Abstract
Plasmodium falciparum, the causative agent of the most deadly form of human malaria, is unable to salvage pyrimidines and must rely on de novo biosynthesis for survival. Dihydroorotate dehydrogenase (DHODH) catalyzes the rate-limiting step in the pyrimidine biosynthetic pathway and represents a potential target for anti-malarial therapy. A high throughput screen and subsequent medicinal chemistry program identified a series of N-alkyl-5-(1H-benzimidazol-1-yl)thiophene-2-carboxamides with low nanomolar in vitro potency against DHODH from P. falciparum, P. vivax, and P. berghei. The compounds were selective for the parasite enzymes over human DHODH, and x-ray structural data on the analog Genz-667348, demonstrated that species selectivity could be attributed to amino acid differences in the inhibitor-binding site. Compounds from this series demonstrated in vitro potency against the 3D7 and Dd2 strains of P. falciparum, good tolerability and oral exposure in the mouse, and ED50 values in the 4-day murine P. berghei efficacy model of 13–21 mg/kg/day with oral twice-daily dosing. In particular, treatment with Genz-667348 at 100 mg/kg/day resulted in sterile cure. Two recent analogs of Genz-667348 are currently undergoing pilot toxicity testing to determine suitability as clinical development candidates.
Keywords: Enzyme Inhibitors, High Throughput Screening, Medicinal Chemistry, Parasite, Pyrimidine, Plasmodium, Dihydroorotate Dehydrogenase, Malaria
Introduction
Malaria is currently one of the world's most severe endemic diseases, and, despite the advent of combination therapy and the introduction of longer acting pharmaceuticals, the need for new targets and relevant anti-malarial agents remains acute. DHODH,3 the enzyme catalyzing the fourth and rate-limiting step in de novo pyrimidine biosynthesis, represents a potential biological target that could be exploited. Pyrimidines are required for the biosynthesis of DNA, RNA, glycoproteins, and phospholipids. Unlike the human host, P. falciparum lacks the ability to salvage pyrimidine bases and thus is entirely dependent on de novo biosynthesis (1, 2). DHODH catalyzes the oxidation of l-dihydroorotate to orotate via a coupled redox reaction with a bound flavin cofactor (3–6). DHODH is ubiquitous to most organisms and exists in two forms. The cytosolic Type 1 enzyme is present in Gram-positive bacteria and Saccharomyces cerevisiae and utilizes fumarate or NAD+ as an electron acceptor (4, 7–10). The membrane-bound Type 2 enzyme is present in eukaryotes and some Gram-negative bacteria (11–14) and utilizes quinones as electron acceptors (15). The eukaryotic enzyme, including the plasmodial form, is localized to the inner mitochondrial space and uses coenzyme Q (16–19). The crucial nature of the reaction catalyzed by PfDHODH is reflected in the fact that, during the intra-erythrocytic stages of P. falciparum development, the sole function of mitochondrial electron transport appears to be regeneration of coenzyme Q as a cofactor for DHODH (20).
Significant differences between the plasmodial and human enzymes are suggested by previous studies with A77 1726, the active metabolite of the immunomodulatory drug leflunomide. Differences in the sequence and alignment of residues in the region of the inhibitor-binding site have been demonstrated for the parasite and human enzymes complexed with A77 1726 and orotate (21, 22) and a recently reported triazolopyrimidine series of PfDHODH-selective inhibitors (23). Furthermore, A77 1726 has been shown to be a preferential inhibitor of the human enzyme with only weak activity against the parasite enzyme (24). These results support the likelihood of finding inhibitors specific for PfDHODH, and combined with the essential nature of this enzyme in the parasite, establish it as a potentially viable and relevant anti-malarial chemotherapeutic target. In fact, small molecule inhibitors of PfDHODH have previously been described, which demonstrate in vitro activity against cultured strains of P. falciparum (25, 26) as well as suppression of parasite growth in an animal model (27).
This report describes current lead compounds from an ongoing medicinal chemistry program that represent analogs of progenitor molecules previously identified as inhibitors of PfDHODH (25). The enzyme assays were expanded to include DHODH from P. berghei and P. vivax, and parasite viability assays were performed not only on P. falciparum but also on P. knowlesi as an initial step toward discovering compounds with potential pan-species activity. The correlation between inhibitory potency against the enzyme and toxicity toward the parasite was assessed. In vitro and in vivo drug absorption, distribution, metabolism, and excretion properties were evaluated, and compound efficacy was assessed in the P. berghei and P. falciparum NOD-scid mouse models.
EXPERIMENTAL PROCEDURES
DHODH Inhibition Assays
DHODH plasmid construction, protein expression and purification, and the initial high throughput screen and its results have been previously described (25). The DHODH activity assay monitored the reduction of 2,6-dichloroindophenol and was conducted in 50 μl of 100 mm HEPES (pH 8.0) containing 150 mm NaCl, 5% glycerol, 0.05% Triton X-100, 175 μm l-dihydroorotate, 18 μm decylubiquinone, and 95 μm 2,6-dichloroindophenol, arrayed in a 384-well format. The concentrations of enzymes used were as follows: P. falciparum, 12.5 nm, P. berghei, 25.5 nm, P. vivax, 19.5 nm, and human, 7 nm. Following a 20-min incubation at room temperature, the absorbance was measured at 600 nm (Envision, PerkinElmer Life Sciences). A sigmoidal dose-response curve was generated by plotting % inhibition as a function of the log of compound concentration (range: 1.5 nm to 30 μm), and an IC50 value representing the concentration at which inhibition was half-maximal was determined.
Expression and Purification of P. falciparum DHODH for Crystallography
Previously, the Phillips laboratory reported that deletion of a surface loop in PfDHODH containing amino acid residues 384–413 facilitated crystallization of the enzyme with the triazolopyrimidine class of inhibitors (23). This construct also contains an N-terminal deletion that removes the mitochondrial membrane-spanning domain as well as residues that are N-terminal to this region. However, the described construct, pET-pfDHODΔ384–413, did not routinely yield good quality co-crystals for inhibitors from other structural classes. The construct was redesigned to shorten the N-terminal tag by replacement of the thrombin site and T7 tag sequence with the TEV protease site. pET28b (Novagen) was digested with Nco1/BamH1 and ligated to the annealed oligomer pairs 1 and 2 containing the TEV protease site to generate pET28b-TEV: 1 (CATGGGCCATCACCATCACCATCACGCTGAGAATCTTTATTTTCAGGGCGCG) and 2 (GATCCGCGCCCTGAAAATAAAGATTCTCAGCGTGATGGTGATGGTGATGGCC). The DNA-encoding PfDHODHΔ384–413 was isolated by BamH1/Sal1 digestion of pET-pfDHODΔ384–413 (23) and ligated with the BamH1/Sal1 fragment of pET28b-TEV to generate the final expression construct pET28b-TEV-pfDHODΔ384–413. This construct yielded protein that could more consistently be crystallized with a wider range of inhibitors than pET-pfDHODΔ384–413. pET28b-TEV-pfDHODΔ384–413 was transformed into Escherichia coli BL21 phage-resistant cells (Novagen), and PfDHODHΔ384–413 was expressed and purified as previously described by using HisTrap HP column (Amersham Biosciences) affinity chromatography followed by gel filtration (23).
Crystallization and Data Collection of PfDHODHΔ384–413 Bound to Inhibitors
Preliminary crystallization conditions were found using the random crystallization screen Cryo suite (Nextal) and detergent screen kits (Hampton Research). Subsequently the conditions were refined by variation of pH, precipitant, detergent, and protein concentrations. Crystals of PfDHODHΔ384–413 were grown by vapor diffusion in a hanging drop at 20 °C. Co-crystals with Genz-667348 were obtained by mixing Reservoir solution A (0.16 m ammonium sulfate, 0.1 m sodium acetate, pH 4.4, 14–15% PEG4000, 25% glycerol, and 10 mm DTT) with an equal volume of PfDHODHΔ384–413 (20 mg/ml) pre-equilibrated with 0.6 mm Genz-667348 and 2 mm dihydroorotate.
