Abstract
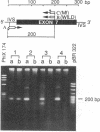
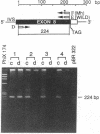
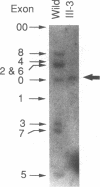
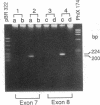
Generalized resistance to thyroid hormone (GRTH) is a syndrome characterized by impaired tissue responsiveness to thyroid hormone. Two distinct point mutations in the hormone binding domain of the thyroid hormone receptor (TR) beta have recently been identified in two unrelated families with GRTH. One, Mf, involves a replacement of the normal glycine-345 for arginine in exon 7 and another, Mh, replaces the normal proline-453 for histidine in exon 8. To probe for the presence of the Mf and Mh defect in 19 unrelated families with GRTH, we applied separate polymerase chain reactions using allele-specific oligonucleotide primers containing the normal and each of the two mutant nucleotides at the 3'-position. A total of 24 affected subjects and 13 normal family members were studied. The mode of inheritance was dominant in 13 families, was unknown in 5 families, and was clearly recessive in 1 family in which only the consanguineous subjects were affected. Primers containing the substitutions specific for Mf and Mh amplified exons 7 and 8, respectively, only in affected members of each of the two index families. Primers containing the normal sequences amplified exons 7 and 8 of the TR beta gene in all subjects except affected members of one family. In this family with recessively inherited GRTH, neither exon could be amplified using any combinations of primers and DNA blot revealed absence of all coding exons. These results indicate a major deletion of the TR beta gene, including both DNA and hormone binding domains. Since heterozygous members of this family are not affected, the presence of a single normal allele is sufficient for normal function of the TR beta. These data also support the hypothesis that in the dominant mode of GRTH inheritance the presence of an abnormal TR beta interferes with the function of the normal TR beta. Distinct mutations are probably responsible for GRTH in unrelated families.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonarakis S. E., Kazazian H. H., Jr The molecular basis of hemophilia A in man. Trends Genet. 1988 Aug;4(8):233–237. doi: 10.1016/0168-9525(88)90156-4. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrook D., Pfahl M. A novel thyroid hormone receptor encoded by a cDNA clone from a human testis library. Science. 1987 Nov 6;238(4828):788–791. doi: 10.1126/science.3672126. [DOI] [PubMed] [Google Scholar]
- Bernal J., Refetoff S., DeGroot L. J. Abnormalities of triiodothyronine binding to lymphocyte and fibroblast nuclei from a patient with peripheral tissue resistance to thyroid hormone action. J Clin Endocrinol Metab. 1978 Dec;47(6):1266–1272. doi: 10.1210/jcem-47-6-1266. [DOI] [PubMed] [Google Scholar]
- Brent G. A., Harney J. W., Chen Y., Warne R. L., Moore D. D., Larsen P. R. Mutations of the rat growth hormone promoter which increase and decrease response to thyroid hormone define a consensus thyroid hormone response element. Mol Endocrinol. 1989 Dec;3(12):1996–2004. doi: 10.1210/mend-3-12-1996. [DOI] [PubMed] [Google Scholar]
- Ceccarelli P., Refetoff S., Murata Y. Resistance to thyroid hormone diagnosed by the reduced response of fibroblasts to the triiodothyronine-induced suppression of fibronectin synthesis. J Clin Endocrinol Metab. 1987 Aug;65(2):242–246. doi: 10.1210/jcem-65-2-242. [DOI] [PubMed] [Google Scholar]
- Chait A., Kanter R., Green W., Kenny M. Defective thyroid hormone action in fibroblasts cultured from subjects with the syndrome of resistance to thyroid hormones. J Clin Endocrinol Metab. 1982 Apr;54(4):767–772. doi: 10.1210/jcem-54-4-767. [DOI] [PubMed] [Google Scholar]
