffin J. Coincidental changes in ventilation and electromyographic activity during consecutive incremental exercise tests. Eur J Appl Physiol Occup Physiol 68: 54–61, 1994 [DOI] [PubMed] [Google Scholar]
- 50. Mateika JH, Duffin J. A review of the control of breathing during exercise. Eur J Appl Physiol Occup Physiol 71: 1–27, 1995 [DOI] [PubMed] [Google Scholar]
- 51. McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Meintjes AF, Nobrega AC, Fuchs IE, Ally A, Wilson LB. Attenuation of the exercise pressor reflex. Effect of opioid agonist on substance P release in L-7 dorsal horn of cats. Circ Res 77: 326–334, 1995 [DOI] [PubMed] [Google Scholar]
- 53. Mense S, Craig AD., Jr Spinal and supraspinal terminations of primary afferent fibers from the gastrocnemius-soleus muscle in the cat. Neuroscience 26: 1023–1035, 1988 [DOI] [PubMed] [Google Scholar]
- 54. Mitchell GS. Ventilatory control during exercise with increased respiratory dead space in goats. J Appl Physiol 69: 718–727, 1990 [DOI] [PubMed] [Google Scholar]
- 55. Mitchell JH, Reeves DR, Jr, Rogers HB, Secher NH. Epidural anaesthesia and cardiovascular responses to static exercise in man. J Physiol 417: 13–24, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Morin D, Viala D. Coordinations of locomotor and respiratory rhythms in vitro are critically dependent on hindlimb sensory inputs. J Neurosci 22: 4756–4765, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ochwadt B, Bücherl E, Kreuzer H, Löschcke HH. Beeinflussung der Atemsteigerung bei Muskelarbeit durch partiellen neuromuskulären Block (Tubocurarine). Pflügers Arch Gesamte Physiol Menschen Tiere 269: 613–621, 1959 [PubMed] [Google Scholar]
- 58. Pan LG, Forster HV, Wurster RD, Murphy CL, Brice AG, Lowry TF. Effect of partial spinal cord ablation on exercise hyperpnea in ponies. J Appl Physiol 69: 1821–1827, 1990 [DOI] [PubMed] [Google Scholar]
- 59. Pomeroy G, Ardell JL, Wurster RD. Spinal opiate modulation of cardiovascular reflexes in the exercising dog. Brain Res 381: 385–389, 1986 [DOI] [PubMed] [Google Scholar]
- 60. Poon CS, Tin C, Yu Y. Homeostasis of exercise hyperpnea and optimal sensorimotor integration: the internal model paradigm. Respir Physiol Neurobiol 159: 1–13, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Punnen S, Willette R, Krieger AJ, Sapru HN. Cardiovascular response to injections of enkephalin in the pressor area of the ventrolateral medulla. Neuropharmacology 23: 939–946, 1984 [DOI] [PubMed] [Google Scholar]
- 62. Rowell LB, Hermansen L, Blackmon JR. Human cardiovascular and respiratory responses to graded muscle ischemia. J Appl Physiol 41: 693–701, 1976 [DOI] [PubMed] [Google Scholar]
- 63. Rowell LB, O'Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol 69: 407–418, 1990 [DOI] [PubMed] [Google Scholar]
- 64. Sato A, Sato Y, Schmidt RF. Heart rate changes reflecting modifications of efferent cardiac sympathetic outflow by cutaneous and muscle afferent volleys. J Auton Nerv Syst 4: 231–247, 1981 [DOI] [PubMed] [Google Scholar]
- 65. Scott JC, Cooke JE, Stanski DR. Electroencephalographic quantitation of opioid effect: comparative pharmacodynamics of fentanyl and sufentanil. Anesthesiology 74: 34–42, 1991 [DOI] [PubMed] [Google Scholar]
- 66. Shannon MT, Ramanathan S. An intravenous fluid bolus is not necessary before administration of intrathecal fentanyl for labor analgesia. J Clin Anesth 10: 452–456, 1998 [DOI] [PubMed] [Google Scholar]
- 67. Smith SA, Querry RG, Fadel PJ, Gallagher KM, Stromstad M, Ide K, Raven PB, Secher NH. Partial blockade of skeletal muscle somatosensory afferents attenuates baroreflex resetting during exercise in humans. J Physiol 551: 1013–1021, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Standl TG, Horn E, Luckmann M, Burmeister M, Wilhelm S, Schulte am Esch J. Subarachnoid sufentanil for early postoperative pain management in orthopedic patients: a placebo-controlled, double-blind study using spinal microcatheters. Anesthesiology 94: 230–238, 2001 [DOI] [PubMed] [Google Scholar]
- 69. Sun SY, Liu Z, Li P, Ingenito AJ. Central effects of opioid agonists and naloxone on blood pressure and heart rate in normotensive and hypertensive rats. Gen Pharmacol 27: 1187–1194, 1996 [DOI] [PubMed] [Google Scholar]
- 70. Swenson JD, Owen J, Lamoreaux W, Viscomi C, McJames S, Cluff M. The effect of distance from injection site to the brainstem using spinal sufentanil. Reg Anesth Pain Med 26: 306–309, 2001 [DOI] [PubMed] [Google Scholar]
- 71. Thornton JM, Guz A, Murphy K, Griffith AR, Pedersen DL, Kardos A, Leff A, Adams L, Casadei B, Paterson DJ. Identification of higher brain centres that may encode the cardiorespiratory response to exercise in humans. J Physiol 533: 823–836, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tibes U. Reflex inputs to the cardiovascular and respiratory centers from dynamically working canine muscles. Some evidence for involvement of group III or IV nerve fibers. Circ Res 41: 332–341, 1977 [DOI] [PubMed] [Google Scholar]
- 73. Waldrop TG, Eldridge FL, Iwamoto GA, Mitchell JH. Central neural control of respiration and circulation during exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, chapt. 9, p. 333–380 [Google Scholar]
- 74. Waldrop TG, Mullins DC, Millhorn DE. Control of respiration by the hypothalamus and by feedback from contracting muscles in cats. Respir Physiol 64: 317–328, 1986 [DOI] [PubMed] [Google Scholar]
- 75. Weissman ML, Whipp BJ, Huntsman DJ, Wasserman K. Role of neural afferents from working limbs in exercise hyperpnea. J Appl Physiol 49: 239–248, 1980 [DOI] [PubMed] [Google Scholar]
- 76. White JM, Irvine RJ. Mechanisms of fatal opioid overdose. Addiction 94: 961–972, 1999 [PubMed] [Google Scholar]
- 77. Winchester PK, Williamson JW, Mitchell JH. Cardiovascular responses to static exercise in patients with Brown-Sequard syndrome. J Physiol 527: 193–202, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yaksh TL. Spinal opiate analgesia: characteristics and principles of action. Pain 11: 293–346, 1981 [DOI] [PubMed] [Google Scholar]
- 79. Yaksh TL, Noueihed R. The physiology and pharmacology of spinal opiates. Annu Rev Pharmacol Toxicol 25: 433–462, 1985 [DOI] [PubMed] [Google Scholar]
- 80. Yaksh TL, Rudy TA. Studies on the direct spinal action of narcotics in the production of analgesia in the rat. J Pharmacol Exp Ther 202: 411–428, 1977 [PubMed] [Google Scholar]
- 81. Yamamoto WS. Looking at the regulation of ventilation as a signaling process. In: Muscular Exercise and the Lung, edited by Dempsey JA, Reed CE. Madison, WI: Univ of Wisconsin Press, 1977, p. 137–149 [Google Scholar]