Abstract
The activation of leukocyte function-associated antigen-1 (LFA-1) plays a critical role in regulating immune responses. The metal ion-dependent adhesion site on the I-domain of LFA-1 αL subunit is the key recognition site for ligand binding. Upon activation, conformation changes in the I-domain can lead LFA-1 from the low affinity state to the high affinity (HA) state. Using the purified HA I-domain locked by disulfide bonds for immunization, we developed an mAb, 2E8, that specifically binds to cells expressing the HA LFA-1. The surface plasmon resonance analysis has shown that 2E8 only binds to the HA I-domain and that the dissociation constant (KD) for HA I-domain is 197 nm. The binding of 2E8 to the HA I-domain is metal ion-dependent, and the affinity decreased as Mn2+ was replaced sequentially by Mg2+ and Ca2+. Surface plasmon resonance analysis demonstrates that 2E8 inhibits the interaction of HA I-domain and ICAM-1. Furthermore, we found that 2E8 can detect activated LFA-1 on both JY and Jurkat cells using flow cytometry and parallel plate adhesion assay. In addition, 2E8 inhibits JY cell adhesion to human umbilical vein endothelial cells and homotypic aggregation. 2E8 treatment reduces the proliferation of both human CD4+ and CD8+ T cells upon OKT3 stimulation without the impairment of their cytolytic function. Taken together, these data demonstrate that 2E8 is specific for the high affinity form of LFA-1 and that 2E8 inhibits LFA-1/ICAM-1 interactions. As a novel activation-specific monoclonal antibody, 2E8 is a potentially useful reagent for blocking high affinity LFA-1 and modulating T cell activation in research and therapeutics.
Keywords: Antibodies, Cell Adhesion, Integrin, Leukocyte, Receptor Structure-Function, Affinity, I-domain, LFA-1, MIDAS, Conformation
Introduction
Leukocyte function-associated antigen-1 (LFA-1,3 αLβ2 integrin, CD11a/CD18) belongs to the β2 subclass of integrins and is important for leukocyte adhesion and T cell activation (1, 2). The ligands for LFA-1 are intercellular adhesion molecule-1 (ICAM-1), ICAM-2, and ICAM-3, which are members of the Ig superfamily (1). Although LFA-1 is constitutively expressed on the surface of leukocytes in an inactive state, binding to its ligands requires cellular activation involving both affinity (conformational change within the molecule) and avidity (receptor clustering) enhancement (3–5). The regulation of LFA-1 activation plays a critical role during inflammatory and immune responses in mice and humans (6–9).
The αL subunit of LFA-1 has two prominent structural features, an inserted-domain (I-domain) of about 200 amino acid residues and three EF hand-like repeats containing putative divalent cation binding sites (1). The I-domain of the LFA-1 αL subunit is the ligand binding site and changes conformation upon activation (10). The changes in the I-domain from the low affinity state to the high affinity state lead to an increased affinity for ligand binding (11–15). The key recognition site for ligand binding is at the metal ion-dependent adhesion site (MIDAS) on the I-domain of the LFA-1 αL subunit (1, 2). Constitutively expressed on the surface of leukocytes in an inactive state, LFA-1 activation undergoes two distinct conformational changes that allow ligand binding: 1) a switchblade-like motion that exposes the I-domain, which increases its accessibility; 2) conformational changes in the I-domain that reorient the divalent cation within the MIDAS to be exposed and thus increase the affinity of LFA-1 to its ligand (10, 11). The conformational states of the I-domain can be classified as low affinity (LA) and high affinity (HA) when in the inactive and active state, respectively. The I-domain exists in open and closed conformations and a recently described intermediate affinity (IA) conformation, which differs in affinity for ligand (16). By introducing disulfide bonds into the I-domain, LFA-1 can be locked in either the open or the closed conformation, which represents the HA (K287C/K297C) or LA (K287C/K294C) state, respectively (13–15). We identified antibodies that are sensitive to the affinity changes in the I-domain of mouse LFA-1 and showed that the activation-dependent epitopes were exposed upon mouse T cell activation (17). Taken together, these data demonstrated that the I-domain of LFA-1 changes to the high affinity state upon leukocyte activation.
The I-domain of LFA-1 has been a therapeutic target for regulating inflammatory responses (18–22). Efalizumab, an mAb blocking non-selectively the HA and LA LFA-1, has been approved for the treatment of psoriasis (21). However, the withdrawal of Efalizumab due to viral-induced progressive multifocal leukoencephalopathy indicates that the effectiveness of anti-LFA-1 therapy might be limited by the inability to target the activated form of LFA-1 (23, 24). With recent advances in understanding the structure and function of LFA-1 activation, developing second generation mAbs that selectively target the HA I-domain may prove clinically advantageous as a result of improved specificity and potency. Herein, we report the generation and characterization of an anti-human LFA-1 mAb 2E8. 2E8 binds to the HA I-domain in a metal ion-dependent manner and blocks the binding of HA I-domain to ICAM-1. In addition, the binding of 2E8 is sensitive to the activation and affinity up-regulation of LFA-1 on human cell lines. 2E8 has differential effects on human T cell proliferation and cytotoxicity.
