Abstract
The relationship between changes in absolute end-expiratory lung volume (EELV) and collapsibility has not been rigorously quantified. We hypothesized that pharyngeal collapsibility varies inversely with absolute lung volume in sleeping humans during 1) conventional and 2) isovolume measurements of passive critical pressure (Pcrit). Eighteen healthy subjects (11 male, 7 female) slept in a negative pressure ventilator for measurements of pharyngeal collapsibility (Pcrit) during non-rapid eye movement sleep. EELV was 1) allowed to vary with changes in nasal pressure for conventional Pcrit measurements and 2) controlled by maintaining a fixed pressure difference across the respiratory system (PRS) from the nose to the body surface for isovolume Pcrit measurements at elevated EELV (PRS = +10 cmH2O), reduced EELV (PRS = −5 cmH2O), and functional residual capacity (FRC; PRS = 0 cmH2O). In each condition, the absolute EELV was determined and the corresponding Pcrit was derived from upper airway pressure-flow relationships. In the entire group, Pcrit varied inversely with EELV (P < 0.001). Pcrit decreased as EELV increased from the conventional to the FRC isovolume condition by −3.5 ± 1.0 cmH2O/l (P < 0.003). Subjects with a conventional Pcrit below −2 cmH2O exhibited greater reductions in EELV and correspondingly greater decreases in the FRC isovolume compared with the conventional Pcrit (P < 0.001). The overall response, ΔPcrit/ΔEELV, was −2.0 ± 0.2 cmH2O/l (P < 0.001) and did not differ between men and women (P = 0.16). Nevertheless, men and women differed significantly in FRC (2.63 ± 0.16 vs. 1.88 ± 0.13 liters, P <0.05) and FRC isovolume Pcrit (−2.3 ± 0.8 vs. −7.2 ± 1.2 cmH2O, P < 0.05), implying that the men had larger lungs and more collapsible airways than the women. The ΔPcrit/ΔEELV response was independent of sex, conventional Pcrit, body mass index, and neck, waist, and hip circumferences. We conclude that Pcrit varies inversely with absolute EELV, which may lead to 1) an underestimation of the magnitude of quantitative differences in Pcrit across the spectrum from health (negative Pcrit) to disease (positive Pcrit) and 2) increases in sleep apnea susceptibility in obesity.
Keywords: pharyngeal collapsibility, sleep apnea, critical pressure
obstructive sleep apnea is a common disorder linked to the increasing prevalence of obesity in Western society (49). Sleep apnea is caused by upper airway obstruction during sleep (30), leading to repeated oxyhemoglobin desaturations and arousals throughout the night. Pharyngeal obstruction is due to elevations in upper airway collapsibility (9, 10, 34, 38, 39, 42), which rises with increases in body weight (19, 34). Nevertheless, the mechanisms linking obesity and upper airway collapsibility during sleep have not been fully elucidated.
In obese individuals, upper airway function could be influenced by adiposity around the pharynx (33) and/or torso (15, 34, 43). When adipose tissue deposits around the neck and peripharyngeal regions, compression of the pharyngeal lumen can lead to narrowing and increases in upper airway collapsibility (14, 33). Central adiposity around the viscera and chest can also decrease end-expiratory lung volume (EELV) (25, 28, 41). Investigators have demonstrated in animal models that reductions in lung volume cause decreases in caudal traction on the upper airway and concomitant increases in upper airway collapsibility (44–46). Similarly, recent evidence suggests that upper airway stability varies directly with EELV (6, 11, 12, 16, 40, 48), which can account for improvements in sleep apnea when lung volume is elevated (11). Recently, investigators demonstrated that increases in EELV were accompanied by decreases in pharyngeal collapsibility, although Pcrit responses to changes in absolute EELV were not assessed (22).
Upper airway collapsibility can be derived from an analysis of pressure-flow relationships in sleeping humans (7, 23, 35). A standardized protocol has been developed for lowering the nasal pressure stepwise and determining the nasal pressure at which the airway occludes (critical pressure, Pcrit). This conventional protocol, however, does not control for concomitant changes in EELV that occur when nasal pressure is lowered. Thus changes in EELV may confound conventional measurements of upper airway collapsibility (Pcrit), and decreases in EELV could account for increases in sleep apnea susceptibility in obesity.
The present study was undertaken to quantify the effects of changes in absolute EELV on upper airway collapsibility. Specifically, we hypothesized that collapsibility varies inversely with absolute lung volume in sleeping humans (36) during conventional and isovolume measurements of Pcrit. To test this hypothesis, we developed a protocol for maintaining a constant EELV (isovolume) while manipulating nasal pressure during Pcrit determinations. In separate protocols, we quantified effects of changes in absolute EELV on 1) conventional and 2) isovolume Pcrit measurements.
METHODS
Subject Selection
Healthy men (n = 11) and women (n = 7) were recruited from the community. Subjects with concomitant medical illness on screening history, physical examination, or pulmonary function testing were excluded. Written informed consent was obtained from each participant for this study, which was approved by the Johns Hopkins Medical Institution Human Investigations Review Board.
Study Design
On separate nights, each subject underwent a standard sleep study to characterize sleep apnea severity and a physiological sleep study to examine effects of lung volume on upper airway collapsibility. To facilitate physiological measurements during sleep, subjects were given a hypnotic agent (Triazolam) at a dose of 0.50 mg 30 min before the onset of the physiological sleep study. Passive Pcrit, a measure of upper airway collapsibility, was determined under conditions of decreased upper airway neuromuscular activity, as previously described (7, 23, 35), as well as at three distinct lung volumes: functional residual capacity (FRC), ∼1 liter above FRC, and ∼0.5 liter below FRC.
Experimental Techniques
Polysomnography.
Standard polysomnographic techniques were employed to define the respiratory pattern during sleep (26, 29). Surface electroencephalographic electrodes placed at C3-A2, C3-O1, and F3-A2, submental electromyographic electrodes, and left and right electrooculograms were used to stage sleep. Respiratory events were scored using impedance thoracoabdominal belts to gauge respiratory efforts, a lightweight, low-deadspace pneumotachometer (Key Technologies, Baltimore, MD) attached to a tight-fitting mask (Mirage Vista, ResMed, Bella Vista, NSW, Australia) open to atmosphere to monitor and measure airflow, and pulse oximetry to monitor arterial oxygen saturation (SaO2). Sleep staging, respiratory events, and arousals were scored using standard criteria (29). Respiratory arousals were identified according to American Academy of Sleep Medicine criteria (3), and respiratory events were identified using previously reported criteria from our laboratory (26). An apnea was defined as a cessation of airflow for 10 s or more. A hypopnea was defined as a discernable reduction in airflow in association with a ≥4% desaturation and/or arousal.
Pulmonary function testing.
