Abstract
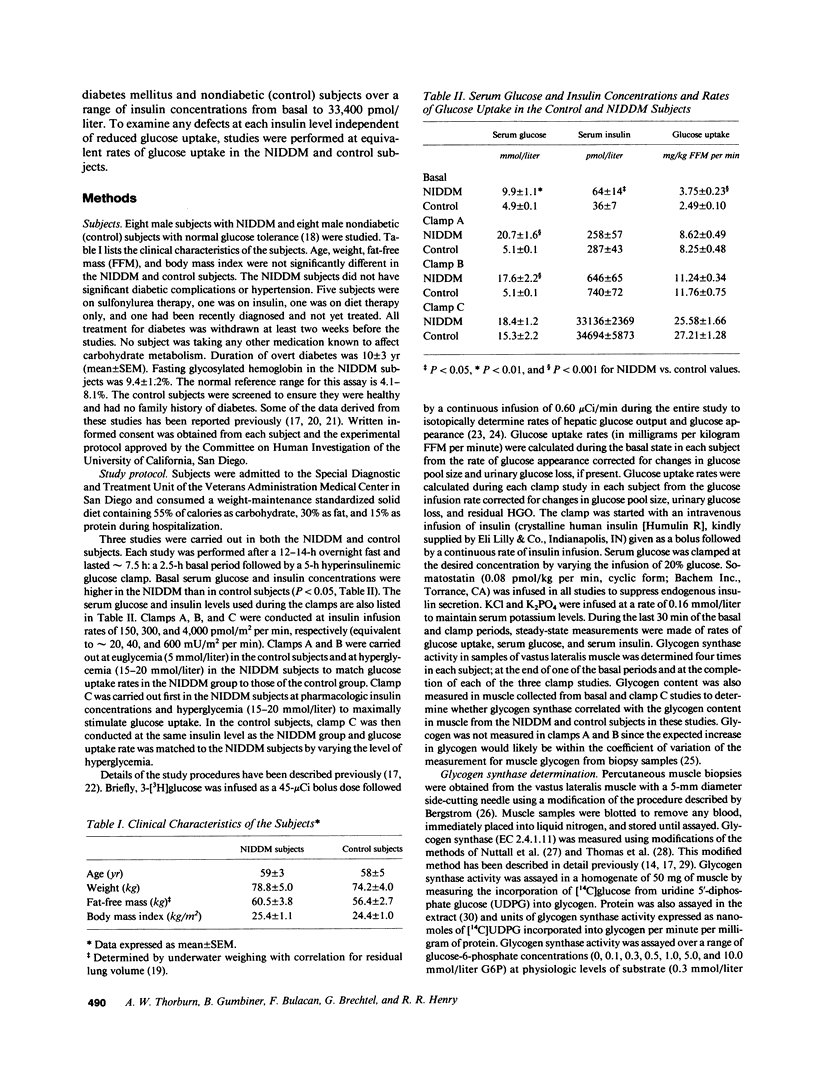
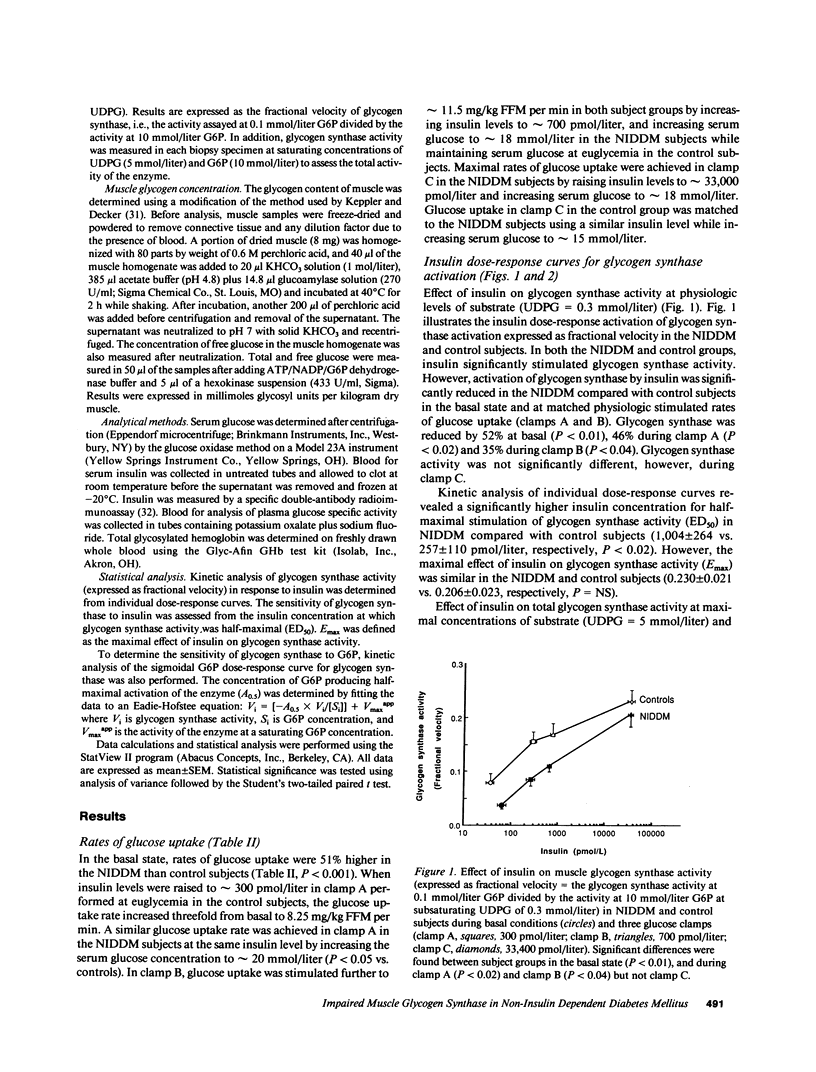
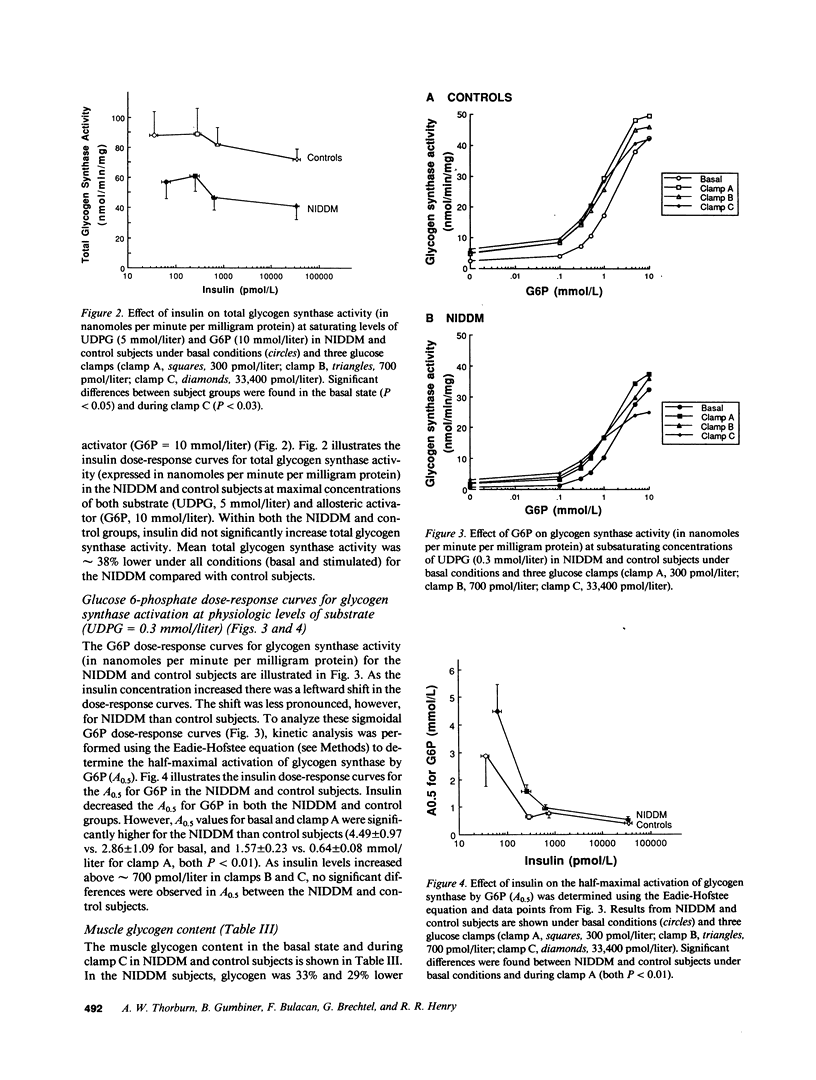
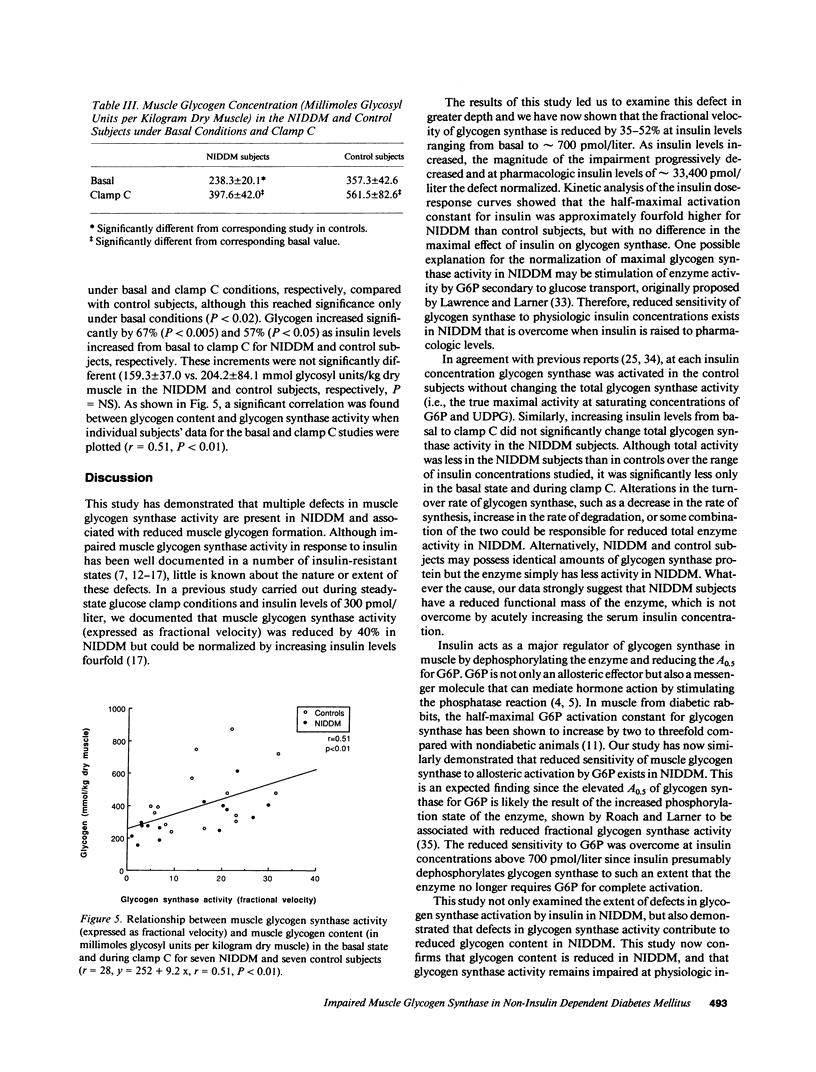
To define the mechanisms of impaired muscle glycogen synthase and reduced glycogen formation in non-insulin dependent diabetes mellitus (NIDDM), glycogen synthase activity was kinetically analyzed during the basal state and three glucose clamp studies (insulin approximately equal to 300, 700, and 33,400 pmol/liter) in eight matched nonobese NIDDM and eight control subjects. Muscle glycogen content was measured in the basal state and following clamps at insulin levels of 33,400 pmol/liter. NIDDM subjects had glucose uptake matched to controls in each clamp by raising serum glucose to 15-20 mmol/liter. The insulin concentration required to half-maximally activate glycogen synthase (ED50) was approximately fourfold greater for NIDDM than control subjects (1,004 +/- 264 vs. 257 +/- 110 pmol/liter, P less than 0.02) but the maximal insulin effect was similar. Total glycogen synthase activity was reduced approximately 38% and glycogen content was approximately 30% lower in NIDDM. A positive correlation was present between glycogen content and glycogen synthase activity (r = 0.51, P less than 0.01). In summary, defects in muscle glycogen synthase activity and reduced glycogen content are present in NIDDM. NIDDM subjects also have less total glycogen synthase activity consistent with reduced functional mass of the enzyme. These findings and the correlation between glycogen synthase activity and glycogen content support the theory that multiple defects in glycogen synthase activity combine to cause reduced glycogen formation in NIDDM.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG W. D., ENGSTROM A., PAUL K. G. Porphyrins and bone. Scand J Clin Lab Invest. 1962;14 (Suppl 64):7–11. [PubMed] [Google Scholar]
