Abstract
Phosphorylation is the most important post-translational event at a cellular level that is regulated by protein kinases. MAPK is a key player in the important cellular signaling pathway. It has been hypothesized that phosphorylation might have a role in the induction of break tolerance against some autoantigens such as SRP72. The aim of this study was to explore the pathways of phosphorylation and overexpression of the SRP72 polypeptide, using an in vitro model of Jurkat cells stimulated by recombinant human (rh)IL-1β in the presence of MAPK inhibitors. We used Jurkat cells as a substrate stimulated with rhIL-1β in the presence of MAPK inhibitors at different concentrations in a time course in vitro experiment by immunoprecipitation, immunoprecipitation-Western blotting, and real time PCR. Our results showed that rhIL-1β causes up-regulation of protein expression and phosphorylation of SRP72 in Jurkat cells. Inhibitors of the MAPK pathway ERK1/2 or p38α/β down-regulate the expression of SRP72 autoantigen in Jurkat cells stimulated by rhIL-1β. Our results highlight the importance of studying the pathways of activation and overexpression of autoantigens. It will be necessary to perform careful research on various kinases pathways, including MAPK in dermatomyositis and other rheumatic diseases, to help to explain the routes of activation and inhibition of autoantigens. The understanding of this process may help to develop new therapies to prevent and control the loss of tolerance toward own normal proteins.
Keywords: Antibodies, Immunology, MAPKs, p38 MAPK, Phosphoprotein Phosphatase
Introduction
The SRP complex is a group of ribonucleoproteins, including the 7SL RNA in association with six polypeptides, 72, 68, 54, 21, 19, and 9 kDa, the main function of which is to attach the translating ribosome to the endoplasmic reticulum (ER)2 and facilitate the translocation and secretion of proteins into the ER lumen (68 and 72 kDa) (1–5). Inside the ER lumen, post-translational events such as glycosylation, methylation, and phosphorylation take place so that a given protein exerts its actions in specific tissues. Phosphorylation is the most important post-translational event at a cellular level regulated by protein kinases (6, 7). MAPK is a key player in the important cellular signaling pathway. Its activation is associated with inflammatory cytokines, genotoxic agents, UV light, and heat shock proteins (8–10). It has been hypothesized that phosphorylation might have a role in the induction of autoimmunity in patients with systemic lupus erythematosus and Sjögren syndrome. Proteolytic cleavage has been identified as a possible mechanism of breaking tolerance in autoimmune rheumatic diseases due to exposure of cryptic epitopes to the immune system triggering autoimmune phenomena such as autoantibodies against self-molecules (11, 12). Other mechanisms that contribute to breaking tolerance toward self-peptides might include release of cytokines and pro-inflammatory mediators able to alter phosphorylation status through kinase pathway activation (13). IL-1β promotes activation of ERK1/2, p38, and JNK (Janus kinases) in rheumatoid arthritis and osteoarthritis inducing joint inflammation, although a clear-cut mechanism remains to be elucidated (14, 15). In patients with dermatomyositis (DM) (16), up-regulation of IL-1β in muscular tissues and peripheral blood has been reported (9, 11, 12). The aim of this work was to explore the phosphorylation status and expression of SRP72 autoantigen, using an in vitro model, previously reported by our group (17), on Jurkat cells that offer an excellent substrate to test models of autoimmune diseases because they are a stable tumor cell line capable of producing human IL-2, which stimulates the long term in vitro proliferation of antigenic specific effector T cells and enhances mitogenic properties; also they offer various antigen and effector specificities. These Jurkat cells were stimulated by recombinant human IL-1β (rhIL-1β). IL-1β is overexpressed in muscle biopsies of patients with DM (18); also it is known to up-regulate the MAPK kinase family; p38, ERK, and JNK (19). Furthermore, we were able to show that specific MAPK inhibitors prevented IL-1β-induced up-regulation of SRP72.
MATERIALS AND METHODS
Cell Culture
Jurkat cells were grown in RPMI 1640 medium (Millipore, Billerica, MA) supplemented with 10% heat-inactivated FBS, 100 units/ml penicillin, 100 μg/ml streptomycin (Invitrogen) under 5% CO2 at 37 °C for 72 h until a confluence of 1 × 106 cells/ml for each experiment. The cell pellet was obtained after centrifugation at 300 × g for 7 min at 4 °C.
Induction of SRP72 Expression with rhIL-1β
In the experiments to examine the effects of rhIL-1β on SRP72 expression, Jurkat cells were cultured in complete RPMI 1640 medium without (control) or with 100 pg/ml rhIL-1β (Millipore) and harvested at 0, 5, 15, 30, 60, 90, 120, 180, and 240 min. The cells were washed with iced-cold PBS and lysate in Nonidet P-40 lysis buffer (150 mm NaCl, 1% Nonidet P-40, 50 mm Tris, pH 8.0) as described elsewhere (7). The total protein concentration of cell extract after centrifugation (13,000 rpm, 10 min at 4 °C) was quantified by Bradford (20).