EXPERIMENTAL PROCEDURES
LFA-1 I-domain Constructs
Human wild type (WT), locked HA (K287C/K297C), and IA (L161C/F299C) mutant I-domain constructs were kindly provided by Dr. Timothy Springer and expressed in supplemented M9 minimal media using Escherichia coli BL21 (DE3) cells (16). The recombinant proteins were subsequently refolded and purified as described (25). Proteins were concentrated using a C10 Centriprep (Millipore Corp.), and sample concentrations were determined by both BCA (Pierce Chemicals, Inc.) and A280 nm. Each LFA-1 I-domain was 15N-labeled for acquisition of 1H/15N heteronuclear single quantum coherence nuclear magnetic resonance spectra for quality assurance prior to surface plasmon resonance (SPR) analysis.
Production and Screening of 2E8
The mAb 2E8 was generated from a hybridoma fusion using draining lymph node lymphocytes from a BALB/c mouse immunized with purified human locked HA (K287C/K297C) mutant I-domain. The mAb 2E8 was then screened by flow cytometry analysis (FACS) for the specific ability to bind only to K562 cells expressing HA (K287C/K297C) LFA-1, but not LA (K287C/K294C) LFA-1 or WT LFA-1 (14).
Binding Specificity of 2E8 Measured by SPR
2E8 was purified by protein A column, fractions were combined, and sample concentration was determined by A280 nm. The 2E8 mAb was coupled to a CM5 sensor chip by amine coupling using the manufacturer's protocols (Biacore/GE Healthcare, Inc.). LFA-1 I-domains (WT, IA, and HA) at concentrations of 100, 200, 400, or 800 nm were subsequently injected over the chip at 10 μl/min with Running Buffer A (10 mm HEPES (pH 7.4), 150 mm NaCl, 2 mm MgSO4, 0.005% (v/v) Tween 20). The samples were run under different metal ions, replacing the MgSO4 with 2 mm CaSO4 or 2 mm MnSO4.
Inhibition of LFA-1 HA I-domain Binding to ICAM-1-Fc by 2E8
ICAM-1-Fc (R&D Systems) was coupled onto a CM5 sensor chip by amine coupling. LFA-1 HA I-domain at a concentration of 2 μm was injected over the chip at 10 μl/min with Running Buffer A. Titrations of 0.5, 1, 2, and 4 μm 2E8 mAb were incubated with LFA-1 HA simultaneously overnight and injected over the chip as stated previously.
Biacore Binding Analysis
All Biacore sensorgrams were analyzed from triplicate runs using a 1:1 Langmuir model by Scrubber (version 2.0; Center for Biomolecular Interaction Analysis, University of Utah). Binding studies were performed using SPR on a Biacore 2000 instrument as described (26).
Parallel Plate Flow Detachment Assay
The parallel plate flow detachment assay was modified from a previous procedure (27). For cell adhesion to mAb, 2E8 at 5 μg/ml was immobilized overnight at 4 °C to 24 × 50-mm plastic slides. The slides were then washed with PBS and blocked with 2% BSA before being assembled to a parallel plate flow chamber. For cell adhesion to human umbilical vein endothelial cells (HUVECs) (Lonza, Switzerland), slides were coated with HUVECs in culture media for 24 h and then stimulated with TNF-α (10 units/ml) in fresh media for 24 h. 4 × 106 cells in running buffer were treated with Mn2+ (5 mm) for 5 min at 37 °C and then injected into the flow chamber and allowed to settle on the slides for 10 min. Using a computer-controlled syringe pump (Harvard Apparatus), an increasing linear gradient of shear flow was pulled over the adhered cells for 300 s, and the number of cells remaining adhered was recorded by digital microscopy. Shear stress calculations were determined every 50 s where the sheer stress in dynes/cm2 was defined as (6 μQ)/(wh2), where μ is the viscosity of the media (0.007), Q is the flow rate in cm3/s, w is the width of the chamber (0.3175 cm), and h is the height of the chamber (0.0254 cm). For each experimental condition, an untreated sample was run on the same slide to assure uniformity between slides tested.
Homotypic Aggregation Assay
The aggregation experiment was performed in flat bottom 96-well plates and scored as described (26). JY cells were washed twice and diluted in medium at a final concentration of 0.5 × 107 cells/ml. Cells were treated with various reagents and then incubated in media at 37 °C in for 1 h. The scores were obtained based on the following criteria: 0 indicated that less than 10% of cells were aggregated; 1 indicated that less than 50% of cells were aggregated; 2 indicated that more than 50% of cells were aggregated; 3 indicated that nearly 100% of cells were in small, loose clusters; 4 indicated that nearly 100% of cells were in large clusters.
T Cell Proliferation Assay
Peripheral blood mononuclear cells (PBMCs) were obtained by sedimentation with Histopaque-1077 (Sigma-Aldrich) using blood from healthy volunteers. All cell preparations were >95% viable by trypan blue exclusion. For the T cell proliferation assay, PBMCs were stimulated with anti-CD3 mAb (OKT3) at 300 ng/ml for 5 days. For the CFSE experiments, PBMCs were washed and resuspended in PBS with 1 μm CFSE (Molecular Probes-Invitrogen). Cell division was monitored by using the FITC channel in a FACScanto II flow cytometer (BD Biosciences).