- Bak J. F., Schmitz O., Sørensen N. S., Pedersen O. Postreceptor effects of sulfonylurea on skeletal muscle glycogen synthase activity in type II diabetic patients. Diabetes. 1989 Nov;38(11):1343–1350. [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Stone K., Mott D. Correlation between muscle glycogen synthase activity and in vivo insulin action in man. J Clin Invest. 1984 Apr;73(4):1185–1190. doi: 10.1172/JCI111304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson J. L., Dietz M. R., Shikama H., Wootten M., Exton J. H. Insulin regulation of skeletal muscle glycogen metabolism. Am J Physiol. 1980 Jul;239(1):E69–E74. doi: 10.1152/ajpendo.1980.239.1.E69. [DOI] [PubMed] [Google Scholar]
- Chiasson J. L., Liljenquist J. E., Lacy W. W., Jennings A. S., Cherrington A. D. Gluconeogenesis: methodological approaches in vivo. Fed Proc. 1977 Feb;36(2):229–235. [PubMed] [Google Scholar]
- DANFORTH W. H. GLYCOGEN SYNTHETASE ACTIVITY IN SKELETAL MUSCLE. INTERCONVERSION OF TWO FORMS AND CONTROL OF GLYCOGEN SYNTHESIS. J Biol Chem. 1965 Feb;240:588–593. [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Freymond D., Bogardus C., Okubo M., Stone K., Mott D. Impaired insulin-stimulated muscle glycogen synthase activation in vivo in man is related to low fasting glycogen synthase phosphatase activity. J Clin Invest. 1988 Nov;82(5):1503–1509. doi: 10.1172/JCI113758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboe D. P., Nuttall F. Q. The role of ATP and glucose 6-phosphate in the regulation of glycogen synthetase D phosphatase. Biochem Biophys Res Commun. 1972 Aug 21;48(4):898–906. doi: 10.1016/0006-291x(72)90693-6. [DOI] [PubMed] [Google Scholar]