Standard techniques for pulmonary function testing were performed in both the supine and upright (seated) positions in each subject (1). Pulmonary function testing equipment was manufactured by W. E. Collins (CPL Lung Function Analyzer; Braintree MA). Spirometry was performed to obtain the forced expiratory volume in 1 s (FEV1) and the forced vital capacity (FVC). Multiple-breath closed-circuit helium dilution was used to measure FRC. At the end of each multiple-breath procedure, slow vital capacity (SVC) and expiratory reserve volume (ERV) were measured in triplicate. Residual volume (RV) was then calculated by subtracting the average ERV from the measured FRC. Total lung capacity (TLC) was determined by adding the largest SVC to the calculated RV (1, 27).
Physiological setup.
During a physiological sleep study, subjects slept supine with one pillow in a head-out rigid shell (Portalung; Lakewood, CO) attached to a negative pressure ventilator (NEV-100; Respironics, Murraysville, PA) and positive pressure generator (BiPAP Synchrony; Respironics). Airflow was monitored with a pneumotachograph (model 4830, 0–400 l/min; Hans Rudolph, Kansas City, MO) attached to a differential pressure transducer (Pneumotach Amplifier 1, series 1110; Hans Rudolph) placed between a tight-fitting nasal mask (Comfort Classic; Respironics) and a continuous positive airway pressure (CPAP) unit custom designed to apply pressures between −20 and +20 cmH2O (ResMed). Respiratory effort was monitored via a Hyatt-type esophageal balloon (Ackrad Laboratories, Cranford, NJ) placed via a perinasal approach for monitoring esophageal pressure. EELV was monitored with respiratory impedance plethysmography. Impedance bands were placed circumferentially around the chest and abdomen and were calibrated using standard noninvasive respiratory maneuvers (Inductotrace; Ambulatory Monitoring, Ardsley, NY). All monitored physiological signals were digitized, acquired, and archived on a computer workstation (Somnologica; Medcare, Buffalo, NY).
Experimental Protocols
Nocturnal EELV monitoring.
The absolute EELV was monitored and controlled throughout the night as follows. Calibrated inductive plethysmography bands (Inductotrace; Ambulatory Monitoring) were utilized to measure nocturnal deviations in EELV from a prior measurement of FRC in the supine position during wakefulness (see Physiological setup). The device was calibrated initially during wakefulness with standardized 1) isovolumetric maneuvers and 2) 800-ml tidal volumes. The validity of the calibration was repeatedly cross-checked during sleep when obstructive apneas were produced by lowering the nasal pressure (see Pcrit protocols described below) and the sum signal remained zero. If the sum deviated from zero, the thoracic and abdominal gains were readjusted to obtain a zero sum signal during the isovolumetric movements of obstructive apneas. Thereafter, nasal pressure was increased to abolish airflow obstruction. Tidal volumes were taken as a reference lung volume for sum signal deflections throughout the entire sleep study and were recalculated in each experimental condition by integrating the airflow signal from a calibrated pneumotachograph through which the subject breathed.
Controlling/manipulating EELV.
EELV was controlled by maintaining a fixed pressure difference across the entire respiratory system (transrespiratory pressure, PRS) from the nose to body surface, as previously described (48). PRS was varied by manipulating both the nasal pressure (PN) through the mask and the extrathoracic pressure (PET) through the Portalung (PRS = PN − PET). Deflections in the EELV signal associated with each level of PRS were measured only after the validity of the sum signal calibration was confirmed during obstructive apneas (see below). To determine the absolute EELV and total respiratory system compliance (CRS) of each subject, we administered a range of positive and negative PRS during non-rapid eye movement (NREM) sleep before and after taking Pcrit measurements.
Passive Pcrit conditions.
CONVENTIONAL PCRIT.
During stable NREM sleep, the nasal mask pressure was increased until flow-limited breathing was abolished (holding pressure) (7, 23, 35). After at least 10 min of stable NREM sleep at the holding pressure, nasal pressure was reduced acutely to discrete levels for five breath intervals, after which the nasal pressure was returned to the holding pressure (see Fig. 1). At least three series of stepwise reductions in nasal pressure that targeted zero airflow (Pcrit) were collected in each subject. If an arousal occurred, the protocol was resumed after the subject reinitiated NREM sleep. Breaths associated with microarousals from sleep were excluded from analysis (23). EELV was allowed to vary with changes in nasal pressure for conventional Pcrit measurements.
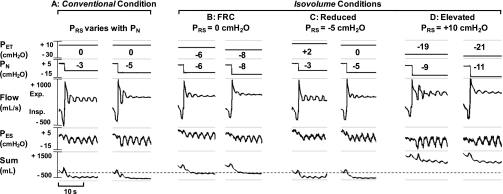
Fig. 1.
Pressure regimens are illustrated for conventional and isovolume conditions. Representative recordings for each experimental condition are shown for 1 female subject during non-rapid eye movement (NREM) sleep. The following signals are shown from top to bottom: extrathoracic pressure (PET), nasal pressure (PN), expiratory (Exp.) and inspiratory (Insp.) airflow (Flow), esophageal pressure (PES), and the inductance plethysmographic sum. Note the dashed line through the sum signal represents functional residual capacity. In each condition, the 2 lowest PN values used to derive the passive critical pressure (Pcrit) are shown. Note that the pressure difference across the respiratory system (PRS) = PN − PET and that in the isovolume conditions (B–D), PET was first lowered or raised to offset the target PN and maintain a fixed PRS during each stepwise reduction in PN. The maximal inspiratory airflow (V̇I max) was measured for the 2nd through 5th breaths after stepwise decreases in PN, when EELV is known to equilibrate (23, 35).
ISOVOLUME PCRIT.
We recognized that manipulations in nasal pressure during the conventional Pcrit protocol lead to concomitant changes in EELV. To prevent these changes in EELV, we counterbalanced the effect of step reductions in nasal pressure on EELV by first applying an equivalent pressure to the extrathoracic space ∼15 s before decreasing the nasal pressure. This approach allowed us to maintain a fixed PRS (PRS = PN − PET) during isovolume Pcrit measurements. The three isovolume conditions were elevated EELV (PRS = +10 cmH2O), reduced EELV (PRS = −5 cmH2O), and FRC (PRS = 0 cmH2O). Pcrit was derived from upper airway pressure-flow relationships during NREM sleep in each isovolume condition. The holding pressure was adjusted between isovolume conditions to 1) prevent inspiratory airflow limitation (i.e., maintain a hypotonic condition) induced by continuous positive extrathoracic pressure (CPEP) or reduced EELV before stepwise reductions in PN and 2) prevent overinflation of the lungs (i.e., maintain PRS < 20 cmH2O) during the application of continuous negative extrathoracic pressure (CNEP).
Pressure-volume relationships.