Diffraction data were collected at 100 K on beamline 19ID at Advanced Photon Source using an ADSC Q315 detector. The crystal of PfDHODH-348 diffracted to 2.4 Å and has a space group of P64 with the cell dimension of a = b = 85.3, c = 138.7, with one molecule of PfDHODH in the asymmetric unit. Diffraction data were integrated, and intensities were scaled with the HKL2000 package (28).
Structure Determination and Refinement of PfDHODH Bound to Inhibitors
Crystallographic phases for PfDHODH inhibitors were solved by molecular replacement with Phaser (29) using the previously reported structure of PfDHODHΔ384–413 bound to DSM1 (PDB ID 3I65) (23), as a search model. Structures were rebuilt with COOT (30) and refined with REFMAC (31). The phases were improved with DM (32) (Table 1S). The final PfDHODH-348 structure contains residues Phe-161 through Leu-565, one molecule of FMN, orotate, Genz-667348, and 37 water molecules. The structure was refined to Rfac of 0.20 and Rfree of 0.23 (Table 1S). A Ramachandran plot generated with Molprobity (33) indicated that 97.3% of all protein residues are in the most favored regions with the remaining 2.7% in allowed regions. Water molecules were added if the density was stronger than 3.4 σ and removed if the density was weaker than 1 σ in the density map generated with ARP/warp (34).
Molecular Modeling
Structures were displayed using the graphics program PyMOL (56). The PfDHODH/Genz-667348 structure was superimposed with the PfDHODH-DSM1 (PDB 3I65), PfDHODH-A77 1726 (PDB 1TV5), and human DHODH bound to brequinar (HsDHODH-Bre, PDB 1D3G) structures by aligning the backbone atoms of full-length structures in DaliLite (35). Root mean square deviation values were calculated for the superimposed structures based on the Cα positions with DaliLite.
In Vitro P. falciparum Viability Assay
A SYBR green assay as previously described by Plouffe et al. (36) was modified for use in 384-well plates. Briefly, parasites were cultured in the presence of serial dilutions of test compounds in 50 μl of RPMI containing 4.16 mg/ml Albumax at a 2.5% hematocrit and an initial parasitemia of 0.3% in black Greiner GNF clear-bottom plates. Following a 72-h incubation at 37 °C under 93% N2, 4% CO2 and 3% O2, SYBR green was added to a dilution of 1:10,000, and plates were stored overnight (or until ready to be read) at −80 °C. Plates were centrifuged at 700 rpm prior to fluorescence measurement (EX 480 nm, EM 530 nm). In this assay, inhibition of parasite replication is reflected in a reduction in the fluorescence intensity of SYBR green bound to parasite DNA.
In Vitro P. knowlesi Viability Assay
Selected compounds were tested against P. knowlesi parasites cultured in Rhesus blood cells as a surrogate for P. vivax infections using the method of Kocken et al. (37). Briefly, P. knowlesi were cultured in 2% Rhesus macaque erythrocytes (New England Primate Research Center) in RPMI culture media supplemented with 10% Human O+ serum (Interstate Blood Bank). Schizont stage parasites were purified by flotation in 60% Percoll (GE Life Sciences) and allowed to reinvade to generate a synchronous population of ring stage parasites. Drug assays were performed by plating ring stage parasites at 0.5% parasitemia in triplicate, in RPMI containing 2.5 μg/ml hypoxanthine. Parasites were incubated for 24 h with serially diluted test compounds. After 24 h, thin smears were made to confirm that reinvasion had occurred and 0.5 μCi of 3H-labeled hypoxanthine were added to each well, and parasites were allowed to progress through S-phase to early schizonts. Cells were then harvested via glass filter plates, and 3H incorporation was measured by scintillation counter. Values were normalized to percentage of no drug controls, and IC50 values were generated.
In Vitro P. berghei Viability Assay
To investigate if compound potency against DHODH was similarly evident in rodent malaria parasites, selected compounds were assessed for in vitro toxicity against P. berghei ANKA using the in vitro drug luminescence (ITDL) assay (38). This assay is based on the quantitation of luciferase activity (luminescence) using the Luciferase Assay System Kit® (Promega), detected in blood samples containing transgenic blood stage parasites that express luciferase under the control of the schizont-specific (ama-1) promoter (transgenic parasite RMgm-32; http://www.pberghei.eu/index.php?rmgm=32). Briefly, ring-stage parasites (39, 40) were cultured for 24 h in serial dilutions of test compounds in 24 well plates. Following centrifugation and removal of supernatant, the cells were lysed by addition of cell culture lysis reagent, after which plates were shaken for 5 min. Luciferase assay substrate was added and luminescence was read for 10 s in a multi-plate reader (Wallac 1420 multilabel counter, PerkinElmer). Measurements were expressed in relative light units (RLU) and represent the average of triplicate samples at each drug dilution.
Mammalian Cytotoxicity
Compounds were tested at 10 dilutions against normal human renal proximal tubule cells (Clonetics #CC-2553) and dermal fibroblasts (NHDF; Clonetics #CC-2509). Cells were incubated with compound for 4 days, until becoming confluent. Viability was then measured using the Alamar Blue assay (TREK Diagnostic Systems), according to the manufacturer's instructions.
Erythrocyte Lysis
Compounds were tested at 10 dilutions against fresh human erythrocytes at 1% hematocrit in Dulbecco's PBS in V-bottomed plates incubated for 24 h at 37 °C. Following incubation, plates were centrifuged 5 min at 2,000 rpm and 50 μl of supernatant was transferred to a fresh flat-bottom plate. The amount of hemoglobin present was determined using the QuantChem Hemoglobin Assay Kit (BioAssay Systems #DIHB-250) according to the manufacturer's recommendations.
In Vitro Pharmaceutical Properties
Solubility was determined using a kinetic method. Briefly, compounds were dissolved in DMSO, diluted to 0.5% in phosphate-buffered saline pH 7.4 (PBS), allowed to equilibrate for 16–24 h, and filtered using a 1.2 μm filter. The concentration was calculated from measurement of absorbance at 254 nm using a 96 well plate UV spectrophotometer.
Passive permeability was measured using the parallel artificial membrane permeation assay (PAMPA). Compound was added to the donor well (pH 6.5 in PBS) which was separated from the acceptor well (pH 7.4 in PBS) by a phospholipid membrane in dodecane. After a 5 h incubation, concentrations were measured in donor and acceptor wells by monitoring UV absorbance from 250–490 nm in order to calculate permeability (41).
Metabolic stability was determined using rat, mouse, and human liver microsomes (BD Gentest) as well as intact hepatocytes (CellzDirect). Test compounds were incubated with microsomes at 0.5 mg protein/ml and NADPH as co-factor, or with hepatocytes at 106 cells/ml. Samples were withdrawn after 0, 5, 15, 30, and 45 min (microsomes) or 0, 15, 30, 60, and 120 min (hepatocytes) of incubation for liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis. Half-life was determined by plotting Ln (Peak Area ratio) versus time (min). The intrinsic clearance was calculated based on the well-stirred model (42) and expressed as ml/min/kg body weight.
Plasma protein binding was calculated by diluting a 25 mm stock solution in DMSO to 1 μm in plasma, dialyzing for 5 h against PBS (pH 7.4) at 37 °C and determining the concentration remaining in each compartment by LC-MS/MS analysis.