- Henry R. R., Gumbiner B., Flynn T., Thorburn A. W. Metabolic effects of hyperglycemia and hyperinsulinemia on fate of intracellular glucose in NIDDM. Diabetes. 1990 Feb;39(2):149–156. doi: 10.2337/diab.39.2.149. [DOI] [PubMed] [Google Scholar]
- Kaslow H. R., Eichner R. D. Fasting and diabetes alter adipose tissue glycogen synthase. Am J Physiol. 1984 Nov;247(5 Pt 1):E581–E584. doi: 10.1152/ajpendo.1984.247.5.E581. [DOI] [PubMed] [Google Scholar]
- Kida Y., Esposito-Del Puente A., Bogardus C., Mott D. M. Insulin resistance is associated with reduced fasting and insulin-stimulated glycogen synthase phosphatase activity in human skeletal muscle. J Clin Invest. 1990 Feb;85(2):476–481. doi: 10.1172/JCI114462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszynska Y. T., Home P. D. Liver and muscle insulin sensitivity, glycogen concentration and glycogen synthase activity in a rat model of non-insulin-dependent diabetes. Diabetologia. 1988 May;31(5):304–309. doi: 10.1007/BF00277412. [DOI] [PubMed] [Google Scholar]
- Kruszynska Y. T., Petranyi G., Alberti K. G. Decreased insulin sensitivity and muscle enzyme activity in elderly subjects. Eur J Clin Invest. 1988 Oct;18(5):493–498. doi: 10.1111/j.1365-2362.1988.tb01045.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larner J. Insulin-signaling mechanisms. Lessons from the old testament of glycogen metabolism and the new testament of molecular biology. Diabetes. 1988 Mar;37(3):262–275. doi: 10.2337/diab.37.3.262. [DOI] [PubMed] [Google Scholar]
- Lawrence J. C., Jr, Larner J. Activation of glycogen synthase in rat adipocytes by insulin and glucose involves increased glucose transport and phosphorylation. J Biol Chem. 1978 Apr 10;253(7):2104–2113. [PubMed] [Google Scholar]
- Mandarino L. J., Madar Z., Kolterman O. G., Bell J. M., Olefsky J. M. Adipocyte glycogen synthase and pyruvate dehydrogenase in obese and type II diabetic subjects. Am J Physiol. 1986 Oct;251(4 Pt 1):E489–E496. doi: 10.1152/ajpendo.1986.251.4.E489. [DOI] [PubMed] [Google Scholar]