In each subject, pressure-volume relationships were delineated in each of four experimental conditions. The Pcrit and upstream resistance (RUS) were measured 1) as previously described (conventional condition; PN is not counterbalanced with the simultaneous application of PET) (7, 23, 35); 2) EELV was maintained at FRC by applying equivalent amounts of PET and PN simultaneously (FRC isovolume condition; PRS was maintained at atmospheric pressure during all PN drops); 3) EELV was raised and maintained at FRC + 1 liter (elevated isovolume condition; PRS was maintained at +10 cmH2O during all PN drops); and 4) EELV was lowered and maintained at FRC − 0.5 liter (reduced isovolume condition; PRS was maintained at −5 cmH2O during all PN drops) (see Fig. 1). To determine specific effects of steady-state changes in EELV, data acquisition for the elevated and reduced isovolume conditions was bracketed by the FRC isovolume condition. No systematic bias was detected between repeated measurements of FRC isovolume Pcrit [95% confidence interval (CI): −1.2 to 1.1 cmH2O, P = 0.76] (23).
Analyses
Measuring relative changes in EELV.
The inductance plethysmograph sum signal was used to measure relative changes in EELV between wake and sleep by subtracting the decrement in EELV at sleep onset (first epoch of N1) from the supine FRC determined by helium dilution during wakefulness (1, 2, 8, 25, 32). The sum signal was also used to measure and track relative changes in EELV throughout the night. It was standardized to actual measurements of tidal volumes during initial 800-ml calibrations (see above) at the start of the sleep study and spontaneous tidal volumes throughout the night. Tidal volumes were calculated by integrating the airflow signal (LabChart Pro; ADInstruments, Colorado Springs, CO). If movement artifacts or drift was present in the sum signal, the calculated tidal volumes were used to calibrate and rescale the sum signal repeatedly throughout the night. The sum signal was then used to measure relative changes in EELV during step changes in PRS.
Calculating absolute EELV.
We recognized that inductance plethysmography is susceptible to changes in baseline and gain between experimental conditions. To address this concern, we used the CRS to calculate deviations in EELV from FRC in each experimental condition as follows: ΔEELV = ΔPRS·CRS. Least-squares linear regression was used to derive CRS as the slope of the relationship between changes in EELV and PRS (R2 values ranged from 0.91 to 0.99 in all subjects). ΔEELV was then calculated and added to the supine FRC associated with sleep onset (described above).
Comparison of static and dynamic compliance of the respiratory system.
We calculated CRS from measurements of EELV during dynamic (acute) drops in nasal pressure and compared these values to CRS derived from measurements of EELV over a similar range of static (steady state) alterations in nasal and extrathoracic pressure. For the group as a whole, we found no significant differences in CRS between dynamic (89 ± 6 ml/cmH2O) and static (96 ± 6 ml/cmH2O) conditions (P = 0.47), regardless of whether the measurement was made by manipulating the nasal or extrathoracic pressure (P = 0.22). Our findings led us to conclude that the lungs rapidly reached an equilibrium volume following asynchronous changes in extrathoracic and nasal pressures (see above).
Passive pressure-flow relationship.
In each experimental condition, the passive pressure-flow relationship was characterized as previously described (23, 24). The maximal inspiratory airflow (V̇I max) was measured for the 2nd through 5th breaths during each stepwise decrease in PN during stable NREM sleep, when EELV is known to equilibrate (23, 35), and the V̇I max vs. PN relationship was constructed using least-squares linear regression. Pcrit was determined by solving for PN at zero flow from the regression equation, and RUS was the inverse of the slope of this relationship. A minimum of three distinct PN levels were required to generate the pressure-flow relationship, and the lowest PN had to be ≤3 cmH2O above the calculated passive Pcrit.
Analytic approach.
The effect of EELV on passive Pcrit and RUS was examined in two ways. First, we compared these parameters from conventional and isovolume measurements to determine the effect of stabilizing EELV at FRC on passive Pcrit and RUS (protocol 1). Second, we compared Pcrit and RUS between reduced, FRC, and elevated isovolume measurements to examine the independent effect of EELV on these parameters (protocol 2). In these analyses, we used multilevel mixed effects linear regression (XTMIXED) to determine how Pcrit and RUS varied across experimental conditions while accounting for repeated measures within subjects. Statistical analyses were performed using STATA 11 (Stata, College Station, TX).
In secondary analyses, we examined the relationships between the absolute EELV during isovolume conditions and the Pcrit and RUS (XTMIXED, STATA 11). The Wilcoxon rank sum test and the Wilcoxon matched-pairs signed-rank test were also used for unpaired and paired comparisons, respectively. The Spearman's rank correlation coefficient was employed to examine the correlation between variables.
RESULTS
Subject Characteristics
In Table 1, demographic, anthropometric, sleep-disordered breathing, and pulmonary function parameters are reported for both men and women, as well as for the entire group. In general, our study sample consisted of young, healthy, lean, nonapneic subjects. Men had significantly greater neck and waist circumferences. During standard polysomnography, men had slightly lower baseline SaO2 and greater oxyhemoglobin desaturations during sleep-disordered breathing events than women, with an overall trend toward a higher total respiratory disturbance index (RDI; P = 0.08). As shown in Table 1, FVC, FEV1, and FRC were significantly greater in the men compared with the women (P < 0.05). FRC, TLC, and SVC decreased significantly from the upright to the supine position (P < 0.05), and RV did not change (P = 0.48). In the entire group, the supine FRC was 2.34 ± 0.14 liters during wakefulness, and the EELV fell minimally to 2.28 ± 0.14 liters during NREM sleep (P = 0.52).
Table 1.