Cytochrome P450 (CYP) inhibition was determined for human P450 isozymes 1A2, 2C9, 2C19, 2D6, and 3A4 by measuring inhibition of each isozyme's ability to process its specific substrate. The assay utilized human liver microsomes (BD Gentest) with the following substrates and incubation times at 37 °C: 1A2: 30 μm phenacetin, 10 min; 2C9: 10 μm diclofenac, 10 min; 2C19: 35 μm S-mephenytoin, 45 min; 2D6: 10 μm bufuralol, 10 min; and 3A4: 5 μm midazolam, 5 min. Following incubation samples were analyzed by LC-MS/MS. A range of test compound concentrations from 0.01 to 10 μm was assayed and IC50 values were calculated.
hERG Channel Activity
Assays were conducted to monitor compound effects on the cardiac hERG potassium ion channel, the inhibition of which can result in potentially fatal arrhythmias (43). CHOK1 cells that stably overexpress the hERG ion channel were tested in a whole cell voltage clamp procedure by measuring the ability of a drug to inhibit the peak current flowing through the hERG channel upon depolarization of the membrane potential. Initially, recordings were made in the presence of control saline to establish a baseline (zero inhibition). This was followed by addition of four increasing concentrations of test drug. Each drug was tested in at least three different cells (n ≥ 3) and the effect of each drug concentration was compared with the baseline to obtain a percent inhibition value. The dose dependent percent inhibition was fitted by a Boltzmann function to generate a dose response curve for the compound and its IC50 was determined.
In Vivo Tolerability/Plasma Exposure
In vivo experiments were conducted to gauge tolerability to a range of doses used in subsequent efficacy studies. These studies were also used to gain preliminary data on drug exposure by measuring plasma levels in response to oral dosing. Compounds were dissolved in 5% lactic acid in ethanol then diluted 1:10 with an aqueous solution of 0.9% sodium lactate/10% hydroxypropyl-β-cyclodextrin. Compounds were administered orally twice-daily 8 h apart to groups of 3 female 4 weeks old CD-1 mice at 50, 100 or 200 mg/kg/day in addition to a vehicle control group. Animals were observed every 15 min for the first hr post-dosing, then hourly for 4 h after the first dose for signs of overt toxicity/poor tolerability. Blood was collected at 1 h after the first dose, 1 h before and 1 h after the second dose, and 18 h after the second dose, and plasma levels of compound were determined.
In Vivo Pharmacokinetic Studies
Pharmacokinetic studies on selected compounds were performed in mice, since this is the host species for the P. berghei efficacy model. Compounds were formulated as described above. Single-dose studies with both an intravenous arm (5 mg/kg) and an oral arm (10 mg/kg) were performed using 25 g male CD-1 mice with 5 animals/dosing group. At specified intervals (0.083, 0.25, 0.5, 1, 2, 6 and 24 h) blood samples were collected into tubes containing dipotassium EDTA as anti-coagulant for LC/MS/MS analysis to determine concentration of compound. Plasma exposure data were used to generate values for a complete set of pharmacokinetic parameters by non-compartment model using WinNonlin.
In Vivo Efficacy Studies, P. berghei Models
The acute P. berghei efficacy model in mice was adapted from Peters' “4-day suppressive test” (44, 45). All initial testing of compounds was conducted at the University of Puerto Rico (UPR), with a limited number of subsequent confirmatory studies performed at the Swiss Tropical and Public Health Institute (Swiss TPH).
The protocol for the studies at UPR was approved by the IACUC of the Medical Sciences Campus, University of Puerto Rico, and all work was conducted in accordance with the “Guide for the Care and Use of Laboratory Animals” and regulations of the PHS Policy on Humane Care and Use of Laboratory Animals (46). Animals were maintained and housed according to NIH guidelines and were allowed to acclimatize for 1 week prior to the commencement of studies. On study day 1, groups of 4–6-week old female Swiss Albino mice (Charles River Laboratories) (n = 5) were infected by tail vein injection with 0.2 ml heparinized blood diluted to contain 1 × 107 P. berghei N-clone parasites. Compounds were formulated as described above and administered by oral gavage. On study day 1 a single dose was given at 9 h post initial infection, and over the subsequent 3 days the dose was split and administered twice daily, with 6 h between doses. Animals in the Control group received vehicle alone. Dose concentration and frequency of dosing were based upon preliminary exposure and tolerability studies (described above). On study day 5 blood was collected by tail-nick, and thin smear microscope slides were prepared and stained using Diff Quick. A minimum of 300 erythrocytes were counted and the percentage of parasitized erythrocytes was determined. Animals lacking detectable parasites on study day 5 were examined every 2–3 days thereafter to determine whether cure was sterile. Animals with no detectable parasites 28 days after cessation of dosing (study day 32) were considered cured; animals were euthanized at the end of the study.
The in vivo studies at the Swiss TPH Institute were performed under a protocol reviewed and approved by the local veterinary authorities of the Canton Basel-Stadt. NMRI mice infected with the ANKA strain MRA-865 (2 × 107 parasites) containing a constitutively-expressed GFP gene (transgenic parasite RMgm-5; http://www.pberghei.eu/index.php?rmgm=5) (47) were used, in contrast to the Swiss Albino mice and N-strain used at UPR. The protocols followed at the two sites were essentially identical with respect to dosing and timing of parasitemia assessments. Parasitologic assessments were made by resuspending 1 μl tail blood in 1 ml PBS buffer and counting the fluorescent cells in a total of 100,000 erythrocytes using a FACScan (Becton Dickinson). Animals that had no detectable parasites on study day 5 were followed out through study day 30 before being declared cured of infection.
In Vivo Efficacy Studies, P. falciparum Model
Efficacy against P. falciparum Pf3D70087/N9 growing in NOD-scid IL-2Rγnull mice engrafted with human erythrocytes was determined as previously described (48). Briefly, groups of 3 animals were infected on day 0 with 2 × 107 parasites. In the test groups, compound was administered twice daily on study days 4–7, while animals in the Control group received no drug. Parasitemia was assessed by FACS as previously described (49).
RESULTS
Identification of Lead Compounds
The N-alkyl-5-(1H-benzimidazol-1-yl)thiophene-2-carboxamides in the present report are analogs derived from Compounds 3 and 4 presented in a previous publication describing a high-throughput screen (25). A manuscript providing details of the analog synthetic pathways and structure-activity relationship is in preparation.
In Vitro Compound Activity
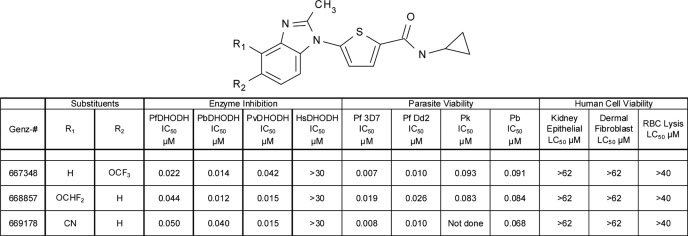
As shown in Table 1, Genz-667348, -668857, and -669178 demonstrated double-digit nanomolar potency against DHODH from P. falciparum, P. berghei and P. vivax, while lacking activity against the human enzyme. These compounds had a profound inhibitory effect on growth of the 3D7 and Dd2 strains of P. falciparum, with single-digit nanomolar IC50 values. To determine whether these compounds showed a similar effect on the in vitro growth of the rodent parasite P. berghei, the in vitro susceptibility of blood stages was determined using the in vitro drug luminescence assay. Genz-667348 and -668857 both efficiently inhibited the growth of blood stage P. berghei, although the IC50 values were 5- to 13-fold higher than those obtained for in vitro growth inhibition of P. falciparum. Due to the interest in treatment and eradication of not just P. falciparum malaria but also the P. vivax-mediated disease, Genz-667348 and -668857 were assessed for their ability to inhibit the growth of a surrogate parasite, P. knowlesi. The IC50 values obtained were equivalent to those for inhibition of the growth of P. berghei. Finally, these compounds demonstrated a total lack of effect on the viability of human kidney epithelial cells and dermal fibroblasts up to the maximum concentration assayed (62 μm), while also exhibiting no lytic effect on human erythrocytes. These observations provide evidence that the species specificity exhibited for DHODH inhibition correlates with a specific effect on the parasites as compared with representative host cells.
TABLE 1.
In vitro activity of DHODH inhibitors
The abbreviations used are: Pf, Plasmodium falciparum; Pb, Plasmodium berghei; Pv, Plasmodium vivax; Pk, Plasmodium knowlesi; Hs, Homo sapiens. Results represent the means of duplicate determinations.