Cytotoxicity Assay
The effector cells were PBMCs generated and primed with OKT3 stimulation for 5 days in vitro. The cytotoxic specificity of human cytolytic T lymphocytes (CTLs) was determined using the flow cytometry-based CTL assay measuring the cleavage of caspase-3 in targets cells (28). The target cells were P815 labeled with a far red fluorescent marker dye, DDAO-SE (Invitrogen), and then incubated with CTLs at different effector:target (E:T) ratios for 3–4 h and fixed, permeabilized, and stained with a phycoerythrin-conjugated anti-cleaved caspase-3 rabbit mAb (BD Biosciences). The stained cells were analyzed using a FACScanto II flow cytometer.
RESULTS
mAb 2E8 Specifically Binds to the HA LFA-1
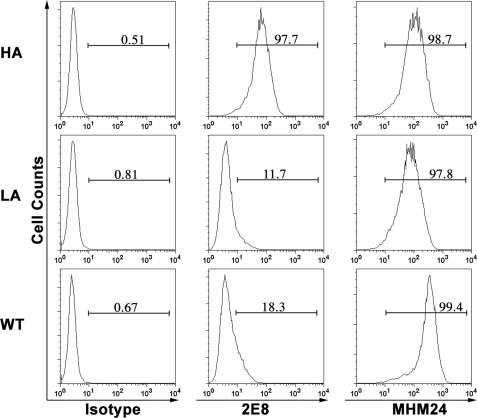
To generate mAb that targets the high affinity LFA-1, purified human HA (K287C/K297C) mutant I-domain was purified for the immunization. 350 hybridoma clones were screened by FACS using K562 cells expressing WT, HA, or LA LFA-1 (14). Only one clone, designated 2E8 with the subtype of IgG1, bound to K562 cells expressing locked HA LFA-1. As shown in Fig. 1, purified 2E8 bound to 97.7% of cells expressing HA LFA-1. There was minimal detectable binding of 2E8 to cells expressing LA (11.7%) and WT (18.3%) LFA-1. MHM24, an mAb for the I-domain of human LFA-1 and humanized for Efalizumab, was used as control (27). In contrast to 2E8, MHM24 bound equally well to cells expressing HA (98.7%), LA (97.8%), or WT (99.4%) LFA-1. Thus, mAb 2E8 specifically binds to the HA LFA-1.
FIGURE 1.
Binding of 2E8 to K562 cells expressing HA LFA-1. The binding of isotype control, 2E8, or MHM24 mAb to K562 cells expressing locked HA (K287C/K297C), LA (K287C/K294C), or WT LFA-1 was determined by flow cytometric analysis. The x axis depicts the relative fluorescence intensity of individual cells, and the y axis represents the cell count. The percentage of gated cells positive for each mAb was displayed in each histogram.
The Binding of 2E8 for HA I-domain Is Metal Ion-dependent
To further determine the specificity for the I-domain, 2E8 was immobilized on a CM5 sensor chip. Various I-domains (WT, IA, and HA) at concentrations of 100 nm were subsequently injected over the chip. As shown in Fig. 2A of the SPR data, 2E8 only bound to the HA I-domain but not to the WT or IA I-domain. Because the IA I-domain is in the inactive state in the absence of ICAM-1 (16), 2E8 therefore specifically bound to the activated I-domain. We determined that the kinetics and the dissociation constant (KD) of 2E8 for HA I-domain is 197 nm (Table 1).
FIGURE 2.
Biacore SPA analysis of 2E8 binding to HA I-domain. A, real time SPR of 2E8 specificity to the HA (red), IA (magenta), or WT (blue) I-domain. 2E8 was coupled to a CM5 sensor chip by amine coupling. LFA-1 I-domains (WT, IA, and HA) at concentrations of 400 nm were subsequently injected over the CM5 chip at 10 μl/min with Running Buffer A. B, real time SPR of divalent metal ion dependence of HA I-domain binding to 2E8. HA I-domain at concentrations of 400 nm was injected over the CM5 chip coupled with 2E8 with Running Buffer A. The samples were run under different metal ions, replacing the MgSO4 in Running Buffer A with CaSO4 or MnSO4. Black, Mn2+; blue, Mg2+; green, Ca2+; red, EDTA.
TABLE 1.
Binding kinetics of 2E8 and HA I-domain
| kon (m−1 × s−1) × 104 | koff (s−1) × 10−3 | KD |
|---|---|---|
| nm | ||
| 2.62 ± 0.22 | 5.16 ± 0.36 | 197 ± 7 |
To examine whether the binding of 2E8 to I-domain is metal ion-dependent, HA I-domain was diluted to a constant concentration of 400 nm with different HBS divalent cation buffers (HBS-Mn2+, HBS-Mg2+, HBS-Ca2+, and HBS-EDTA). As shown in Fig. 2B, 2E8 bound to the HA I-domain in the presence of divalent cations. The affinity decreased as Mn2+ was replaced sequentially by Mg2+ and Ca2+, and the binding was abolished in the presence of EDTA. Thus, the binding of 2E8 to the HA I-domain is metal ion-dependent.