Subect characteristics and pulmonary function parameters
| Men | Women | All | |
|---|---|---|---|
| n | 11 | 7 | 18 |
| Age, yr | 27.6 ± 7.1 | 27.4 ± 10.6 | 27.5 ± 8.3 |
| Anthropometrics | |||
| BMI, kg/m2 | 25.9 ± 3.7 | 23.1 ± 2.2 | 24.8 ± 3.4 |
| Neck, cm | 38.2 ± 1.6* | 32.1 ± 1.1 | 35.9 ± 3.4 |
| Waist, cm | 87.6 ± 6.7* | 77.6 ± 9.0 | 83.7 ± 9.0 |
| Hip, cm | 99.9 ± 5.0 | 95.4 ± 5.0 | 98.2 ± 5.4 |
| Waist-to-hip ratio | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 |
| Sleep architecture | |||
| Total sleep time, min | 337.3 ± 44.6 | 332.0 ± 33.5 | 335.1 ± 39.3 |
| Sleep efficiency, % | 90.8 ± 7.5 | 87.3 ± 5.1 | 89.4 ± 6.7 |
| WASO, min | 25.4 ± 23.4 | 26.7 ± 14.2 | 26.0 ± 19.6 |
| N1 (stage 1), % | 13.1 ± 7.8 | 7.4 ± 2.4 | 10.8 ± 6.7 |
| N2 (stage 2), % | 52.9 ± 5.0 | 51.8 ± 5.2 | 52.4 ± 5.0 |
| N3 (stage 3/4), % | 19.0 ± 9.1 | 24.9 ± 5.9 | 21.4 ± 8.2 |
| NREM, % | 85.0 ± 8.5 | 84.1 ± 5.8 | 84.6 ± 7.3 |
| REM, % | 15.0 ± 8.5 | 16.0 ± 5.8 | 15.4 ± 7.3 |
| RDI | |||
| NREM, events/h | 6.2 ± 5.5 | 2.9 ± 4.7 | 4.8 ± 5.3 |
| REM, events/h | 11.0 ± 10.9† | 6.2 ± 9.1 | 8.7 ± 10.1 |
| Total, events/h | 7.0 ± 4.5 | 3.1 ± 4.0 | 5.4 ± 4.6 |
| Baseline SaO2 | |||
| NREM, % | 96.8 ± 0.6* | 97.8 ± 1.0 | 97.2 ± 0.9 |
| REM, % | 97.1 ± 0.7† | 97.8 ± 1.0 | 97.4 ± 0.9 |
| Total, % | 96.8 ± 0.6* | 97.9 ± 1.1 | 97.3 ± 1.0 |
| Average low SaO2 | |||
| NREM, % | 94.0 ± 1.3* | 96.0 ± 2.1 | 94.8 ± 1.9 |
| REM, % | 94.1 ± 1.8*† | 96.1 ± 1.7 | 94.9 ± 2.0 |
| Total, % | 94.0 ± 1.4* | 96.3 ± 1.9 | 95.0 ± 1.9 |
| ΔSaO2 | |||
| NREM, % | 2.8 ± 1.1* | 1.7 ± 1.2 | 2.4 ± 1.3 |
| REM, % | 3.0 ± 1.3*† | 1.8 ± 0.7 | 2.5 ± 1.2 |
| Total, % | 2.8 ± 1.2* | 1.6 ± 0.9 | 2.3 ± 1.2 |
| Pulmonary function | |||
| FVC, liters (upright) | 5.98 ± 0.27* | 3.67 ± 0.22 | 5.03 ± 0.33 |
| %Predicted | 113.6 ± 3.3 | 100.1 ± 2.9 | 108.1 ± 2.8 |
| FEV1, liters (upright) | 4.88 ± 0.21* | 3.14 ± 0.19 | 4.16 ± 0.26 |
| %Predicted | 107.6 ± 2.1 | 99.5 ± 2.8 | 104.3 ± 1.9 |
| FRC, liters (supine) | 2.63 ± 0.16* | 1.88 ± 0.13 | 2.34 ± 0.14 |
| %Upright | 73.3 ± 3.2 | 73.4 ± 4.2 | 73.3 ± 2.5 |
Values are means ± SD; n = no. of subects. BMI, body mass index; REM, rapid eye movement sleep; NREM, non-rapid eye movement sleep; WASO, wake after sleep onset; RDI, respiratory disturbance index; SaO2, arterial oxygen saturation; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; FRC, functional residual capacity.
P < 0.05, men vs. women.
Two men had no REM sleep.
Pressure-Flow Recordings
In Fig. 1, sleep recordings are shown for conventional and isovolume conditions in one subject and illustrate changes in airway patency with alterations in lung volume. In the conventional condition (Fig. 1A), step decreases in PN led to reductions in V̇I max with near airway closure at −5 cmH2O. Note that EELV decreased below FRC when PN was lowered to subatmospheric levels while PET remained at atmospheric level (see sum signal). Conversely, in the FRC isovolume condition shown (Fig. 1B), FRC was maintained during each reduction in PN by first lowering PET to the target PN level (PRS = 0 cmH2O). Note that EELV was higher than that in the conventional condition (Fig. 1A). Furthermore, when FRC was maintained, the decrease in airflow was less pronounced at comparable levels of PN (compare Fig. 1B, left, with Fig. 1A, right). In fact, a reduction in PN to −8 cmH2O was required to produce a comparable degree of airway obstruction (near occlusion) to that found at a PN of −5 cmH2O in the conventional condition. Conversely, positive PET was applied to lower EELV below FRC in the reduced isovolume condition (Fig. 1C, PRS = −5 cmH2O), leading to airway occlusion at −5 cmH2O rather than −8 cmH2O in the FRC isovolume condition (Fig. 1B). Finally, PET was lowered to raise EELV above FRC in the elevated isovolume condition (Fig. 1D, PRS = +10 cmH2O), resulting in near airway occlusion at a PN of −11 cmH2O.
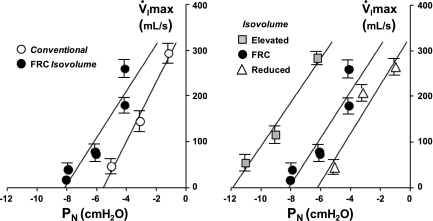
In Fig. 2, pressure-flow plots are shown for each condition illustrated in Fig. 1. At left, the V̇I max vs. PN relationships demonstrate a leftward shift of the FRC isovolume curve compared with the conventional curve. At right, isovolume V̇I max vs. PN relationships demonstrate a progressive leftward shift in the curves as EELV increased from a reduced EELV to FRC and to an elevated EELV. The parallel shift in these curves indicates that the primary effect of increasing EELV was to decrease the passive Pcrit without changing RUS, as demonstrated by a decrease in the zero-flow intercepts.
Fig. 2.
V̇I max vs. PN relationships are shown for conventional and isovolume conditions in 1 representative female subject. Values are means ± SE for the 2nd through 5th breaths from each run. At left, pressure-flow curves are shown for conventional and functional residual capacity (FRC) isovolume conditions. Note the leftward shift in this relationship as end-expiratory lung volume (EELV) was maintained at FRC in the isovolume condition. At right, all 3 isovolume pressure-flow curves are shown. Note the parallel, leftward shift in these relationships as EELV increased from a reduced to FRC and to an elevated isovolume.
Conventional vs. FRC Isovolume Passive Pcrit (Protocol 1)
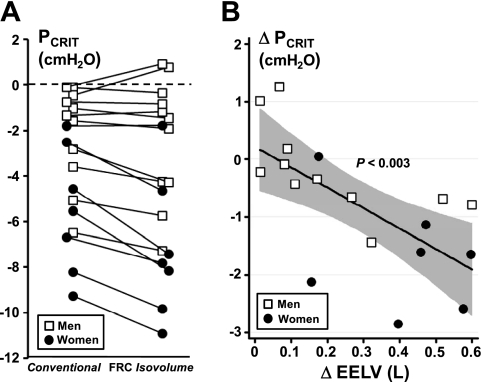
In Fig. 3A, conventional and FRC isovolume measurements of Pcrit are shown for each subject. For the group as a whole, both Pcrit and EELV differed significantly between conventional and FRC isovolume conditions (P < 0.001). Nevertheless, we found that the passive Pcrit fell as EELV rose from the conventional to FRC isovolume condition (Fig. 3B) by −3.5 ± 1.0 cmH2O/l (95% CI: −5.6 to −1.4 cmH2O/l, P < 0.003). Subjects with the more negative conventional Pcrit (below −2 cmH2O) exhibited greater reductions in EELV and correspondingly greater decreases in the FRC isovolume compared with the conventional Pcrit (Fig. 3, A and B, P < 0.003). In contrast, those with a conventional Pcrit near atmospheric demonstrated little change in EELV and Pcrit during the FRC isovolume Pcrit measurement. Differences in Pcrit were significant in the women (but not men), since a more negative conventional Pcrit in the women was associated with correspondingly greater decreases in EELV during the conventional compared with FRC isovolume Pcrit measurement (P < 0.001). RUS trended upward from the conventional to the FRC isovolume condition (P = 0.07) and from men to women (P = 0.08).