- Damm K., Thompson C. C., Evans R. M. Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature. 1989 Jun 22;339(6226):593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- Dayton A. I., Selden J. R., Laws G., Dorney D. J., Finan J., Tripputi P., Emanuel B. S., Rovera G., Nowell P. C., Croce C. M. A human c-erbA oncogene homologue is closely proximal to the chromosome 17 breakpoint in acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4495–4499. doi: 10.1073/pnas.81.14.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eil C., Fein H. G., Smith T. J., Furlanetto R. W., Bourgeois M., Stelling M. W., Weintraub B. D. Nuclear binding of [125I]triiodothyronine in dispersed cultured skin fibroblasts from patients with resistance to thyroid hormone. J Clin Endocrinol Metab. 1982 Sep;55(3):502–510. doi: 10.1210/jcem-55-3-502. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass C. K., Lipkin S. M., Devary O. V., Rosenfeld M. G. Positive and negative regulation of gene transcription by a retinoic acid-thyroid hormone receptor heterodimer. Cell. 1989 Nov 17;59(4):697–708. doi: 10.1016/0092-8674(89)90016-0. [DOI] [PubMed] [Google Scholar]
- Gómez Sáez J. M., Fernández Castañer M., Navarro M. A., Bonnin M. R., Soler Ramón J., Roca A. Resistencia parcial a las hormonas tiroideas con bocio y eutiroidismo. Med Clin (Barc) 1981 May 10;76(9):412–416. [PubMed] [Google Scholar]
- Hodin R. A., Lazar M. A., Chin W. W. Differential and tissue-specific regulation of the multiple rat c-erbA messenger RNA species by thyroid hormone. J Clin Invest. 1990 Jan;85(1):101–105. doi: 10.1172/JCI114398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodin R. A., Lazar M. A., Wintman B. I., Darling D. S., Koenig R. J., Larsen P. R., Moore D. D., Chin W. W. Identification of a thyroid hormone receptor that is pituitary-specific. Science. 1989 Apr 7;244(4900):76–79. doi: 10.1126/science.2539642. [DOI] [PubMed] [Google Scholar]
- Hughes I. A., Ichikawa K., Degroot L. J., John R., Jones M. K., Hall R., Scanlon M. F. Non-adenomatous inappropriate TSH hypersecretion and euthyroidism requires no treatment. Clin Endocrinol (Oxf) 1987 Oct;27(4):475–483. doi: 10.1111/j.1365-2265.1987.tb01176.x. [DOI] [PubMed] [Google Scholar]
- Ichikawa K., Hughes I. A., Horwitz A. L., DeGroot L. J. Characterization of nuclear thyroid hormone receptors of cultured skin fibroblasts from patients with resistance to thyroid hormone. Metabolism. 1987 Apr;36(4):392–399. doi: 10.1016/0026-0495(87)90214-9. [DOI] [PubMed] [Google Scholar]
- Kaplowitz P. B., D'Ercole A. J., Utiger R. D. Peripheral resistance to thyroid hormone in an infant. J Clin Endocrinol Metab. 1981 Nov;53(5):958–963. doi: 10.1210/jcem-53-5-958. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Warne R. L., Brent G. A., Harney J. W., Larsen P. R., Moore D. D. Isolation of a cDNA clone encoding a biologically active thyroid hormone receptor. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5031–5035. doi: 10.1073/pnas.85.14.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberg B. A., Liewendahl K. Thyroid hormone resistance. Ann Clin Res. 1980 Oct;12(5):243–253. [PubMed] [Google Scholar]
- Magner J. A., Petrick P., Menezes-Ferreira M. M., Stelling M., Weintraub B. D. Familial generalized resistance to thyroid hormones: report of three kindreds and correlation of patterns of affected tissues with the binding of [125I] triiodothyronine to fibroblast nuclei. J Endocrinol Invest. 1986 Dec;9(6):459–470. doi: 10.1007/BF03346968. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi T., Tennyson G. E., Nikodem V. M. Alternative splicing generates messages encoding rat c-erbA proteins that do not bind thyroid hormone. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5804–5808. doi: 10.1073/pnas.85.16.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y., Refetoff S., Flink I. L., Charbonneau M., Murata Y., Seo H., Morkin E., Dussault J. H. Detection of the thyroxine-binding globulin (TBG) gene in six unrelated families with complete TBG deficiency. J Clin Endocrinol Metab. 1988 Oct;67(4):727–733. doi: 10.1210/jcem-67-4-727. [DOI] [PubMed] [Google Scholar]