X-ray Structure Determination of Genz-667348 in Complex with PfDHODH
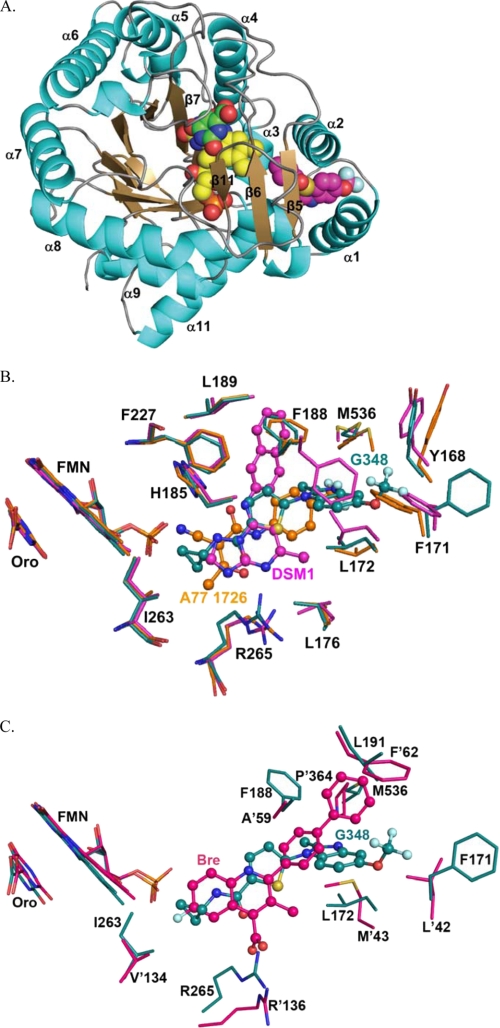
The overall structure of PfDHODH complexed with Genz-667348 (RCSB ID rcsb060790, PDB ID 3O8A) is similar to the previously reported structures of PfDHODH bound to the triazolopyrimidine inhibitors (e.g. DSM1) (23) and A77 1726 (50). A short hydrophobic N-terminal helical domain (residues 163–194) precedes a classic β/α barrel domain that begins with β-strand 3 (Fig. 1A). The Fo − Fc difference density map showed strong, interpretable density for the bound inhibitor (Genz-667348) between the two N-terminal helices (α1 and α2) and the α/β barrel domain (supplemental Fig. 1S). The inhibitor-binding site is formed adjacent to the FMN site by helices α1 and α2 in the N-terminal extension and helices α3 and α10 and strand β 5 in the α/β barrel (Fig. 1, A and B). The cyclopropyl ring of Genz-667348 contacts β5 and is within Van der Waals distance of the FMN, whereas the trifluoromethoxy group extends toward α1 and α2. The cyclopropyl binds a largely hydrophobic pocket formed by Val-532, Ile-272, and Ile-263. Adjacent to this site are two residues that form the only non-hydrophobic contacts with the inhibitor in the pocket. These include ion pair H-bonds between His-185 and the N of methylformamide, and between Arg-265 and the O of methylformamide. The benzimidazole ring extends toward the protein surface between α1 and α2 and is bound in a hydrophobic pocket formed by Tyr-168, Cys-175, Phe-171, Leu-172, Phe-188, Leu-191, and Leu-531.
FIGURE 1.
X-ray structure of Genz-667348 in complex with PfDHODH. A, ribbon diagram of Genz-667348 bound to PfDHODH. α-Helices are displayed in teal, β-strands are displayed in sand, ligands are displayed as space-filling balls. Atoms are colored as follows: carbon atoms are yellow in FMN, pink in G348, and green in orotate; nitrogen is blue; oxygen is red; fluorine is light blue; and sulfur is yellow. B, comparison of PfDHODH inhibitor-binding sites. PfDHODH-G348 (teal) superimposed with PfDHODH-DSM1 (pink) and A77 1726 (orange). Colors refer to carbon atoms, other atoms are colored as in A. Inhibitors are displayed as balls and sticks. A subset of residues within 4 Å of the bound inhibitor is displayed. C, comparison with the human DHODH inhibitor-binding site. PfDHODH-G348 (teal) superimposed with hDHODH-bre (pink). Residue numbers for hDHODH are marked with an apostrophe, while PfDHODH numbers are not. Inhibitors are displayed as balls and sticks.
Comparison to A77 1726 and DSM1-bound PfDHODH Structures
PfDHODH-348 was superimposed with the structures of PfDHODH bound to A77 1726 (1TV5) and the triazolopyrimidine DSM1 (3I65) with root mean square deviations of 0.7 and 0.6 Å, respectively (Fig. 1B). We previously noted that the inhibitor-binding site of PfDHODH is composed of two regions: the H-bond site that contains His-185 and Arg-265, and the hydrophobic pocket (23). The position of the hydrophobic pocket is variable and depends on the conformation of Phe-188, with DSM1 occupying one pocket and A77 1726 occupying the other. These new structures of the enzyme bound to Genz-667348 show that all three inhibitor classes overlap in the H-bond pocket forming interactions with His-185 and Arg-265. Genz-667348 occupies the same hydrophobic pocket as A77 1726 with Phe-188 observed in the up conformation, and thus makes distinct interactions that are not observed in the structures of the enzyme bound to the triazolopyrimidine series of inhibitors. However, as is the case for DSM1, an edge-to-face stacking interaction between Genz-667348 and Phe-188 is present (Fig. 1B), suggesting this interaction likely contributes to the potent binding of the inhibitor series. In addition to the previously identified conformational flexibility of Phe-188, the PfDHODH-348 structure demonstrates that Phe-171 is also capable of conformational flexibility. This ring adopts several conformations allowing the protein to accommodate substituents of variable size into the hydrophobic pocket adjacent to this residue.
Comparison to the Human DHODH Structure Bound to Brequinar
To provide insight into the strong species selectivity that is observed for the series, PfDHODH-348 was superimposed with the structure of human DHODH bound to brequinar (21) (Fig. 1C). The two structures superimpose with a root mean square deviation of 1.8 Å. The structural comparison suggests that the selective binding of Genz-667348 to PfDHODH is due to amino acid substitutions in the benzimidazole ring-binding site, including substitution of Leu-46 and Met-43 in human DHODH for Cys-175 and Leu-172 in PfDHODH. The structural alignment suggests that the human residues in these positions overlap the benzimidazole-binding site, providing a structural rationale for the poor inhibition by these compounds of the human enzyme. As noted previously the substitutions of Met-563, Leu-191, and Phe-188 in PfDHODH for Pro-364, Phe-62, and Ala-59 in human DHODH close off the pocket accessed by brequinar and impede binding in the PfDHODH structure (23).
In Vitro Pharmaceutical Properties
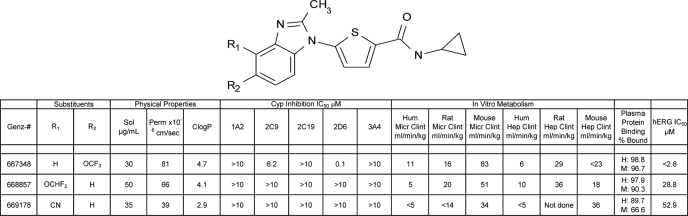
The three lead compounds in the N-alkyl-5-(1H-benzimidazol-1-yl)thiophene-2-carboxamide series were assessed for a number of in vitro drug absorption, distribution, metabolism, and excretion parameters, and the results are presented in Table 2. All three compounds demonstrated moderate solubility (30–50 μg/ml) and good permeability (>30 × 10−6 cm/sec), and they tended to be slightly more hydrophobic than optimal (computed partition coefficient between 3 and 5). Inhibition of cytochrome P450 occurred only for Genz-667348, with inhibition of 2D6 (IC50 = 0.1 μm) and to a lesser extent, 2C9 (IC50 = 6.2 μm). Intrinsic clearance for these compounds ranged from good to moderate as calculated from experiments using both microsomes and hepatocytes from human, rat, and mouse. Genz-667348 demonstrated hERG channel inhibition, with an IC50 value of <2.8 μm. Primarily on the basis of good activity in the in vitro enzyme and parasite assays and the prediction of reasonable stability in the mouse, Genz-667348 was selected for advancement into in vivo drug metabolism and pharmacokinetics and efficacy studies and evaluation of crystal structure. Two later analogs, Genz-668857 and -669178, exhibited increasingly favorable profiles with respect to cytochrome P450 and hERG inhibition while maintaining good activity, and these compounds were also subjected to further in vivo testing.