Fig. 3.
A: conventional and FRC isovolume measurements of Pcrit are shown for all subjects (n = 18). Note that conventional Pcrit and FRC isovolume Pcrit differed significantly below a threshold of −2 cmH2O in all subjects (P < 0.001). B: the difference between conventional and FRC isovolume Pcrit (ΔPcrit) is plotted against the corresponding difference in EELV (ΔEELV; n = 18). Note the inverse relationship for the group as a whole (solid line); when EELV is maintained at FRC in the isovolume condition, Pcrit falls directly as ΔEELV widens (P < 0.003). The shaded region represents the 95% confidence interval; the regression equation is ΔPcrit = −3.5(ΔEELV) + 0.2, where ΔPcrit and ΔEELV are measured in cmH2O and liters, respectively.
Effects of Absolute EELV on Passive Pcrit (Isovolume Conditions, Protocol 2)
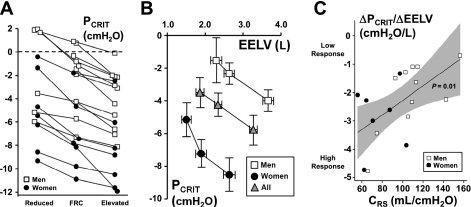
In Table 2, changes in EELV and PRS are reported for reduced, FRC, and elevated isovolume conditions. Pcrit decreased progressively and consistently from the reduced to elevated isovolume conditions in all subjects (Fig. 4A, P < 0.001). Pcrit also varied inversely with EELV across all isovolume conditions in both men and women and for the group as a whole (Fig. 4B, P < 0.001). RUS did not differ between isovolume conditions (P = 0.10). Nonetheless, RUS did decrease by 2.4 cmH2O·l−1·s−1 (95% CI: −4.2 to −0.7 cmH2O·l−1·s−1) per liter increase in EELV (P < 0.01) for the entire group, possibly reflecting pharyngeal dilation at higher lung volumes (6, 13, 40).
Table 2.
Conventional and isovolume parameters
| n | EELV, liters | Pcrit, cmH2O | RUS, cmH2O·l−1·s−1 | ΔEELV, ml | ΔPRS, cmH2O | CRS, ml/cmH2O | |
|---|---|---|---|---|---|---|---|
| Men | |||||||
| Conventional | 11 | 2.43 ± 0.17* | −2.1 ± 0.6* | 16.8 ± 1.4 | −205 ± 61* | −2.1 ± 0.6* | 108 ± 8* |
| Isovolume | |||||||
| Reduced | 6 | 2.29 ± 0.25*‡ | −1.5 ± 1.5‡ | 18.3 ± 3.9 | −417 ± 58‡ | −4.0 ± 0.5‡ | |
| FRC | 11 | 2.63 ± 0.16* | −2.3 ± 0.8* | 19.5 ± 1.6 | 0 ± 0 | 0.0 ± 0.0 | |
| Elevated | 11 | 3.67 ± 0.19*‡ | −4.0 ± 0.8*‡ | 17.2 ± 1.5* | 1035 ± 72*‡ | 9.6 ± 0.1‡ | |
| Women | |||||||
| Conventional | 7 | 1.48 ± 0.13† | −5.5 ± 1.0† | 22.6 ± 2.9 | −405 ± 67† | −5.5 ± 1.0† | 77 ± 7 |
| Isovolume | |||||||
| Reduced | 7 | 1.50 ± 0.11‡ | −5.1 ± 1.3‡ | 25.4 ± 3.3 | −384 ± 31‡ | −5.0 ± 0.1‡ | |
| FRC | 7 | 1.88 ± 0.13 | −7.2 ± 1.2 | 25.5 ± 2.8 | 0 ± 0 | 0.0 ± 0.0 | |
| Elevated | 7 | 2.63 ± 0.18‡ | −8.5 ± 1.3‡ | 24.7 ± 3.6 | 748 ± 66‡ | 9.7 ± 0.1‡ | |
| All subects | |||||||
| Conventional | 18 | 2.06 ± 0.16† | −3.4 ± 0.7† | 19.1 ± 1.5 | −283 ± 50† | −3.4 ± 0.7† | 96 ± 6 |
| Isovolume | |||||||
| Reduced | 13 | 1.86 ± 0.17‡ | −3.5 ± 1.1‡ | 22.1 ± 2.6 | −400 ± 31‡ | −4.5 ± 0.3‡ | |
| FRC | 18 | 2.34 ± 0.14 | −4.2 ± 0.9 | 21.9 ± 1.6 | 0 ± 0 | 0.0 ± 0.0 | |
| Elevated | 18 | 3.26 ± 0.18‡ | −5.7 ± 0.8‡ | 20.1 ± 1.8 | 924 ± 60‡ | 9.6 ± 0.1‡ |
Values are means ± SE; n = no. of subects. Pcrit, passive critical pressure; RUS, upstream resistance; ΔEELV, change in end-expiratory lung volume from FRC; ΔPRS, change in transrespiratory pressure; CRS, total respiratory system compliance. Conventional conditions are nonisovolume. Reduced, FRC, and elevated conditions are isovolume at reduced EELV, FRC, and elevated EELV, respectively.
P < 0.05, men vs women.
P < 0.001, conventional vs. FRC isovolume.
P < 0.001, elevated or reduced vs. FRC isovolume.
Fig. 4.
A: reduced (n = 13), FRC (n = 18), and elevated (n = 18) isovolume measurements of Pcrit are shown for all subjects. Note that each isovolume Pcrit differed significantly in all subjects (P < 0.001). B: isovolume relationships between the Pcrit and absolute EELV are represented for men (n = 11), women (n = 7), and the entire group. Note the inverse relationship between Pcrit and EELV (P < 0.001). Also note the curve representing the men is shifted upward and to the right relative to that representing the women (P < 0.005). Nevertheless, the change in Pcrit per change in EELV (ΔPcrit/ ΔEELV) was not different between men and women (P = 0.16). Reduced isovolume measurements were not obtained in 5 men due to the potential effects of positive PET on the neck (n = 3) or incomplete data (n = 2). C: the Pcrit response to changes in lung volume (ΔPcrit/ΔEELV) is plotted against respiratory system compliance (CRS) for all subjects. Note the ΔPcrit/ΔEELV response was inversely associated with CRS (P = 0.01) such that subjects with lower compliances had greater responses (more negative) than those with higher compliances. The shaded region represents the 95% confidence interval; the regression equation is ΔPcrit/ΔEELV = 0.026(CRS) − 4.9, where ΔPcrit/ΔEELV and CRS are measured in cmH2O/l and ml/cmH2O, respectively.