2E8 Inhibits the Binding of HA I-domain to ICAM-1
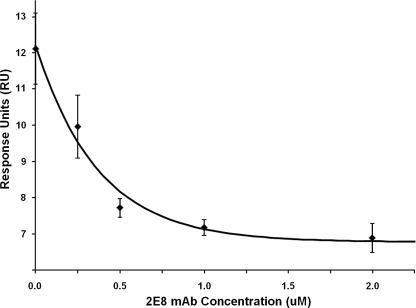
To determine whether 2E8 can inhibit the binding of LFA-1 I-domain to its ligand ICAM-1, ICAM-1-Fc was coupled onto a CM5 sensor chip. HA I-domain at a concentration of 2 μm with the titrated 2E8 was injected over the chip simultaneously. As shown in Fig. 3, the SPR analysis showed an exponential decay of the binding response of the HA I-domain to ICAM-1-Fc as the 2E8 concentration increased. These data demonstrated that 2E8 inhibits the interaction of HA I-domain and ICAM-1.
FIGURE 3.
The exponential decay of HA I-domain binding to ICAM-1-Fc in the presence of 2E8. ICAM-1-Fc was immobilized on the SPR chip, and 2 μm HA I-domain was run across the chip. Then 2 μm HA I-domain was titrated simultaneously with 0.25, 0.50, 1.0, and 2.0 μm 2E8, and the binding of HA I-domain binding to ICAM-1-Fc was measured. Data points were averages of three experiments with error bars denoting S.D. The exponential decay fit was computed by a first-order exponential decay fit using MicroCal Origin v6.0.
2E8 Binds to Activated LFA-1 on Jurkat and JY Cells
Our data demonstrated that 2E8 specifically bound the locked HA mutant I-domains and blocked ICAM-1 interaction. We further investigated whether the similar binding specificity can be achieved for activated WT LFA-1 on human cell lines. As shown in Fig. 4A, the percentage of Jurkat cells binding to 2E8 was significantly increased when LFA-1 was activated in the presence of Mn2+ when compared with untreated control (70.6% versus 21.1%), whereas MHM24 binding remained unchanged. The same trend was observed for the mean fluorescence intensity of 2E8 binding cells (mean fluorescence intensity: 19 versus 8). Similar results were obtained from JY cells with increased 2E8 binding for cells activated by Mn2+ when compared with the untreated cells (Fig. 4B). Thus, 2E8 is an activation-sensitive mAb and recognizes Mn2+-activated LFA-1.
FIGURE 4.
Binding of 2E8 to activated LFA-1 on cell lines. Jurkat or JY cells were left untreated (NT) or stimulated with Mn2+ (5 mm) in the presence of 2E8, MHM24, or isotype control mAb at the concentration of 1 μg/ml and then incubated at 4 °C for 30 min. Cells were stained with secondary Alexa Fluor488-conjugated goat anti-mouse IgG (Invitrogen) and then washed and resuspended in PBS with 2% paraformaldehyde for 20 min at 4 °C. All experimental conditions were performed in triplicate and analyzed by flow cytometry using a FACSCalibur (BD Biosciences). A, binding of 2E8 and MEM24 to Jurkat cells. Staining of 2E8 and MHM24 is shown in solid lines, and isotype control is shown in dashed lines. The mean fluorescence intensity (MFI) and the percentage of gated cells positive for mAbs are representative of three independent experiments. B, the mean fluorescence intensity of 2E8 binding to Jurkat and JY cells. Results were mean and S.D. of three independent experiments. The asterisk represents data with p value less than 0.05 in the Student's t test.
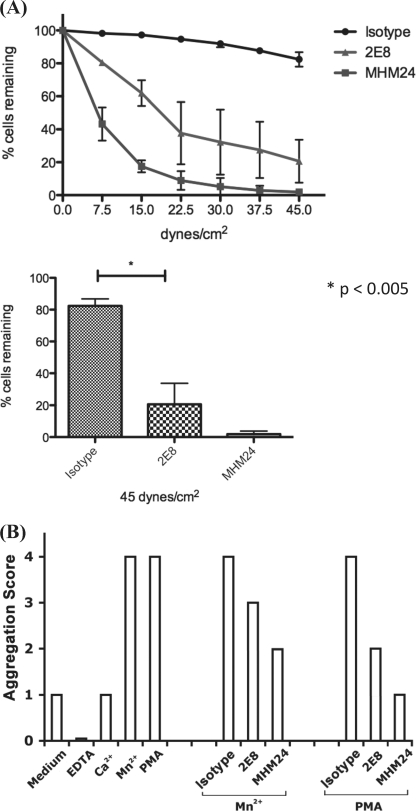
In addition, we examined whether LFA-1 can bind to 2E8 under shear force using parallel plate flow, which is a physiologic model for the study of cell rolling and adhesion mediated by integrins (29). JY cells were first injected into the flow chamber and allowed to adhere to slides coated with mAbs. As shown in Fig. 5A (top), when cells were treated with Mn2+, there was no increased adhesion to isotype control when compared with untreated sample (NT). In addition, JY cells persistently adhered to MHM24 up to 45 dynes/cm2 in the absence and presence of Mn2+. Although the adhesion of JY cells to 2E8 was similar to BSA control in the untreated sample, adding Mn2+ increased the adhesion to 100% at low shear forces, and the adhesion slowly decreased to about 60% at 45 dynes/cm2 (Fig. 5A, bottom). Similar results were observed using Jurkat cells (Fig. 5B). The data demonstrated that 2E8 binds to Mn2+-activated LFA-1 and that the binding is resistant to shear stress.