- Murata Y., Refetoff S., Horwitz A. L., Smith T. J. Hormonal regulation of glycosaminoglycan accumulation in fibroblasts from patients with resistance to thyroid hormone. J Clin Endocrinol Metab. 1983 Dec;57(6):1233–1239. doi: 10.1210/jcem-57-6-1233. [DOI] [PubMed] [Google Scholar]
- Nakai A., Sakurai A., Bell G. I., DeGroot L. J. Characterization of a third human thyroid hormone receptor coexpressed with other thyroid hormone receptors in several tissues. Mol Endocrinol. 1988 Nov;2(11):1087–1092. doi: 10.1210/mend-2-11-1087. [DOI] [PubMed] [Google Scholar]
- Newton C. R., Graham A., Heptinstall L. E., Powell S. J., Summers C., Kalsheker N., Smith J. C., Markham A. F. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS). Nucleic Acids Res. 1989 Apr 11;17(7):2503–2516. doi: 10.1093/nar/17.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W. C., Liepnieks J. J., McKusick V. A., Benson M. D. Direct sequencing of the gene for Maryland/German familial amyloidotic polyneuropathy type II and genotyping by allele-specific enzymatic amplification. Genomics. 1989 Oct;5(3):535–540. doi: 10.1016/0888-7543(89)90020-7. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Coulombe P., Schwartz H. L., Gutfeld N. W. Nonlinear (amplified) relationship between nuclear occupancy by triiodothyronine and the appearance rate of hepatic alpha-glycerophosphate dehydrogenase and malic enzyme in the rat. J Clin Invest. 1978 Apr;61(4):987–997. doi: 10.1172/JCI109024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliara A. S., Caplan R. H., Gundersen C. B., Wickus G. G., Elston A. C., 3rd Peripheral resistance to thyroid hormone in a family: heterogeneity of clinical presentation. J Pediatr. 1983 Aug;103(2):228–232. doi: 10.1016/s0022-3476(83)80350-3. [DOI] [PubMed] [Google Scholar]
- Refetoff S., DeGroot L. J., Benard B., DeWind L. T. Studies of a sibship with apparent hereditary resistance to the intracellular action of thyroid hormone. Metabolism. 1972 Aug;21(8):723–756. doi: 10.1016/0026-0495(72)90121-7. [DOI] [PubMed] [Google Scholar]
- Refetoff S., DeWind L. T., DeGroot L. J. Familial syndrome combining deaf-mutism, stuppled epiphyses, goiter and abnormally high PBI: possible target organ refractoriness to thyroid hormone. J Clin Endocrinol Metab. 1967 Feb;27(2):279–294. doi: 10.1210/jcem-27-2-279. [DOI] [PubMed] [Google Scholar]
- Refetoff S., Degroot L. J., Barsano C. P. Defective thyroid hormone feedback regulation in the syndrome of peripheral resistance to thyroid hormone. J Clin Endocrinol Metab. 1980 Jul;51(1):41–45. doi: 10.1210/jcem-51-1-41. [DOI] [PubMed] [Google Scholar]
- Refetoff S., Matalon R., Bigazzi M. Metabolism of L-thyroxine (T4) and L-triiodothyronine (T3) by human fibroblasts in tissue culture: evidence for cellular binding proteins and conversion of T4 to T3. Endocrinology. 1972 Oct;91(4):934–947. doi: 10.1210/endo-91-4-934. [DOI] [PubMed] [Google Scholar]
- Refetoff S., Salazar A., Smith T. J., Scherberg N. H. The consequences of inappropriate treatment because of failure to recognize the syndrome of pituitary and peripheral tissue resistance to thyroid hormone. Metabolism. 1983 Aug;32(8):822–834. doi: 10.1016/0026-0495(83)90114-2. [DOI] [PubMed] [Google Scholar]
- Refetoff S. The syndrome of generalized resistance to thyroid hormone (GRTH). Endocr Res. 1989;15(4):717–743. doi: 10.3109/07435808909036358. [DOI] [PubMed] [Google Scholar]