The overall Pcrit response to changes in lung volume (ΔPcrit/ΔEELV) was −2.0 ± 0.2 cmH2O/l (95% CI: −2.5 to −1.5 cmH2O/l, P < 0.001) and did not differ between men and women (Fig. 4B, P = 0.16). Nevertheless, men and women differed significantly in FRC (2.63 ± 0.16 vs. 1.88 ± 0.13 liters, P < 0.05) and FRC isovolume Pcrit (−2.3 ± 0.8 vs. −7.2 ± 1.2 cmH2O, P < 0.05), leading to an upward, rightward shift in the isovolume Pcrit vs. EELV relationship for the men relative to the women (see Fig. 4B and Table 2, P < 0.005).
The ΔPcrit/ΔEELV response was inversely associated with CRS (Fig. 4C, P = 0.01) and exhibited a similar trend with specific compliance (CRS/FRC, P < 0.08). Specifically, subjects with lower compliances had greater Pcrit responses to changes in lung volume than those with higher compliances, suggesting that a less compliant respiratory system produces greater changes in axial airway tension and Pcrit when the lungs inflate. The ΔPcrit/ΔEELV response, however, was independent of sex, conventional Pcrit, body mass index (BMI), and neck, waist, and hip circumferences for the group as a whole.
DISCUSSION
The current study quantified the effects of absolute EELV on upper airway collapsibility (Pcrit) during sleep. Comparing conventional and FRC isovolume measurements of Pcrit, we found that Pcrit changed as a function of EELV and became more negative when FRC was maintained by offsetting the nasal with extrathoracic pressure. Isovolume measurements of Pcrit also revealed significant decreases in Pcrit of ∼2 cmH2O per liter increase in EELV (Fig. 4B). The Pcrit response to changes in lung volume was associated with respiratory system compliance and was independent of sex, intrinsic collapsibility (conventional Pcrit), BMI, and neck, waist, and hip circumferences. These findings quantify the effects of absolute EELV on Pcrit and suggest that upper airway collapsibility varies inversely with EELV during sleep. Moreover, they also suggest that conventional Pcrit measurements have consistently underestimated the magnitude of quantitative differences in Pcrit across the spectrum from health to disease.
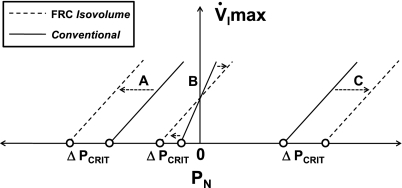
Pharyngeal critical pressures are derived from measurements of maximal inspiratory airflow at several levels of nasal pressure (7, 9, 10, 23, 34, 35, 38, 39, 42). Because nasal pressures were varied experimentally in these protocols, changes in EELV might have influenced measurements of maximal inspiratory airflow if pharyngeal collapsibility (Pcrit) varies with EELV. In prior work, we found that step reductions in nasal pressure produced a gradual roll off in maximal inspiratory airflow as lung volume decreased (35). In the present study, we have demonstrated systematic differences between isovolume Pcrit measurements and demonstrated an inverse relationship between the passive Pcrit and EELV. Furthermore, our findings establish the impact of EELV on conventional measurements of passive Pcrit and suggest that concomitant decreases in lung volume during conventional measurements in subjects with a negative Pcrit make Pcrit less negative than when lung volume is maintained at FRC (see Fig. 5, arrow A) (9, 39, 42). This effect would be most pronounced in normal individuals with a markedly negative Pcrit, leading to as much as a 3- to 4-cmH2O overestimation of Pcrit in the conventional compared with isovolume measurement. This bias would be somewhat less pronounced in asymptomatic snorers and patients with obstructive hypopneas, whose conventional Pcrit is less negative (9) (see Fig. 5, arrow B). Conversely, concomitant increases in lung volume during conventional measurements in apneic patients with a positive Pcrit would make Pcrit more positive than when lung volume is maintained at FRC (see Fig. 5, arrow C). These effects would have led investigators to underestimate the magnitude of quantitative differences in Pcrit across the spectrum from health to disease, since conventional methods would bias markedly positive and negative Pcrit measurements toward atmospheric (Fig. 5).
Fig. 5.
A conceptual illustration of V̇I max vs. PN relationships at 3 Pcrit levels under conventional (solid lines) and FRC isovolume conditions (dashed lines). At A, maintaining EELV at FRC when subatmospheric PN is applied results in a leftward, parallel shift in the pressure-flow relationship and a more negative Pcrit. At C, maintaining EELV at FRC when positive PN is applied results in a rightward, parallel shift in the pressure-flow relationship and a more positive Pcrit. Conversely, at B a change in both the slope (RUS) and Pcrit is represented as PN traverses atmospheric, since positive pressures would elevate and negative pressures would reduce EELV in the conventional condition.
Passive changes in upper airway collapsibility can be attributed to elevations in axial tension on upper airway structures (44–46). Mechanical linkage can stabilize upper airway patency during lung inflation as caudal traction is applied to the trachea through mediastinal structures. Caudal tracheal traction also decreases upper airway collapsibility (passive Pcrit) by stretching the soft palate and pharyngeal mucosa, making it better able to withstand collapsing pressures exerted by surrounding tissues. In fact, we found that subjects with lower compliances had greater Pcrit responses to changes in lung volume, leading us to speculate that a stiff, noncompliant respiratory system would exert greater traction on upper airway structures as lung volume increases. Mucosal tension can also augment this decrease in critical pressure when radial dilating forces are applied to upper airway structures (17, 18, 31). Mechanical attachments between the rib cage and cervical strap muscles may contribute to decreases in tissue pressure surrounding the pharynx (17, 45), which would further reduce its collapsibility. These mechanical effects can also account for breath-to-breath increases in passive Pcrit as lung volume falls gradually over an abrupt decrease in nasal pressure (37). Our Pcrit measurements were performed under passive (hypotonic) conditions, suggesting that observed changes in Pcrit reflect passive mechanical effects of lung inflation on upper airway structures rather than alterations in neuromuscular activity. Furthermore, observed changes in passive Pcrit of our sleeping subjects were comparable in magnitude to those demonstrated in subjects during complete neuromuscular blockade (43).