Activation of Jurkat Cells and Inhibition of MAPK Pathway
Another series of experiments, effects of MAPK pathway inhibition (ERK1/2, p38, and JNK) on rhIL-1β-stimulated Jurkat cells, was examined by culturing cells with ERK1/2-MAPK inhibitors PD98059 (Selleck Chemicals LLC, Houston, TX) (MEK1 and MEK2 (1, 5, 10 μm)), HA1077 (Sigma) (RISK2 (p90 ribosomal S6 kinase family of serine/threonine number 2) (1, 10, 20 μm)), p38 MAPK inhibitors SB203580 (Selleck Chemicals LLC) (p38α (1, 5, 10 μm)), SB202190 (Selleck Chemicals LLC) (p38β2 (1, 5, 10 μm)), or JNK-MAPK inhibitor SP600125 (Sigma) (MAPK 9-JNK (1, 10, 20 μm)). Cells were harvested at 0, 120, and 240 min. The experiments were repeated three times.
Western Blot (WB) Analysis and Quantification of Protein Bands
Cell lysate that contains 20 μg of protein in SDS-Laemmli loading buffer (7) per lane was fractionated by 12% SDS-PAGE using mini Protean 3 electrophoresis system (Bio-Rad). The proteins were transferred to a nitrocellulose membrane (Millipore). The filter was blocked in TBS (20 mm Tris base, pH 7.6, 150 mm NaCl) containing 5% nonfat milk (Bio-Rad), followed by incubation with the primary antibody goat polyclonal IgG (200 μg/ml) to human SRP72 epitope mapping near the C terminus of SRP72 of human origin (Santa Cruz Biotechnology, 1:2500), human SRP54 (Sigma) (1:2500), which represents the nonphosphorylated SRP protein and human GAPDH (Syd Labs, Boston) (1:3000). The filters were then incubated with HRP-conjugated rabbit anti-goat IgG antibody (Santa Cruz Biotechnology) and developed using ECL system (Thermo Fisher Scientific Inc.). The protein band was quantified using the Kodak 1D Imaging System, version 3.5 software, and expressed as relative units of area (RUA).
IP-WB Analysis
Lysates from Jurkat cells treated with different MAPK inhibitors and rhIL-1β (100 pg/ml) were immunoprecipitated as described previously (7). Briefly, 2 μl (2 μg) of anti-goat SRP19 antibody (Aviva Systems Biology LLC, San Diego) plus 2 μl (2 μg) of rabbit anti-goat IgG (Thermo Fisher Scientific Inc.) incubated with 30 μl of protein A-Sepharose beads (BioVision, Mountain View, CA) were used to immunoprecipitate the SRP complex that contained six polypeptides: 72, 68, 54, 21, 19, and 9 kDa. The IP-WB was performed using anti-phosphoserine antibodies (dilution 1:3000, Santa Cruz Biotechnology), followed by incubation with HRP-conjugated secondary antibody (Santa Cruz Biotechnology), and was developed by ECL (Thermo Fisher Scientific Inc.).
RNA Extraction and Real Time RT-Quantitative PCR
Jurkat cells (1 × 106) were treated in the same experimental conditions as described before. Total RNA extraction was carried out using the RNeasy kit for isolation of total RNA (QiagenTM, Germantown, MD) according to the manufacturer's instructions. Total RNA (1 μg) was reverse-transcribed to cDNA using Superscript II reverse transcriptase reagent and oligo(dT) (Invitrogen). The cycle included 5 min at 65 °C, 2 min at 37 °C, 50 min at 37 °C, and 15 min at 70 °C. cDNA obtained from the RT reaction was subjected to PCR using TaqMan Universal PCR master mix and ABI PRISM 7300 sequence detection system (Applied Biosystems, Foster City, CA). The primer and probe sequences and concentrations were optimized according to the manufacturer's guidelines in TaqMan Universal PCR master mix, protocol number HS00974354_g1, for human SRP72, containing FAMTM (fluorescent reporter dye 6-carboxyfluorescein, 518 nm) as reporter dye, and human GAPDH was obtained from TaqMan human GAPDH reagents kit protocol part 4326317E (Applied Biosystems, Foster City, CA), containing VIC® dye (552 nm) as reporter. PCR parameters were as follows: incubation at 50 °C for 2 min, incubation at 95 °C for 10 min, and then 40 cycles of denaturation at 95 °C for 15 s and annealing and extension at 60 °C for 1 min. Each sample was tested in duplicate. Relative mRNA levels of genes of interest were then calculated from the standard curve. The experiment was repeated three times and was analyzed with ABI prism software.