- Ridgway E. C., Kourides I. A., Chin W. W., Cooper D. S., Maloof F. Augmentation of pituitary thyrotrophin response to thyrotrophin releasing hormone during subphysiological tri-iodothyroinine therapy in hypothyroidism. Clin Endocrinol (Oxf) 1979 Apr;10(4):343–353. doi: 10.1111/j.1365-2265.1979.tb02089.x. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sakurai A., Nakai A., DeGroot L. J. Expression of three forms of thyroid hormone receptor in human tissues. Mol Endocrinol. 1989 Feb;3(2):392–399. doi: 10.1210/mend-3-2-392. [DOI] [PubMed] [Google Scholar]
- Sakurai A., Nakai A., DeGroot L. J. Structural analysis of human thyroid hormone receptor beta gene. Mol Cell Endocrinol. 1990 Jun 18;71(2):83–91. doi: 10.1016/0303-7207(90)90245-4. [DOI] [PubMed] [Google Scholar]
- Sakurai A., Takeda K., Ain K., Ceccarelli P., Nakai A., Seino S., Bell G. I., Refetoff S., DeGroot L. J. Generalized resistance to thyroid hormone associated with a mutation in the ligand-binding domain of the human thyroid hormone receptor beta. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8977–8981. doi: 10.1073/pnas.86.22.8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Sarne D. H., Refetoff S., Rosenfield R. L., Farriaux J. P. Sex hormone-binding globulin in the diagnosis of peripheral tissue resistance to thyroid hormone: the value of changes after short term triiodothyronine administration. J Clin Endocrinol Metab. 1988 Apr;66(4):740–746. doi: 10.1210/jcem-66-4-740. [DOI] [PubMed] [Google Scholar]
- Sarne D. H., Sobieszczyk S., Ain K. B., Refetoff S. Serum thyrotropin and prolactin in the syndrome of generalized resistance to thyroid hormone: responses to thyrotropin-releasing hormone stimulation and short term triiodothyronine suppression. J Clin Endocrinol Metab. 1990 May;70(5):1305–1311. doi: 10.1210/jcem-70-5-1305. [DOI] [PubMed] [Google Scholar]
- Schueler P. A., Schwartz H. L., Strait K. A., Mariash C. N., Oppenheimer J. H. Binding of 3,5,3'-triiodothyronine (T3) and its analogs to the in vitro translational products of c-erbA protooncogenes: differences in the affinity of the alpha- and beta-forms for the acetic acid analog and failure of the human testis and kidney alpha-2 products to bind T3. Mol Endocrinol. 1990 Feb;4(2):227–234. doi: 10.1210/mend-4-2-227. [DOI] [PubMed] [Google Scholar]
- Theophilus B. D., Latham T., Grabowski G. A., Smith F. I. Comparison of RNase A, a chemical cleavage and GC-clamped denaturing gradient gel electrophoresis for the detection of mutations in exon 9 of the human acid beta-glucosidase gene. Nucleic Acids Res. 1989 Oct 11;17(19):7707–7722. doi: 10.1093/nar/17.19.7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usala S. J., Bale A. E., Gesundheit N., Weinberger C., Lash R. W., Wondisford F. E., McBride O. W., Weintraub B. D. Tight linkage between the syndrome of generalized thyroid hormone resistance and the human c-erbA beta gene. Mol Endocrinol. 1988 Dec;2(12):1217–1220. doi: 10.1210/mend-2-12-1217. [DOI] [PubMed] [Google Scholar]
- Usala S. J., Tennyson G. E., Bale A. E., Lash R. W., Gesundheit N., Wondisford F. E., Accili D., Hauser P., Weintraub B. D. A base mutation of the C-erbA beta thyroid hormone receptor in a kindred with generalized thyroid hormone resistance. Molecular heterogeneity in two other kindreds. J Clin Invest. 1990 Jan;85(1):93–100. doi: 10.1172/JCI114438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Weiss R. E., Balzano S., Scherberg N. H., Refetoff S. Neonatal detection of generalized resistance to thyroid hormone. JAMA. 1990 Nov 7;264(17):2245–2250. [PubMed] [Google Scholar]
- Wortsman J., Premachandra B. N., Williams K., Burman K. D., Hay I. D., Davis P. J. Familial resistance to thyroid hormone associated with decreased transport across the plasma membrane. Ann Intern Med. 1983 Jun;98(6):904–909. doi: 10.7326/0003-4819-98-6-904. [DOI] [PubMed] [Google Scholar]