- Mandarino L. J., Wright K. S., Verity L. S., Nichols J., Bell J. M., Kolterman O. G., Beck-Nielsen H. Effects of insulin infusion on human skeletal muscle pyruvate dehydrogenase, phosphofructokinase, and glycogen synthase. Evidence for their role in oxidative and nonoxidative glucose metabolism. J Clin Invest. 1987 Sep;80(3):655–663. doi: 10.1172/JCI113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall F. Q., Barbosa J., Gannon M. C. The glycogen synthase system in skeletal muscle of normal humans and patients with myotonic dystrophy: effect of glucose and insulin administration. Metabolism. 1974 Jun;23(6):561–568. doi: 10.1016/0026-0495(74)90084-5. [DOI] [PubMed] [Google Scholar]
- Okubo M., Bogardus C., Lillioja S., Mott D. M. Adenosine 3',5'-monophosphate-dependent protein kinase activity decreases in human muscle after insulin infusion. J Clin Endocrinol Metab. 1989 Oct;69(4):798–803. doi: 10.1210/jcem-69-4-798. [DOI] [PubMed] [Google Scholar]
- Okubo M., Bogardus C., Lillioja S., Mott D. M. Glucose-6-phosphate stimulation of human muscle glycogen synthase phosphatase. Metabolism. 1988 Dec;37(12):1171–1176. doi: 10.1016/0026-0495(88)90196-5. [DOI] [PubMed] [Google Scholar]
- Oron Y., Larner J. Insulin action in intact mouse diaphragm. I. Activation of glycogen synthase through stimulation of sugar transport and phosphorylation. Mol Cell Biochem. 1980 Nov 20;32(3):153–160. [PubMed] [Google Scholar]
- Roach P. J., Larner J. Rabbit skeletal muscle glycogen synthase. II. Enzyme phosphorylation state and effector concentrations as interacting control parameters. J Biol Chem. 1976 Apr 10;251(7):1920–1925. [PubMed] [Google Scholar]