Effects of p38 and ERK1/2 MAPK Inhibitors on Proliferation of Jurkat Cells
Effects of p38 and ERK1/2 MAPK inhibitors on Jurkat cell proliferation were evaluated by counting the concentration of cells at time 0, 12, 24, 48, and 72 h of culture. SB203580 p38 inhibitor (Promega Corp.) and ERK1/2 inhibitor PD98059 (Selleck Chemicals LLC) were added at 1, 5, 10 μm concentration to Jurkat cells without stimulation with rhIL-β. ConA (5 μg/ml)-stimulated Jurkat cells in complete RPMI 1640 medium were used as a control. There was no toxic effect of inhibitors on cell viability nor on cell proliferation.
Statistical Analysis
Statistical analysis was performed by one-way analysis of variance with Dunnett's multiple comparisons test using Prism 5.0 for Windows (GraphPad). Differences were considered significant at p < 0.05.
RESULTS
Recombinant Human IL-1β Induces Overexpression of SRP72 in Jurkat Cells
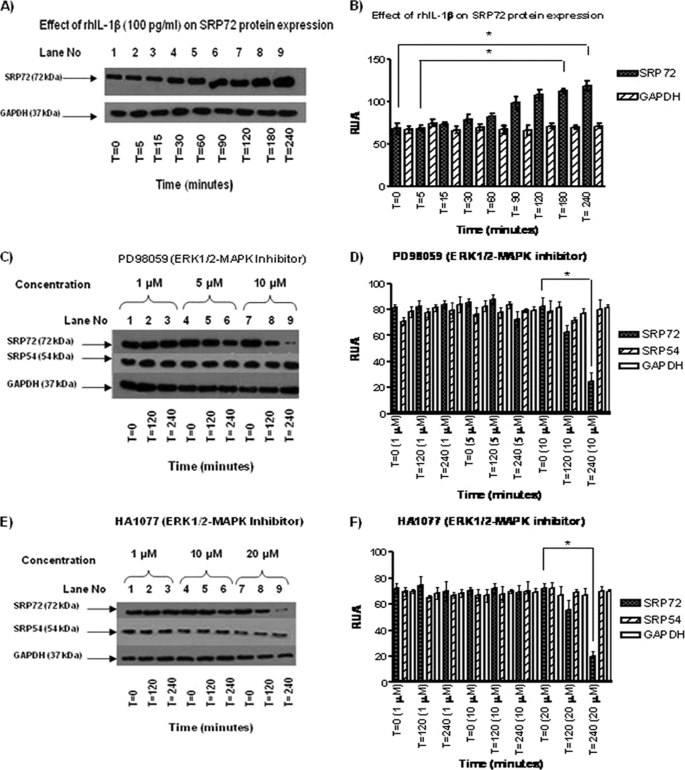
The SRP72 protein expression was increased between 90 and 240 min of rhIL-1β stimulation with a peak of expression at 240 min (Fig. 1A, lanes 6–9; corresponding values of RUA are shown in Fig. 1B, closed bars; 0 versus 240 and 5 versus 180 min, p < 0.05).
FIGURE 1.
A, effects of rhIL-1β on SRP72 protein expression. Jurkat cells were stimulated with rhIL-1β at different time points. WB was carried out using antibodies against human SRP72 and human GAPDH as control. We found an increase of expression of SRP72 protein at 90, 90 and 240 min (lanes 6–9), being statistically different at time 0 and 5 versus 240 and 180 min, respectively (p < 0.05). B, in contrast, GAPDH expression did not show any change. C, effect of ERK1/2 inhibitor PD98059 (1, 5, and 10 μm) on SRP72 protein expression was evaluated on Jurkat cells stimulated with rhIL-1β. The cells were harvested at 0, 120, and 240 min, and the protein expression was verified by WB using antibodies against human SRP72, human SRP54 (nonphosphorylated SRP protein), and human GAPDH as constitutive protein. A decreased SRP72 expression at 10 μm and 240 min was found. D, reduction of SRP72 expression was confirmed by RUA (closed bars) at 240 versus 0 min, 10 μm. E, effect of HA1077 (1, 10, 20 μΜ) on SRP72 protein expression by WB was evaluated at 0, 120, and 240 min. A decreased intensity of SRP72 band was found when using a concentration of 20 μm at 240 min. F, results were analyzed and RUA illustrated, finding significant results at 20 μm, 240 min (closed bars). * indicates p < 0.05. The experiments were repeated three times.