TABLE 2.
DMPK properties of DHODH inhibitors
The abbreviations used are: Sol, solubility; Perm, permeability in PAMPA membranes; CLogP, computed partition coefficient; Micr Clint, intrinsic clearance, microsomal study; Hep Clint, intrinsic clearance, hepatocyte study; H, human, M, mouse. Results represent single determinations.
In Vivo Tolerability/Plasma Exposure
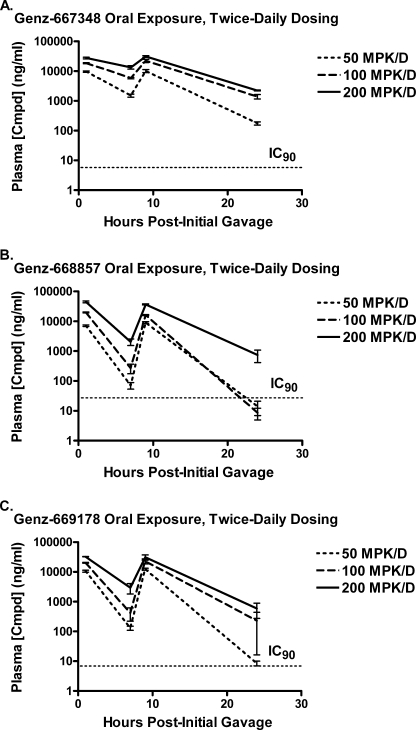
Genz-667348, -668857, and -669178 were administered to mice by oral gavage using twice-daily dosing, and doses up to 200 mg/kg/day elicited no signs of pain, distress, or local or systemic toxicity. Plasma levels of compound were determined, and comparisons with IC90 values derived from in vitro anti-parasite assays are shown in Fig. 2. Values for area under the curve and plasma compound concentrations at peak and trough are given in Table 3. The values for area under the curve were not significantly different between compounds within a given dose. In addition, the maximum concentrations were similar for all three compounds within a given dose, although the levels for Genz-668857 at 200 mg/kg/day tended to be relatively higher. The most noticeable difference for these compounds was observed in the trough levels, where Genz-667348 at all three doses maintained the highest relative concentrations. At the high dose of 200 mg/kg/day the trough concentration of each compound at 24 h remained above the IC90. For each of these three compounds the exposure data were sufficiently favorable to warrant further in vivo testing.
FIGURE 2.
Oral exposure of DHODH inhibitors in the mouse. Genz-667348 (A), -668857 (B), and -669178 (C) were administered to mice by oral gavage using twice daily dosing for a single day, and plasma samples were collected over a 24-h period for analysis as described under “Experimental Procedures.” Exposure levels are shown relative to the IC90 values derived from in vitro parasite viability studies. Results represent the means for three animals per dose ± S.D.
TABLE 3.
Plasma exposure in mice
Abbreviations are: AUC, area under the curve; Cmax1, concentration at 1 h (1 h post initial dose); Cmax2, concentration at 9 h (1 h post second dose); Ctrough1, concentration at 7 h (1 h pre second dose); Ctrough2, concentration at 24 h. Results represent the means (S.D.) of results obtained from five animals/group.
| AUC | Cmax1 | Cmax2 | Ctrough1 | Ctrough2 | |
|---|---|---|---|---|---|
| h*μg/ml | ng/ml | ng/ml | ng/ml | ng/ml | |
| 200 mg/kg/day | |||||
| Genz-667348 | 418 (53) | 27694 (3140) | 31116 (3696) | 13447 (3054) | 2235 (124) |
| Genz-668857 | 460 (22) | 44804 (5238) | 36736 (2511) | 2035 (870) | 747 (579) |
| Genz-669178 | 378 (99) | 32519 (612) | 31134 (10793) | 2927 (1922) | 586 (539) |
| 100 mg/kg/day | |||||
| Genz-667348 | 279 (46) | 18511 (1424) | 22324 (4796) | 5859 (550) | 1394 (423) |
| Genz-668857 | 198 (8) | 19763 (1044) | 16243 (1222) | 271 (167) | 9 (6) |
| Genz-669178 | 259 (68) | 20199 (973) | 23035 (7765) | 419 (345) | 229 (367) |
| 50 mg/kg/day | |||||
| Genz-667348 | 122 (13) | 9691 (1005) | 10095 (2058) | 1513 (310) | 177 (37) |
| Genz-668857 | 98 (12) | 7099 (761) | 9061 (1127) | 70 (31) | 14 (12) |
| Genz-669178 | 137 (12) | 10631 (1318) | 12385 (1649) | 130 (35) | 9 (3) |
In Vivo Pharmacokinetic Studies
The results of the low dose in vivo pharmacokinetic studies are presented in Table 4. All three compounds exhibited rapid (Tmax = 0.25–1 h) and efficient (F = 78–145%) oral uptake with comparable values for Cmax (4.23–6.81 μg/ml). The overall exposure for Genz-667348 appeared to be somewhat greater than the other two compounds as reflected by a higher value for area under the curve, although the half-lives of ∼1–3 h for both the intravenous and oral portions of the studies indicated a slightly higher than optimal rate of plasma clearance for all compounds. Clearance was reasonably low with values of 7–18 ml/min/kg, and the values for steady-state volume of distribution (Vss) of 0.45–0.88 liter/kg suggested a uniform distribution throughout the body for each of the compounds.
TABLE 4.
Pharmacokinetic properties in mice
Abbreviations are: Tmax, time to maximum compound concentration; Cmax, maximum compound concentration; T½, terminal half-life of the compound; AUClast, area under the curve to last quantifiable concentration; AUCINF, area under the curve extrapolated to infinite time; F, % of compound orally bioavailable; CL, calculated rate of clearance from plasma; VSS, steady-state volume of distribution. The results were derived from curves incorporating data from five animals/compound.
| Genz# | 10 MPK Oral |
5 MPK Intravenous |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Tmax | Cmax | T½ | AUClast | AUCINF | F | CL | VSS | T½ | |
| h | μg/ml | h | h*μg/ml | h* μg/ml | % | ml/min/kg | liters/kg | h | |
| 667348 | 0.50 | 5.22 | 1.92 | 35.10 | 35.10 | 145 | 6.9 | 0.88 | 1.67 |
| 668857 | 0.25 | 6.81 | 3.20 | 14.00 | 14.00 | 78 | 9.3 | 0.45 | 1.83 |
| 669178 | 1.00 | 4.23 | 1.00 | 9.40 | 9.50 | 102 | 18.0 | 0.71 | 0.73 |
In Vivo Efficacy Studies, P. berghei Models
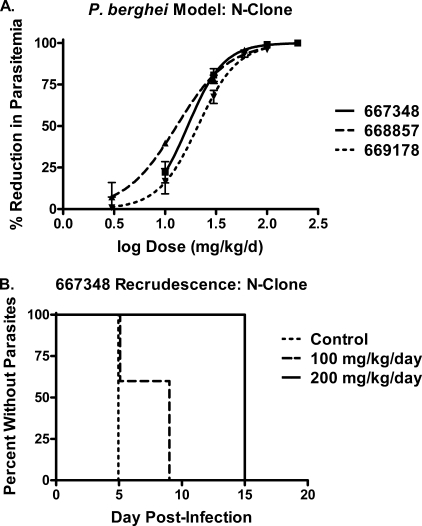
Genz-667348, -668857, and -669178 were assessed for in vivo efficacy in the 4-day murine P. berghei (N-clone) model, and the results are shown in Fig. 3 and Table 5. All three compounds were potent in this animal model, exhibiting ED50, ED90, and ED99 values of 13–21 mg/kg/day, 40–56 mg/kg/day, and 108–192 mg/kg/day, respectively. As shown in Fig. 3A, Genz-667348 at doses of 100–200 mg/kg/day reduced parasitemia by 99–100%. Because parasitemia may initially be reduced below the limit of microscopic detection even after treatment with sub-curative doses, it is necessary to monitor the levels of parasites in the blood following cessation of dosing, determining the first day at which parasites again become detectable (recrudescence). Animals that have no detectable parasites through day 30 are deemed cured. The results are typically presented in a Kaplan-Meyer plot, following recrudescence for a given population as a function of dose over time. As shown in Fig. 3B, Genz-667348 reduced parasitemia to undetectable levels in 3 of 5 mice at day 5, with recrudescence occurring by day 9. At a dose of 200 mg/kg/day parasitemia was undetectable in all 5 animals at day 5, with recrudescence occurring by day 15. Genz-668857 and -669178 were only dosed to a maximum of 100 mg/kg/day, at which level measurable parasitemia existed in all animals at day 5 (0.4 ± 0.2% and 1.6 ± 1.1%, respectively). As a result, there was no recrudescence study phase for these two compounds.