- Rossetti L., Lauglin M. R. Correction of chronic hyperglycemia with vanadate, but not with phlorizin, normalizes in vivo glycogen repletion and in vitro glycogen synthase activity in diabetic skeletal muscle. J Clin Invest. 1989 Sep;84(3):892–899. doi: 10.1172/JCI114250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Sheorain V. S., Khatra B. S., Soderling T. R. Hormonal regulation of glycogen synthase phosphorylation in rabbit skeletal muscle. J Biol Chem. 1982 Apr 10;257(7):3462–3470. [PubMed] [Google Scholar]
- Shulman G. I., Rothman D. L., Jue T., Stein P., DeFronzo R. A., Shulman R. G. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990 Jan 25;322(4):223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Thorburn A. W., Gumbiner B., Brechtel G., Henry R. R. Effect of hyperinsulinemia and hyperglycemia on intracellular glucose and fat metabolism in healthy subjects. Diabetes. 1990 Jan;39(1):22–30. doi: 10.2337/diacare.39.1.22. [DOI] [PubMed] [Google Scholar]
- Thorburn A. W., Gumbiner B., Bulacan F., Wallace P., Henry R. R. Intracellular glucose oxidation and glycogen synthase activity are reduced in non-insulin-dependent (type II) diabetes independent of impaired glucose uptake. J Clin Invest. 1990 Feb;85(2):522–529. doi: 10.1172/JCI114468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K. S., Beck-Nielsen H., Kolterman O. G., Mandarino L. J. Decreased activation of skeletal muscle glycogen synthase by mixed-meal ingestion in NIDDM. Diabetes. 1988 Apr;37(4):436–440. doi: 10.2337/diab.37.4.436. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Mott D., Young A. A., Stone K., Bogardus C. Regulation of glycogen synthase and phosphorylase activities by glucose and insulin in human skeletal muscle. J Clin Invest. 1987 Jul;80(1):95–100. doi: 10.1172/JCI113069. [DOI] [PMC free article] [PubMed] [Google Scholar]