FIGURE 5.
Adhesion of activated LFA-1 to 2E8 under shear force in parallel plate flow assay. JY (A) or Jurkat (B) cells were either untreated (NT) or stimulated with Mn2+ (5 mm) before being injected into the flow chamber and allowed to adhere to slides coated with mAbs or BSA. Top, a linear gradient of shear flow increasing from 0–51 dynes/cm2 was perfused over the adhered cells, and the extent of adhesion was determined by the percentage of cells remaining at particular shear forces. The baseline of cell adhesion for each mAb was established with the untreated sample (NT), which showed a linear steady rate of detachment as the shear forces increase over time. A linear gradient of shear flow was applied to the adhered cells, and the percentages of cells remaining were enumerated every 50 s. The data represent the average of three independent experiments with error bars. Bottom, the percentage of cells remaining adhered to the coated slides at 45 dynes/cm2. The error bars indicate the S.E., and the p value (**) was generated using the Student's t test.
2E8 Inhibits the Adhesion and Homotypic Aggregation of JY Cells
We further examined the effect of 2E8 on LFA-1-mediated adhesion to HUVECs using a parallel plate flow assay. HUVECs were first plated on the slides and cultured in media overnight. Subsequently TNF-α (10 units/ml) was added in the fresh media to stimulate HUVECs for 24 h before placing the cells in the flow chamber. Activated JY cells were pretreated with mAb and then injected into the flow chamber and allowed to adhere to slides coated with HUVECs. As shown in Fig. 6A, MHM24 blocked about 80% of JY cell adhesion to HUVECs at low shear force of 15 dynes/cm2 and achieved near 100% blockade at high shear force of 45 dynes/cm2. Although 2E8 exhibited the ability to inhibit JY cell adhesion under various shear forces, there were about 20% of cells adhered to HUVECs at 45 dynes/cm2. In addition, we examined the effect of 2E8 on cell homotypic aggregation. JY cells were stimulated with Mn2+ or phorbol 12-myristate 13-acetate in flat bottom 96-well plates in the presence of 2E8, MHM24, or isotype control. As shown in Fig. 6B, both 2E8 and MHM24 blocked the homotypic aggregation of JY cells, but 2E8 was less efficient when compared with MHM24 at equivalent concentrations. Thus, our data demonstrated that 2E8 can inhibit JY cell adhesion to HUVECs and homotypic aggregation.
FIGURE 6.
Inhibition of JY cell adhesion and homotypic aggregation by 2E8. A, JY cell adhesion to TNF-α stimulated HUVECs. HUVECs were cultured on the slides and stimulated with TNF-α (10 units/ml) for 24 h. JY cells were pretreated with isotype control, 2E8, or MHM24 mAb (1 μg/ml) upon the addition of Mn2+ (5 mm). Activated JY cells were injected into the flow chamber and allowed to adhere to slides coated with HUVECs. Top, a linear gradient of shear flow increasing from 0 to 51 dynes/cm3 was perfused over the adhered cells, and the extent of adhesion was determined by enumerating the percentage of cells remaining at 50-s intervals. The data represent the average of three independent experiments with error bars. Bottom, the percentage of cells remaining adhered to HUVECs at 45 dynes/cm2. The error bars indicate the S.E., and the p value was generated using the Student's t test. B, homotypic aggregation. JY cells were treated with Mn2+ (1 mm) or phorbol 12-myristate 13-acetate (PMA) (10 ng/ml) in the presence of 50 nm of isotype control, 2E8, or MHM24 mAb. Cells treated with 1 mm Mn2+, 10 ng/ml phorbol 12-myristate 13-acetate, 1 mm Ca2+, or 1 mm EDTA, or cells without any treatment were used as controls. Aggregation was scored as described under “Experimental Procedures.” Three independent experiments showed identical results.