Our findings highlight the potential impact of obesity on sleep apnea and postural changes on upper airway patency and sleep apnea susceptibility (15). They suggest that obesity, which lowers EELV substantially, can increase sleep apnea susceptibility markedly by increasing pharyngeal collapsibility (19). This effect might be most marked when obese subjects sleep in the supine position, when FRC is the lowest, rather than in lateral decubitus or semirecumbent positions (47). In fact, this postural effect was associated with an ∼30% reduction in FRC (supine compared with upright) in our lean subjects (0.9 liter). A decrease of this magnitude would be associated with an ∼1.8 cmH2O increase in critical pressure during sleep, leading us to speculate that changes in lung volume may contribute to postural influences on upper airway patency during sleep. We can also estimate effects of lung volume on pharyngeal collapsibility in morbidly obese subjects (e.g., BMI ≥40 kg/m2). In this group, reductions in FRC of ∼0.8 liter (25) would produce elevations in passive Pcrit of ∼1.6 cmH2O (Fig. 4B) and increase sleep apnea susceptibility accordingly (16, 19, 36). These findings imply that reductions in EELV in obesity, especially in central adiposity, can play a major role in sleep apnea pathogenesis by increasing upper airway collapsibility and compromising pharyngeal patency during sleep. The effects of lung volume on upper airway collapsibility also account for recent findings that upper airway collapsibility and sleep apnea improve when lung volume is elevated experimentally (11, 22).
There are several advantages in the present study that extend our understanding of lung volume's effect on upper airway control. First, we utilized the method of Younes et al. (48) to offset changes in lung volume during nasal pressure manipulations and controlled lung volume experimentally. This approach eliminated Pcrit confounding by concomitant alterations in lung volume. Second, the effects of lung volume on Pcrit were assessed during sleep (6) rather than wakefulness (40), suggesting that changes in lung volume are implicated in the pathogenesis of sleep apnea. Third, we quantified the extent to which changes in lung volume confound conventional Pcrit measurements over a wide range of Pcrit (∼10 cmH2O). Fourth, the effects of lung volume on passive Pcrit were quantified under isovolumetric conditions. Pcrit responses to changes in absolute lung volume were quantified in an effort to predict the potential impact of lung volume (11, 12, 22), posture (7, 20, 21), and obesity (19) on sleep apnea susceptibility.
This study has several limitations. First, we cannot exclude neuromuscular effects of changes in lung volume or benzodiazepines, which could potentially influence the sensitivity of the Pcrit response to changes in lung volume (16). We utilized a hypnotic agent to enhance sleep continuity, which may have elevated passive Pcrit above baseline natural sleeping conditions. Nevertheless, passive Pcrit was measured under hypotonic conditions, which presumably minimized neuromuscular influences on the response. Moreover, prior studies have demonstrated that measurements of passive Pcrit are comparable in benzodiazepine-induced and natural sleep (4, 5, 30). Second, we acknowledge that absolute measurements of lung volume may not fully account for reductions in lung volume that may occur in the supine position at sleep onset. Nonetheless, we did not detect any significant reductions in lung volume in our subjects. Third, although Pcrit responses to changes in lung volume did not differ significantly by sex, we recognize that our limited sample size would preclude detecting modest differences between men and women. Nonetheless, we utilized rigorous methods that allowed us to discern significant elevations in both FRC (0.75 liter) and Pcrit (4.9 cmH2O) in normal men compared with age- and weight-matched normal women. Fourth, positive extrathoracic pressure can increase pressure around the neck; an increase in pharyngeal surrounding pressure rather than a decrease in EELV could confound our assessment of Pcrit responses to reductions in lung volume. This effect, which we observed initially in three men, was minimized by preventing positive pressure exposure to the base of the neck. Finally, the nasal and extrathoracic pressures could not be manipulated simultaneously to achieve the targeted lung volume during the “isovolume” measurements. Nevertheless, we were able to quantify and account for changes in lung volume following asynchronous alterations in nasal and extrathoracic pressures (see methods).
In summary, we have demonstrated methods for quantifying effects of EELV on passive Pcrit under isovolumetric conditions. We speculate that reductions in lung volume can lead to marked increases in passive Pcrit and might account for increased sleep apnea susceptibility in obese compared with lean individuals. Modeling the effects of changes in absolute lung volume on passive Pcrit before and after weight loss could help determine whether obesity increases airway collapsibility by decreasing lung volume, increasing surrounding tissue pressures, or both (43). Further work relating changes in upper airway collapsibility to absolute measures of lung volume will be important to dissect the contribution of obesity, lung disease, adipose distribution, sex, age, and menopausal status on intermediate physiological measures of sleep apnea susceptibility.
ACKNOWLEDGMENTS
We appreciate the support of Dano Carbone RRT, BS (Portalung Inc., Lakewood, CO) for providing a negative pressure ventilator (Portalung), Edward Polites at Phillips Respironics for providing a negative pressure pump (NEV-100), and Dr. Adam Benjafield and Dr. Glenn Richards at ResMed Ltd. for building a customized device for manipulating nasal pressures. We also thank Gary Paxton (Johns Hopkins University) for help with pulmonary function testing.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL50381, HL37379, and HL077137.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. American Thoracic Society Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med 152: 1107–1136, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Aronson RM, Alex CG, Onal E, Lopata M. Changes in end-expiratory lung volume during sleep in patients with occlusive apnea. J Appl Physiol 63: 1642–1647, 1987 [DOI] [PubMed] [Google Scholar]
- 3. Atlas Task Force EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 15: 174–184, 1992 [PubMed] [Google Scholar]
- 4. Ayuse T, Hoshino Y, Inazawa T, Oi K, Schneider H, Schwartz AR. A pilot study of quantitative assessment of mandible advancement using pressure-flow relationship during midazolam sedation. J Oral Rehabil 33: 813–819, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Ayuse T, Hoshino Y, Kurata S, Ayuse T, Schneider H, Kirkness JP, Patil SP, Schwartz AR, Oi K. The effect of gender on compensatory neuromuscular response to upper airway obstruction in normal subjects under midazolam general anesthesia. Anesth Analg 109: 1209–1218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Begle RL, Badr S, Skatrud JB, Dempsey JA. Effect of lung inflation on pulmonary resistance during NREM sleep. Am Rev Respir Dis 141: 854–860, 1990 [DOI] [PubMed] [Google Scholar]
- 7. Boudewyns A, Punjabi N, Van de Heyning PH, De Backer WA, O'Donnell CP, Schneider H, Smith PL, Schwartz AR. Abbreviated method for assessing upper airway function in obstructive sleep apnea. Chest 118: 1031–1041, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Chadha TS, Watson H, Birch S, Jenouri GA, Schneider AW, Cohn MA, Sackner MA. Validation of respiratory inductive plethysmography using different calibration procedures. Am Rev Respir Dis 125: 644–649, 1982 [DOI] [PubMed] [Google Scholar]