FIGURE 3.
Compound efficacy in the P. berghei mouse model (N-clone). Animals were dosed once on day 1 and with split twice daily dosing on days 2–4. A, reduction in parasitemia for Genz-667348, -668857, and -669178 as a function of dose. ED50 values: 667348, 16.7 mg/kg/day; 668857, 13.0 mg/kg/day; and 669178, 21.0 mg/kg/day. Results represent the means for five animals per dose ± S.D. B, Kaplan-Meyer plot demonstrating the effect of dose of Genz-667348 on time to recrudescence. The high dose of 200 mg/kg/day delayed the reappearance of parasites until day 15; a sterile cure was not achieved.
TABLE 5.
In vivo efficacy in mouse models
Efficacy values expressed as mg/kg/day. In P. berghei N-clone studies (University of Puerto Rico) animals were dosed once on day 1 and with split twice daily dosing on days 2–4. In P. berghei ANKA strain studies (Swiss Tropical and Public Health Institute) animals were dosed twice daily. In the P. falciparum study (GlaxoSmithKline) NOD-scid IL-2Rγnull mice were engrafted with human erythrocytes, and animals were dosed twice daily. ED50 values were derived from dose-response curves incorporating data from five animals/group. ED90 and ED99 values were calculated from ED50 values and slopes of the curves using GraphPad software.
| Genz-667348 |
Genz-668857 |
Genz-669178 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ED50 | ED90 | ED99 | ED50 | ED90 | ED99 | ED50 | ED90 | ED99 | |
| mg/kg/day | |||||||||
| P. berghei N | 16.7 | 40.9 | 108.7 | 13.0 | 47.1 | 192.3 | 21.0 | 56.5 | 165.9 |
| P. berghei ANKA | 6.1 | 12.7 | 28.3 | NDa | ND | ND | ND | ND | ND |
| P. falciparum 3D70087/N9 | 5.3 | 11.0 | 24.5 | ND | ND | ND | ND | ND | ND |
a ND, not determined.
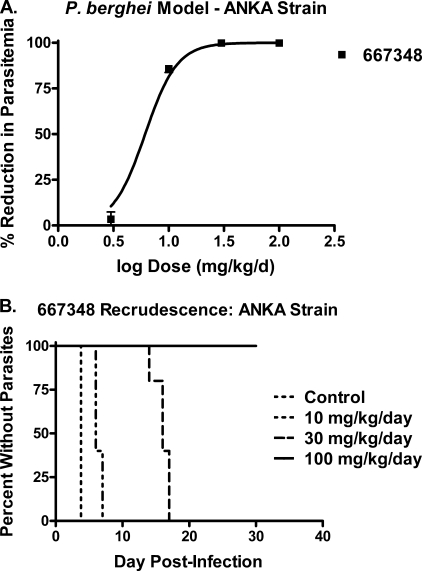
One compound, Genz-667348, was chosen for evaluation in a mouse efficacy model using the ANKA strain of P. berghei. The results of this study are shown in Fig. 4 and Table 5. The values for ED50, ED90, and ED99 were 3- to 4-fold lower than the corresponding values obtained in the N-clone study, suggesting a greater potency against the ANKA strain. A Kaplan-Meyer plot showing the onset of recrudescence as a function of dose is shown in Fig. 4B, with a clear indication that the length of time for relapse was proportional to the dose of compound administered. Of particular importance is the fact that sterile cure was achieved in this model at a dose of 100 mg/kg/day, as defined by a failure for recrudescence to occur within thirty days after infection.
FIGURE 4.
Efficacy of Genz-667348 in the P. berghei mouse model (ANKA strain). Animals were dosed twice daily. A, reduction in parasitemia for Genz-667348 as a function of dose. ED50 value: 6.1 mg/kg/day. Results represent the means for five animals per dose ± S.D. B, Kaplan-Meyer plot demonstrating the effect of dose of Genz-667348 on time to recrudescence. A sterile cure was achieved at 4 days of twice daily dosing at 100 mg/kg/day.
In Vivo Efficacy Studies, P. falciparum Model
Genz-667348 was tested for efficacy against P. falciparum Pf3D70087/N9 growing in NOD-scid IL-2Rγnull mice engrafted with human erythrocytes, and the results are shown in Table 5. The values for ED50, ED90, and ED99 were similar to the corresponding values derived from the P. berghei studies, and they were virtually identical to those from the ANKA strain study. These results are important in that they demonstrate direct activity for the compound against the human parasite rather than a surrogate organism. The similarity of the results for the two species of Plasmodium provides additional validation for use of the P. berghei rodent model as a tool for discovery of agents to treat the human disease.
DISCUSSION
Malaria is one of the world's most devastating endemic diseases, particularly in developing countries, and there is an acute need for new therapeutic approaches because of rapidly increasing resistance to current pharmaceutics. DHODH is a critical enzyme for survival of the parasite, and it represents a potential target for an anti-malarial therapy, as indicated by several reports in the literature. The importance of the target is underscored by the fact that multiple unique chemical series are currently undergoing lead optimization programs. Phillips et al. described a promising series of triazolopyrimidine compounds with inhibitory activity against the enzyme and potency against the parasite both in vitro and in animal models (26, 27). The screening campaign performed at Genzyme and the Broad Institute and described previously identified several additional diverse chemical scaffolds with activity against the enzyme (25), the most promising of which is the N-alkyl-5-(1H-benzimidazol-1-yl)thiophene-2-carboxamide series described in the current report. Data presented here demonstrate that these compounds are highly potent DHODH inhibitors, possess comparable activity in a parasite viability assay, exhibit favorable drug metabolism and pharmacokinetics properties, and are efficacious in animal models of the disease. In particular, sterile cure was achieved in the P. berghei ANKA strain mouse model with the compound Genz-667348, which to the best of our knowledge is the first evidence of this effect with a plasmodial DHODH inhibitor.
Potency against both the enzyme and the parasite was highly correlated throughout this compound class. Previous studies on the original indole screen hit (Genz-582463) and some closely related analogs using a transgenic strain of P. falciparum expressing DHODH from S. cerevisiae confirmed inhibition of this enzyme as the primary mode of action (25), and this finding is currently being confirmed for several later benzimidazole analogs, including those described herein. The interaction between one of the most potent analogs, Genz-667348, and PfDHODH was elucidated by crystallography. The x-ray structure of the enzyme-inhibitor complex reveals extensive contacts, consistent with the observed potent binding of the series. Conformational flexibility allows Genz-667348 to access an alternative hydrophobic pocket from the triazolopyrimidines (e.g. DSM1), enabling the enzyme to bind two alternative chemical scaffolds with high potency. The structural flexibility enhances the value of PfDHODH as a drug target by expanding the chemical space that can be evaluated for the discovery of suitable inhibitors with the potential to lead to drugs.