2E8 Has Differential Effects on Human T Cell Proliferation and Cytotoxicity
LFA-1, as a costimulatory molecule, is important in regulating T cell activation and cytolytic function in the context of the immunological synapse (30, 31). We examined whether 2E8 treatment affects human T cell proliferation upon T cell receptor stimulation. First, we examined the effect of 2E8 at various concentrations on the proliferation of both CD4+ and CD8+ T cells using CFSE-labeled cells stimulated with OKT3. As shown in Fig. 7A (solid line), the inhibition of T cell proliferation by 2E8 is dose-dependent. With the increase of 2E8 concentration from 0.5 μg/ml to 5 μg/ml and 50 μg/ml, the frequency of dividing cells were decreased from 83% to 73 and 67% for CD4+ T cells and from 88% to 78 and 62% for CD8+ T cells, respectively. However, in the presence of 5 μg/ml MHM24 (shadow), the majority of T cells remained undivided (the first peak on the left). The cell division index was determined based on the proliferation kinetics and normalized to the isotype control (Fig. 7B). Although both 2E8 and MHM24 can decrease the division index about 20% in CD4+ T cells, the p value was not significant. For CD8+ T cells, 2E8 and MHM24 significantly reduced the division index to about 60 and 20% of the isotype control, respectively. Thus, 2E8 can inhibit the proliferation of human T cells upon T cell receptor stimulation but less efficiently than MHM24.
FIGURE 7.
Effect of 2E8 and MHM24 on human T cell proliferation. PBMCs labeled with CFSE were stimulated by OKT3 (300 ng/ml) for 5 days in the presence of various concentrations of 2E8 (solid line: 0.5, 5, or 50 μg/ml) or MHM24 (shadow: 5 μg/ml). A, T cell proliferation was assessed by CFSE dye dilution following sequential gating on lymphocytes (by scatter) and CD4+ or CD8+ T cells. Each histogram plot represents the cell division overlay in the presence of different concentrations of 2E8 (solid line) and 5 μg/ml MHM24 (shadow). The percentage of dividing cells in the presence of various concentration of 2E8 was shown on the histogram. Representative sample data of three independent experiments were presented. B, the relative division index of CD4+ and CD8+ T cells in the presence of 2E8 and MHM24. The proliferation kinetics were analyzed by FlowJo, and cell proliferation models were generated based on the CFSE histogram data. The cell division index was calculated by FlowJo based on the proliferation kinetics. Data shown here are representative of three independent experiments from three different samples. Results were mean and S.D. of three independent samples. The asterisk represents data with p value less than 0.05 in the Student's t test.
To determine whether 2E8 affects cytotoxicity, we assessed the cytolytic capacity of effector cells from PBMCs primed and expanded with OKT3. Using P815 cells as a target, we compared the specific lysis in the presence of 2E8 and MHM24 using cytotoxicity assay (28). As shown in Fig. 8A, the specific lysis of P815 by a representative human CTLs was measured in the presence of 2E8 (20 μg/ml), and the killing of target cells at various E:T ratios was not compromised. However, MHM24 at the concentration of 0.2 μg/ml was already effective in inhibiting the cytotoxicity, and we observed similar results using EL-4 as the target cells (data not shown). The inhibition of cytotoxicity (relative percentage of caspase-3 positive cells normalized to isotype control) by 2E8 and MHM24 at E:T ration of 20:1 was summarized in Fig. 8B, showing that 2E8 treatment did not affect the cytolytic function, whereas MHM24 significantly inhibited the specific lysis of target cells.
FIGURE 8.
Effect 2E8 and MHM24 on the cytolytic function of human T cells. The effector cells were generated from PBMCs primed with OKT3 stimulation for 5 days. Target cells were P815 cells labeled with DDAO-SE and incubated for 4 h with effector cells at the E:T ratios indicated, in the presence of 20 μg/ml isotype control, 2E8, or MHM24 mAb. Lysis of P815 was measured by the percentage of caspase-3-positive cells. A, representative data of specific lysis of P815 by human CTLs, with mean and S.D. of three independent experiments from one CTL sample. B, the reduction of specific cytotoxicity in the presence of 2E8 or MHM24 mAb at E:T ratio of 20:1. Results were mean and S.D. of three different samples from nine independent experiments. The asterisk represents data with p value less than 0.02 in the Student's t test.
DISCUSSION
We report a novel mAb, 2E8, that specifically binds to the HA I-domain of LFA-1. In addition, 2E8 blocks the interaction of LFA-1 to its ligand ICAM-1. The binding of 2E8 is metal ion-dependent, and thus the binding site is likely associated with the MIDAS. Furthermore, 2E8 recognizes Mn2+-activated LFA-1 but not LFA-1 in the resting state on human cells. Therefore, 2E8 is an activation-sensitive mAb and demonstrates the appearance of activation-induced conformational changes in the I-domain. More importantly, we have shown here that 2E8 can inhibit human T cell proliferation without affecting their cytolytic function.
LFA-1 is an important molecule in the immune system, and its activation state influences the outcome of T cell activation (30, 31). There has been long standing interest in LFA-1 as a therapeutic target for regulating immunity. Efalizumab binds to LFA-1 with high affinity (KD: 2 nm) for both active HA and inactive LA I-domain and sterically inhibits the binding of LFA-1 to ICAM-1 (32). Although Efalizumab showed promise in treating psoriasis patients, it was voluntarily removed from the market due to patients developing progressive multifocal leukoencephalopathy, possibly through completely down-regulating T cell function. Developing the second generation of antibodies, such as 2E8, that specifically target the HA LFA-1 I-domain as therapeutic inhibitors is the logical next step.