- 9. Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis 143: 1300–1303, 1991 [DOI] [PubMed] [Google Scholar]
- 10. Gold AR, Schwartz AR. The pharyngeal critical pressure. The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest 110: 1077–1088, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Heinzer RC, Stanchina ML, Malhotra A, Fogel RB, Patel SR, Jordan AS, Schory K, White DP. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med 172: 114–117, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heinzer RC, Stanchina ML, Malhotra A, Jordan AS, Patel SR, Lo YL, Wellman A, Schory K, Dover L, White DP. Effect of increased lung volume on sleep disordered breathing in sleep apnoea patients. Thorax 61: 435–439, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoffstein V, Zamel N, Phillipson EA. Lung volume dependence of pharyngeal cross-sectional area in patients with obstructive sleep apnea. Am Rev Respir Dis 130: 175–178, 1984 [DOI] [PubMed] [Google Scholar]
- 14. Isono S. Contribution of obesity and craniofacial abnormalities to pharyngeal collapsibility in patients with obstructive sleep apnea. Sleep Biol Rhythms 2: 17–21, 2004 [Google Scholar]
- 15. Isono S. Obstructive sleep apnea of obese adults: pathophysiology and perioperative airway management. Anesthesiology 110: 908–921, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Jordan AS, White DP, Lo YL, Wellman A, Eckert DJ, Yim-Yeh S, Eikermann M, Smith SA, Stevenson KE, Malhotra A. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep 32: 361–368, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kairaitis K, Byth K, Parikh R, Stavrinou R, Wheatley JR, Amis TC. Tracheal traction effects on upper airway patency in rabbits: the role of tissue pressure. Sleep 30: 179–186, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Kairaitis K, Parikh R, Stavrinou R, Garlick S, Kirkness JP, Wheatley JR, Amis TC. Upper airway extraluminal tissue pressure fluctuations during breathing in rabbits. J Appl Physiol 95: 1560–1566, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Kirkness JP, Schwartz AR, Schneider H, Punjabi NM, Maly JJ, Laffan AM, McGinley BM, Magnuson T, Schweitzer M, Smith PL, Patil SP. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol 104: 1618–1624, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McEvoy RD, Sharp DJ, Thornton AT. The effects of posture on obstructive sleep apnea. Am Rev Respir Dis 133: 662–666, 1986 [DOI] [PubMed] [Google Scholar]
- 21. Oksenberg A, Khamaysi I, Silverberg DS, Tarasiuk A. Association of body position with severity of apneic events in patients with severe nonpositional obstructive sleep apnea. Chest 118: 1018–1024, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Owens RL, Malhotra A, Eckert DJ, White DP, Jordan AS. The influence of end-expiratory lung volume on measurements of pharyngeal collapsibility. J Appl Physiol 108: 445–451, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patil SP, Punjabi NM, Schneider H, O'Donnell CP, Smith PL, Schwartz AR. A simplified method for measuring critical pressures during sleep in the clinical setting. Am J Respir Crit Care Med 170: 86–93, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol 102: 547–556, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Pelosi P, Croci M, Ravagnan I, Tredici S, Pedoto A, Lissoni A, Gattinoni L. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg 87: 654–660, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Punjabi NM, O'hearn DJ, Neubauer DN, Nieto FJ, Schwartz AR, Smith PL, Bandeen-Roche K. Modeling hypersomnolence in sleep-disordered breathing. A novel approach using survival analysis. Am J Respir Crit Care Med 159: 1703–1709, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Punjabi NM, Shade D, Wise RA. Correction of single-breath helium lung volumes in patients with airflow obstruction. Chest 114: 907–918, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K. Effects of obesity on respiratory function. Am Rev Respir Dis 128: 501–506, 1983 [DOI] [PubMed] [Google Scholar]
- 29. Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington, DC: Public Health Service, U.S. Government Printing Office, 1968 [Google Scholar]
- 30. Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 44: 931–938, 1978 [DOI] [PubMed] [Google Scholar]
- 31. Rowley JA, Permutt S, Willey S, Smith PL, Schwartz AR. Effect of tracheal and tongue displacement on upper airway airflow dynamics. J Appl Physiol 80: 2171–2178, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Sackner MA, Watson H, Belsito AS, Feinerman D, Suarez M, Gonzalez G, Bizousky F, Krieger B. Calibration of respiratory inductive plethysmograph during natural breathing. J Appl Physiol 66: 410–420, 1989 [DOI] [PubMed] [Google Scholar]
- 33. Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med 152: 1673–1689, 1995 [DOI] [PubMed] [Google Scholar]
- 34. Schwartz AR, Gold AR, Schubert N, Stryzak A, Wise RA, Permutt S, Smith PL. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis 144: 494–498, 1991 [DOI] [PubMed] [Google Scholar]
- 35. Schwartz AR, O'Donnell CP, Baron J, Schubert N, Alam D, Samadi SD, Smith PL. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am J Respir Crit Care Med 157: 1051–1057, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc 5: 185–192, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schwartz AR, Rowley JA, Thut DC, Permutt S, Smith PL. Structural basis for alterations in upper airway collapsibility. Sleep 19: S184–S188, 1996 [DOI] [PubMed] [Google Scholar]
- 38. Schwartz AR, Schubert N, Rothman W, Godley F, Marsh B, Eisele D, Nadeau J, Permutt L, Gleadhill I, Smith PL. Effect of uvulopalatopharyngoplasty on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis 145: 527–532, 1992 [DOI] [PubMed] [Google Scholar]
- 39. Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol 64: 535–542, 1988 [DOI] [PubMed] [Google Scholar]
- 40. Series F, Marc I. Influence of lung volume dependence of upper airway resistance during continuous negative airway pressure. J Appl Physiol 77: 840–844, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Sharp JT, Henry JP, Sweany SK, Meadows WR, Pietras RJ. Effects of mass loading the respiratory system in man. J Appl Physiol 19: 959–966, 1964 [DOI] [PubMed] [Google Scholar]
- 42. Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol 64: 789–795, 1988 [DOI] [PubMed] [Google Scholar]
- 43. Tagaito Y, Isono S, Remmers JE, Tanaka A, Nishino T. Lung volume and collapsibility of the passive pharynx in patients with sleep-disordered breathing. J Appl Physiol 103: 1379–1385, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Thut DC, Schwartz AR, Roach D, Wise RA, Permutt S, Smith PL. Tracheal and neck position influence upper airway airflow dynamics by altering airway length. J Appl Physiol 75: 2084–2090, 1993 [DOI] [PubMed] [Google Scholar]
- 45. Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol 65: 2124–2131, 1988 [DOI] [PubMed] [Google Scholar]
- 46. Van de Graaff WB. Thoracic traction on the trachea: mechanisms and magnitude. J Appl Physiol 70: 1328–1336, 1991 [DOI] [PubMed] [Google Scholar]
- 47. Watson RA, Pride NB. Postural changes in lung volumes and respiratory resistance in subjects with obesity. J Appl Physiol 98: 512–517, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Younes M, Sanii R, Patrick W, Marantz S, Webster K. An approach to the study of upper airway function in humans. J Appl Physiol 77: 1383–1394, 1994 [DOI] [PubMed] [Google Scholar]
- 49. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328: 1230–1235, 1993 [DOI] [PubMed] [Google Scholar]