The species selectivity of this series was evaluated and showed that these compounds also inhibited DHODH from P. berghei and P. vivax, important in the first instance for validation of the animal model and in the second instance for the potential development of a candidate with pan-parasite activity. In addition, whereas activity against the parasite enzymes translated into toxicity against their respective organisms, there was no effect on the human enzyme. The structural basis for this species selectivity is evident from the co-crystal structure of PfDHODH with Genz-667348. The requirement for an accelerated de novo synthesis of pyrimidines in certain rapidly dividing human cells such as activated lymphocytes (51) makes it desirable to identify compounds specific for the parasite DHODH, and the opportunity to achieve an adequate therapeutic window appears to be excellent.
The compounds in this series differed in their in vitro drug metabolism and pharmacokinetics properties, most notably with regard to cytochrome P450 inhibition. Because of rapidly evolving resistance, anti-malarials are unlikely to be administered as monotherapy, with the result that drug-drug interactions are an important consideration. Cytochromes 2C9 and 2D6 were the most likely isozymes to be affected by the compounds described herein, and a number of substrates and inducers across several compound classes have been described for both (52). However, neither cytochrome P450 plays a significant role in the disposition of anti-malarials (53), so issues would primarily concern other co-therapies. Nevertheless, a number of analogs in this compound series exhibited an absence of activity against these enzymes, and the ultimate goal would be the selection of a candidate with little to no cytochrome P450 inhibition.
The DHODH inhibitors in this report exhibited variability with regard to metabolic stability predicted from studies with microsomes and hepatocytes. The earliest compound in this series to undergo in vivo testing, Genz-667348, was chosen primarily on the basis of good activity against both the enzyme and the parasite along with the prediction of reasonable stability. Although potent, this compound produced significant inhibition of CYP2D6 as well as the cardiac hERG channel, rendering it somewhat less desirable as a development candidate. Later analogs, Genz-668857 and -669178, were selected on the basis of equivalent activity, and more favorable cytochrome P450 and hERG inhibition properties when compared with Genz-667348. Exposure studies monitoring plasma levels of compound for 24 h after twice daily dosing demonstrated that each compound maintained plasma levels above the IC90 for the entire study period, and pharmacokinetic studies indicated that each compound was readily taken up into plasma and maintained a reasonable residence time. Tolerability was excellent at all doses tested (up to 200 mg/kg/day), and the compounds were carried forward into efficacy studies.
Genz-667348, -668857, and -669178 underwent testing in the murine P. berghei N-clone model and demonstrated similar potencies, with ED50 values of 13–21 mg/kg/day. In this model, chloroquine and artemisinin are curative at 30 and 100 mg/kg/day, respectively (data not shown). Genz-667348 was further studied in the murine P. berghei ANKA strain model, and sterile cure was achieved following 4 days of twice daily dosing for a total dose of 100 mg/kg/day. To the best of our knowledge this is the first report of sterile cure in a murine model in response to treatment with an inhibitor of DHODH. It is interesting that sterile cure was achieved when the infecting parasite was the ANKA strain of P. berghei but not the N-clone, although it is conceivable that higher concentrations or longer treatment would have yielded cure in the latter model as well. Genz-667348 did demonstrate a 3- to 4-fold greater in vitro potency against the ANKA strain as compared with the N-clone, and this difference may be substantial enough to account for the selective cure. Alternatively, the difference in the susceptibility of the parasites may lie in differences between the original source of these isolates or in the laboratory history of these lines. The N-clone has been derived from the K173 strain that has been kept in the laboratory by mechanical passage for prolonged periods (54), whereas the lines of the ANKA strain are derived from the original ANKA isolate (55). The causes of the different response to DHODH inhibitors in the two lines are yet unknown, but plans are in place to incorporate the ANKA strain model into the assay cascade.
The efficacy of the compounds described in this report is particularly encouraging in light of the fact that any inhibitor of DHODH developed for use in humans would most probably represent one portion of a combination therapy. Because the approach being utilized for these compounds is inhibition of an enzyme crucial for the viability of the parasite, the development of resistance, potentially via mutation of the enzyme, is a possibility. For this reason resistance selection studies are underway on the lead compounds from this series. Nevertheless, evidence of sterile cure in the mouse with a compound from this series used as a monotherapy suggests that DHODH inhibition should be a valuable addition to anti-malarial strategies.
In conclusion, we have demonstrated that the N-alkyl-5-(1H-benzimidazol-1-yl)thiophene-2-carboxamide series offers a potential scaffold for the development of anti-malarial agents. These compounds derive from an iterative medicinal chemistry program, which originated with a high throughput screen of 215,000 diverse compounds contained within the Genzyme library, and they represent novel structures not previously described for this application. Further refinement of the structure-activity relationship within this series is underway, with Genz-668857 and -669178 undergoing further testing to determine if they represent suitable candidates for preclinical development. In addition, the search for closely related compounds representing distinct new scaffolds with anti-DHODH activity is currently ongoing.
Acknowledgment
We thank Dr. Leonard D. Shultz and The Jackson Laboratory for providing access to NSG mice.
This work was supported, in whole or in part, by National Institutes of Health Grants U01AI075594 and AI053680 (to M. A. P.). This work was also supported by the Medicines for Malaria Venture and the Humanitarian Assistance for Neglected Diseases Initiative of Genzyme Corp.
The atomic coordinates and structure factors (code 3O8A) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1S.
- DHODH
- dihydroorotate dehydrogenase
- PfDHODH
- P. falciparum DHODH
- DHO
- dihydroorotate
- CoQ
- coenzyme Q
- PfDHODH-348
- the x-ray structure of PfDHODH bound to Genz-667348
- DSM1
- 5-methyl-[1,2,4]triazolo[1,5-a]pyrimidin-7-yl)naphthalen-2-yl-amine
- A77 1726
- 2-cyano-3-hydroxy-N-[4-(trifluoromethyl)phenyl]-2-butenamide
- hERG
- human Ether-a-go-go Related gene.