AL-57, an mAb against both the HA and the IA I-domain of LFA-1, was developed recently by phage display and recognizes the affinity-up-regulated I-domain in a ligand mimetic fashion (32, 33). The crystal structures (3HI5 and 3HI6) of the Fab fragment of AL-57 in complex with the locked IA I-domain demonstrates that AL-57 coordinates with the MIDAS in a similar geometry as ICAM-1 and that the binding site of AL-57 overlaps the ICAM-1 binding site on the I-domain (34). Additionally, a hydrophobic patch further stabilizes the AL-57/I-domain interface to outcompete ICAM-1 for the MIDAS site (32, 34).
Similar to ICAM-1, the binding of 2E8 to the I-domain requires the presence of a divalent cation and thus is metal ion-dependent (32). This indicates that the 2E8/I-domain binding possibly acts in a typical ligand/integrin MIDAS interaction, which requires an acidic residue interacting with the metal ion bound I-domain (16, 35). 2E8 may have a primary binding site located at the MIDAS and is most likely mediated through a glutamic or aspartic acid carboxyl group. A comparison of binding kinetics demonstrates that the KD of 2E8 to HA I-domain is an order of magnitude weaker than that of AL-57 (197 versus 23 nm, respectively) but close to the KD of ICAM-1 to HA I-domain at 310 nm (26).
An important attribute of 2E8 is the specificity for the HA I-domain over both the IA and the WT forms. In contrast to AL-57, 2E8 does not bind to the IA I-domain (Fig. 2A). Moreover, we have shown that 2E8 not only blocks the binding of HA I-domain to ICAM-1 but also prevents LFA-1-mediated cell aggregation. 2E8 preferentially recognizes the active conformation of the I-domain and selectively binds activated LFA-1 on cells; thus, the binding is in an activation-specific manner.
In addition to functioning as an adhesion molecule for leukocyte migration and adhesion, LFA-1 plays a critical role in regulating T cell function in the context of immunological synapse. Specifically, LFA-1 is a costimulatory molecule mediating T cell proliferation and cytotoxicity (30, 31). We demonstrated the affinity changes in the I-domain of LFA-1 during mouse T cell activation (17). However, little is known on the role of high affinity LFA-1 in human T cell function, although Efalizumab has been used in the clinic to target the I-domain of LFA-1 in inflammatory responses (18–22). It binds to both the low and the high affinity form of I-domain (23, 24). We found that 2E8 can block both human primary CD4+ and human primary CD8+ T cell proliferation, albeit less efficiently than MHM24. Interestingly, the cytolytic activity of human T cells remains intact in the presence of 2E8, which is in contrast to MHM24, which can significantly inhibit specific lysis of target cells. The data suggest that there might be differential requirement of LFA-1 activation in T cell proliferation and cytolytic function, which remains to be investigated and understood in the future.
In summary, we developed and characterized 2E8 that specifically binds to the MIDAS site of the high affinity I-domain of LFA-1. Our study improves the understanding of the structure and function aspects of LFA-1 biology. Furthermore, 2E8 is a potentially novel reagent for blocking high affinity LFA-1 and modulating T cell activation.
Acknowledgments
We thank Dr. Timothy Springer for providing reagents. The animal experiments were approved by the Institutional Animal Care and Use Committee at University of Texas M.D. Anderson Cancer Center.
The work was supported by American Cancer Society Grant RSG-08-183-01-LIB (to Q. M.).
- LFA-1
- leukocyte function-associated antigen-1
- MIDAS
- metal ion-dependent adhesion site
- LA
- low affinity
- HA
- high affinity
- IA
- intermediate affinity
- SPR
- surface plasmon resonance
- HUVEC
- human umbilical vein endothelial cell
- PBMC
- peripheral blood mononuclear cell
- HBS
- Hanks’ balanced salt solution
- CTL
- cytolytic T lymphocyte
- CFSE
- carboxyfluorescein succinimidyl ester.
REFERENCES
- 1.Springer T. A. (1994) Cell. 76, 301–314 [DOI] [PubMed] [Google Scholar]
- 2.Dustin M. L. (2003) Ann. N.Y. Acad. Sci. 987, 51–59 [DOI] [PubMed] [Google Scholar]
- 3.Carman C. V., Springer T. A. (2003) Curr. Opin. Cell Biol. 15, 547–556 [DOI] [PubMed] [Google Scholar]
- 4.Hogg N., Smith A., McDowall A., Giles K., Stanley P., Laschinger M., Henderson R. (2004) Immunol. Lett. 92, 51–54 [DOI] [PubMed] [Google Scholar]
- 5.Hynes R. O. (2003) Science. 300, 755–756 [DOI] [PubMed] [Google Scholar]
- 6.Schmits R., Kündig T. M., Baker D. M., Shumaker G., Simard J. J., Duncan G., Wakeham A., Shahinian A., van der Heiden A., Bachmann M. F., Ohashi P. S., Mak T. W., Hickstein D. D. (1996) J. Exp. Med. 183, 1415–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berlin-Rufenach C., Otto F., Mathies M., Westermann J., Owen M. J., Hamann A., Hogg N. (1999) J. Exp. Med. 189, 1467–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blazar B. R., Taylor P. A., Panoskaltsis-Mortari A., Gray G. S., Vallera D. A. (1995) Blood 85, 2607–2618 [PubMed] [Google Scholar]
- 9.Lebwohl M., Tyring S. K., Hamilton T. K., Toth D., Glazer S., Tawfik N. H., Walicke P., Dummer W., Wang X., Garovoy M. R., Pariser D. (2003) N. Engl. J. Med. 349, 2004–2013 [DOI] [PubMed] [Google Scholar]
- 10.Shimaoka M., Takagi J., Springer T. A. (2002) Annu. Rev. Biophys. Biomol. Struct. 31, 485–516 [DOI] [PubMed] [Google Scholar]
- 11.Legge G. B., Morris G. M., Sanner M. F., Takada Y., Olson A. J., Grynszpan F. (2002) Proteins. 48, 151–160 [DOI] [PubMed] [Google Scholar]
- 12.Luo B. H., Carman C. V., Springer T. A. (2007) Annu. Rev. Immunol. 25, 619–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu C., Shimaoka M., Zang Q., Takagi J., Springer T. A. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 2393–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimaoka M., Lu C., Palframan R. T., von Andrian U. H., McCormack A., Takagi J., Springer T. A. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 6009–6014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Q., Shimaoka M., Lu C., Jing H., Carman C. V., Springer T. A. (2002) J. Biol. Chem. 277, 10638–10641 [DOI] [PubMed] [Google Scholar]
- 16.Shimaoka M., Xiao T., Liu J. H., Yang Y., Dong Y., Jun C. D., McCormack A., Zhang R., Joachimiak A., Takagi J., Wang J. H., Springer T. A. (2003) Cell. 112, 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., Li D., Nurieva R., Yang J., Sen M., Carreño R., Lu S., McIntyre B. W., Molldrem J. J., Legge G. B., Ma Q. (2009) J. Biol. Chem. 284, 12645–12653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicolls M. R., Gill R. G. (2006) Am. J. Transplant. 6, 27–36 [DOI] [PubMed] [Google Scholar]
- 19.Scheinfeld N. (2006) Expert. Opin. Drug. Saf. 5, 197–209 [DOI] [PubMed] [Google Scholar]
- 20.Matthews J. B., Ramos E., Bluestone J. A. (2003) Am. J. Transplant. 3, 794–803 [DOI] [PubMed] [Google Scholar]
- 21.Gordon K. B., Papp K. A., Hamilton T. K., Walicke P. A., Dummer W., Li N., Bresnahan B. W., Menter A., Efalizumab Study Group (2003) JAMA 290, 3073–3080 [DOI] [PubMed] [Google Scholar]
- 22.Giblin P. A., Lemieux R. M. (2006) Curr. Pharm. Des. 12, 2771–2795 [DOI] [PubMed] [Google Scholar]
- 23.Pugashetti R., Koo J. (2009) J. Dermatolog. Treat. 20, 132–136 [DOI] [PubMed] [Google Scholar]
- 24.Major E. O. (2010) Annu. Rev. Med. 61, 35–47 [DOI] [PubMed] [Google Scholar]
- 25.Legge G. B., Kriwacki R. W., Chung J., Hommel U., Ramage P., Case D. A., Dyson H. J., Wright P. E. (2000) J. Mol. Biol. 295, 1251–1264 [DOI] [PubMed] [Google Scholar]
- 26.Carreño R., Li D., Sen M., Nira I., Yamakawa T., Ma Q., Legge G. B. (2008) J. Biol. Chem. 283, 10642–10648 [DOI] [PubMed] [Google Scholar]
- 27.Hildreth J. E., Gotch F. M., Hildreth P. D., McMichael A. J. (1983) Eur. J. Immunol. 13, 202–208 [DOI] [PubMed] [Google Scholar]
- 28.He L., Hakimi J., Salha D., Miron I., Dunn P., Radvanyi L. (2005) J. Immunol. Methods. 304, 43–59 [DOI] [PubMed] [Google Scholar]
- 29.Mitchell J. S., Brown W. S., Woodside D. G., Vanderslice P., McIntyre B. W. (2009) Immunol. Cell Biol. 87, 324–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huppa J. B., Davis M. M. (2003) Nat. Rev. Immunol. 3, 973–983 [DOI] [PubMed] [Google Scholar]
- 31.Dustin M. L. (2007) Curr. Opin. Cell Biol. 19, 529–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimaoka M., Kim M., Cohen E. H., Yang W., Astrof N., Peer D., Salas A., Ferrand A., Springer T. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 13991–13996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang L., Shimaoka M., Rondon I. J., Roy I., Chang Q., Po M., Dransfield D. T., Ladner R. C., Edge A. S., Salas A., Wood C. R., Springer T. A., Cohen E. H. (2006) J. Leukoc. Biol. 80, 905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H., Liu J. H., Yang W., Springer T., Shimaoka M., Wang J. H. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 18345–18350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song G., Yang Y., Liu J. H., Casasnovas J. M., Shimaoka M., Springer T. A., Wang J. H. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 3366–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]