REFERENCES
- 1.Subbayya I. N., Ray S. S., Balaram P., Balaram H. (1997) Indian J. Med. Res. 106, 79–94 [PubMed] [Google Scholar]
- 2.Reyes P., Rathod P. K., Sanchez D. J., Mrema J. E., Rieckmann K. H., Heidrich H. G. (1982) Mol. Biochem. Parasitol. 5, 275–290 [DOI] [PubMed] [Google Scholar]
- 3.Caroline D. F. (1969) J. Bacteriol. 100, 1371–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor M. L., Taylor W. H., Eames D. F., Taylor C. D. (1971) J. Bacteriol. 105, 1015–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams J. C., O'Donovan G. A. (1973) J. Bacteriol. 115, 1071–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones M. E. (1980) Annu. Rev. Biochem. 49, 253–279 [DOI] [PubMed] [Google Scholar]
- 7.van der Plas J., Hellingwerf K. J., Seijen H. G., Guest J. R., Weiner J. H., Konings W. N. (1983) J. Bacteriol. 153, 1027–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skophammer R. G., Servin J. A., Herbold C. W., Lake J. A. (2007) Mol. Biol. Evol. 24, 1761–1768 [DOI] [PubMed] [Google Scholar]
- 9.Denis-Duphil M. (1989) Biochem. Cell Biol. 67, 612–631 [DOI] [PubMed] [Google Scholar]
- 10.Jordan D. B., Bisaha J. J., Picollelli M. A. (2000) Arch. Biochem. Biophys. 378, 84–92 [DOI] [PubMed] [Google Scholar]
- 11.Chen J. J., Jones M. E. (1976) Arch. Biochem. Biophys. 176, 82–90 [DOI] [PubMed] [Google Scholar]
- 12.Gero A. M., O'Sullivan W. J., Brown D. J. (1985) Biochem. Med. 34, 60–69 [DOI] [PubMed] [Google Scholar]
- 13.Knecht W., Löffler M. (1998) Biochem. Pharmacol. 56, 1259–1264 [DOI] [PubMed] [Google Scholar]
- 14.Taylor W. H., Taylor M. L. (1964) J. Bacteriol. 88, 105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Löffler M., Jöckel J., Schuster G., Becker C. (1997) Mol. Cell Biochem. 174, 125–129 [PubMed] [Google Scholar]
- 16.Eakin R. T., Mitchell H. K. (1969) Arch. Biochem. Biophys. 134, 160–171 [DOI] [PubMed] [Google Scholar]
- 17.Kennedy J. (1973) Arch. Biochem. Biophys. 157, 369–373 [DOI] [PubMed] [Google Scholar]
- 18.Miller R. W., Curry J. R. (1969) Can J. Biochem. 47, 725–734 [DOI] [PubMed] [Google Scholar]
- 19.Fry M., Beesley J. E. (1991) Parasitology 102, 17–26 [DOI] [PubMed] [Google Scholar]
- 20.Painter H. J., Morrisey J. M., Mather M. W., Vaidya A. B. (2007) Nature 446, 88–91 [DOI] [PubMed] [Google Scholar]
- 21.Liu S., Neidhardt E. A., Grossman T. H., Ocain T., Clardy J. (2000) Structure 8, 25–33 [DOI] [PubMed] [Google Scholar]
- 22.Hurt D. E., Sutton A. E., Clardy J. (2006) Bioorg. Med. Chem. Lett. 16, 1610–1615 [DOI] [PubMed] [Google Scholar]
- 23.Deng X., Gujjar R., El Mazouni F., Kaminsky W., Malmquist N. A., Goldsmith E. J., Rathod P. K., Phillips M. A. (2009) J. Biol. Chem. 284, 26999–27009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baldwin J., Farajallah A. M., Malmquist N. A., Rathod P. K., Phillips M. A. (2002) J. Biol. Chem. 277, 41827–41834 [DOI] [PubMed] [Google Scholar]
- 25.Patel V., Booker M., Kramer M., Ross L., Celatka C. A., Kennedy L. M., Dvorin J. D., Duraisingh M. T., Sliz P., Wirth D. F., Clardy J. (2008) J. Biol. Chem. 283, 35078–35085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips M. A., Gujjar R., Malmquist N. A., White J., El Mazouni F., Baldwin J., Rathod P. K. (2008) J. Med. Chem. 51, 3649–3653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gujjar R., Marwaha A., El Mazouni F., White J., White K. L., Creason S., Shackleford D. M., Baldwin J., Charman W. N., Buckner F. S., Charman S., Rathod P. K., Phillips M. A. (2009) J. Med. Chem. 52, 1864–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otwinowski Z., Minor W. (1997) Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 29.McCoy A. J. (2007) Acta Crystallogr. D Biol. Crystallogr. 63, 32–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 31.Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 32.Cowtan K. (1998) Acta Crystallogr. D Biol. Crystallogr. 54, 750–756 [DOI] [PubMed] [Google Scholar]
- 33.Davis I. W., Leaver-Fay A., Chen V. B., Block J. N., Kapral G. J., Wang X., Murray L. W., Arendall W. B., 3rd, Snoeyink J., Richardson J. S., Richardson D. C. (2007) Nucleic Acids Res. 35, W375–W383, web server issue [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleywegt G. J., Jones T. A. (1994) CCP4/ESF-EACBM Newsletter on Protein Crystallography 31, 9–14 [Google Scholar]
- 35.Holm L., Park J. (2000) Bioinformatics 16, 566–567 [DOI] [PubMed] [Google Scholar]
- 36.Plouffe D., Brinker A., McNamara C., Henson K., Kato N., Kuhen K., Nagle A., Adrián F., Matzen J. T., Anderson P., Nam T. G., Gray N. S., Chatterjee A., Janes J., Yan S. F., Trager R., Caldwell J. S., Schultz P. G., Zhou Y., Winzeler E. A. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 9059–9064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kocken C. H., Ozwara H., van der Wel A., Beetsma A. L., Mwenda J. M., Thomas A. W. (2002) Infect. Immun. 70, 655–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franke-Fayard B., Djokovic D., Dooren M. W., Ramesar J., Waters A. P., Falade M. O., Kranendonk M., Martinelli A., Cravo P., Janse C. J. (2008) Int. J. Parasitol. 38, 1651–1662 [DOI] [PubMed] [Google Scholar]
- 39.Janse C. J., Waters A. P. (1995) Parasitol. Today 11, 138–143 [DOI] [PubMed] [Google Scholar]
- 40.Janse C. J., Franke-Fayard B., Mair G. R., Ramesar J., Thiel C., Engelmann S., Matuschewski K., van Gemert G. J., Sauerwein R. W., Waters A. P. (2006) Mol. Biochem. Parasitol. 145, 60–70 [DOI] [PubMed] [Google Scholar]
- 41.Kansy M., Senner F., Gubernator K. (1998) J. Med. Chem. 41, 1007–1010 [DOI] [PubMed] [Google Scholar]
- 42.Obach R. S., Baxter J. G., Liston T. E., Silber B. M., Jones B. C., MacIntyre F., Rance D. J., Wastall P. (1997) J. Pharmacol. Exp. Ther. 283, 46–58 [PubMed] [Google Scholar]
- 43.Sanguinetti M. C., Tristani-Firouzi M. (2006) Nature 440, 463–469 [DOI] [PubMed] [Google Scholar]
- 44.Peters W. (1975) Ann. Trop. Med. Parasitol. 69, 155–171 [PubMed] [Google Scholar]
- 45.Peters W. (1987) Chemotherapy and Drug Resistance in Malaria, 2nd Ed., pp. 145–273, Academic Press, Orlando, FL [Google Scholar]
- 46.National-Research-Council (1996) Guide for the Care and Use of Laboratory Animals, National Academy Press, Washington, D. C [Google Scholar]
- 47.Franke-Fayard B., Trueman H., Ramesar J., Mendoza J., van der Keur M., van der Linden R., Sinden R. E., Waters A. P., Janse C. J. (2004) Mol. Biochem. Parasitol. 137, 23–33 [DOI] [PubMed] [Google Scholar]
- 48.Angulo-Barturen I., Jiménez-Díaz M. B., Mulet T., Rullas J., Herreros E., Ferrer S., Jiménez E., Mendoza A., Regadera J., Rosenthal P. J., Bathurst I., Pompliano D. L., Gómez de las Heras F., Gargallo-Viola D. (2008) PLoS ONE 3, e2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiménez-Díaz M. B., Mulet T., Gómez V., Viera S., Alvarez A., Garuti H., Vázquez Y., Fernández A., Ibáñez J., Jiménez M., Gargallo-Viola D., Angulo-Barturen I. (2009) Cytometry A 75, 225–235 [DOI] [PubMed] [Google Scholar]
- 50.Hurt D. E., Widom J., Clardy J. (2006) Acta Crystallogr. D Biol. Crystallogr. 62, 312–323 [DOI] [PubMed] [Google Scholar]
- 51.Fairbanks L. D., Bofill M., Ruckemann K., Simmonds H. A. (1995) J. Biol. Chem. 270, 29682–29689 [PubMed] [Google Scholar]
- 52.Hersh E. V., Moore P. A. (2004) J. Am. Dent. Assoc. 135, 298–311 [DOI] [PubMed] [Google Scholar]
- 53.Giao P. T., de Vries P. J. (2001) Clin. Pharmacokinet. 40, 343–373 [DOI] [PubMed] [Google Scholar]
- 54.Saul A., Prescott N., Smith F., Cheng Q., Walliker D. (1997) Mol. Biochem. Parasitol. 84, 143–147 [DOI] [PubMed] [Google Scholar]
- 55.Janse C. J., Ramesar J., Waters A. P. (2006) Nat. Protoc. 1, 346–356 [DOI] [PubMed] [Google Scholar]
- 56.DeLano W. L. (2002) The PyMOL Molecular Graphics System, DeLano Scientific LLC, San Carlos, CA [Google Scholar]