ERK1/2 PD98059 and HA1077 Reduced SRP72 Protein Expression
The effects of MAPK inhibitors ERK1/2 PD98059 and HA1077 on rhL-1β-stimulated Jurkat cells were evaluated by WB using antibodies against SRP72, SRP54 (nonphosphorylated form of SRP), and GAPDH (loading control). Reduction of SRP72 protein expression by MAPK inhibitor was apparent at 120–240 min after culturing with both PD98059 (10 μm, Fig. 1C) and HA1077 (20 μm, Fig. 1E). The reduction of SRP72 expression was confirmed by quantitative analysis as shown in RUA (Fig. 1, D and F). In contrast, expression of SRP54 and the constitutive protein GAPDH was not changed, indicating specific inhibition of SRP72 expression by these MAPK inhibitors.
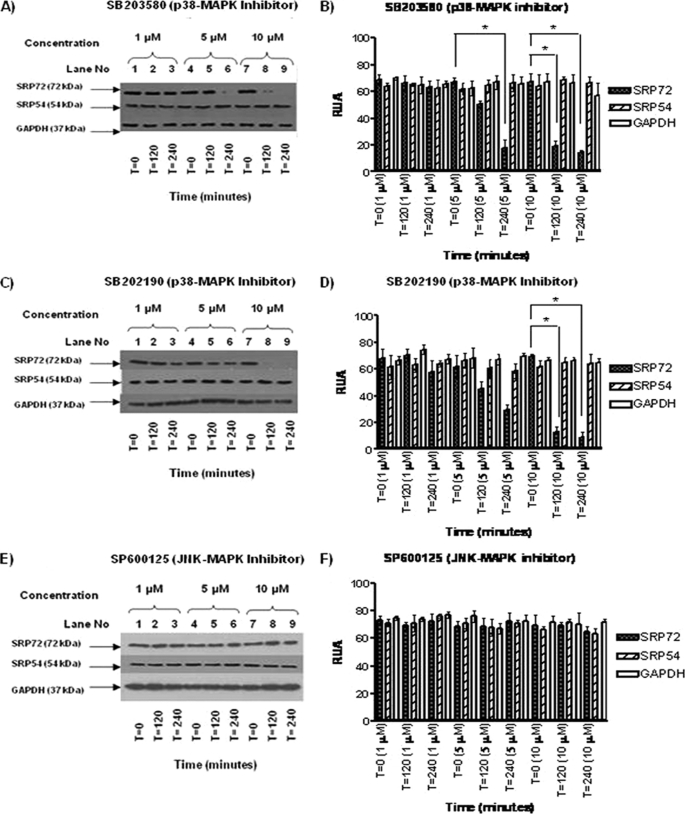
Reduced SRP72 Protein Expression by p38 MAPK Inhibitors but Not by JNK-MAPK Inhibitor
Next, the effect of p38 inhibitors SB203580 (Fig. 2A) and SB202190 (Fig. 2C) on SRP72, SRP54, and GAPDH protein expression was examined by WB. Both inhibitors induced a concentration-dependent reduction of SRP72 expression but did not affect SRP54 and GAPDH levels. 10 μm SB203580 and 10 μm SB202190 induced the maximum reduction of SRP72 expression (Fig. 2, A and C, respectively). A quantitative analysis by densitometry scanning (RUA) verified a statistically significant reduction of SRP72 expression by SB203580 (Fig. 2B) and SB202190 (p < 0.05, Fig. 2D). SRP54 and GAPDH expression was not affected, suggesting that the reduction of SRP72 by the MAPK inhibitors was not due to nonspecific protein degradation but rather the effects were specific for SRP72 protein expression. In contrast, JNK inhibitor SP600125 did not show any effect on expression of SRP72, SRP54, and GAPDH (Fig. 2, E and F).
FIGURE 2.
A, effects of p38 inhibitor SB203580 (1, 5, and 10 μm) on SRP72 protein expression was evaluated by WB using antibodies against human SRP72, SRP54, and GAPDH. A decreased intensity of SRP72 bands was noted when using the inhibitor at 5 μm concentration at 240 min (lane 6) and 10 μm at 120 and 240 min (lanes 8 and 9). B, results were analyzed and RUA illustrated, finding significant results at 5 μm, 240 versus 0 min, and 10 μm, 240 versus 0 and 120 versus 0 min, respectively, (p < 0.05). C, Jurkat cells with SB202190 at 1, 5, and 10 μm were tested, and a decreased SRP72 expression was found when using at 10 μm (lanes 8 and 9). D, results were analyzed and RUA illustrated, finding significant results at 10 μm at 240 versus 0 and 120 versus 0 min (p < 0.05). E, JNK inhibitor SP600125 (1, 5, and 10 μm, 0, 120, and 240 min); no difference in intensity of bands corresponding to SRP72, SRP54, and GAPDH was found. F, RUA of JNK inhibitor by WB was illustrated, without significant changes in protein expression of SRP72, SRP54, and GAPDH. The experiments were repeated three times.
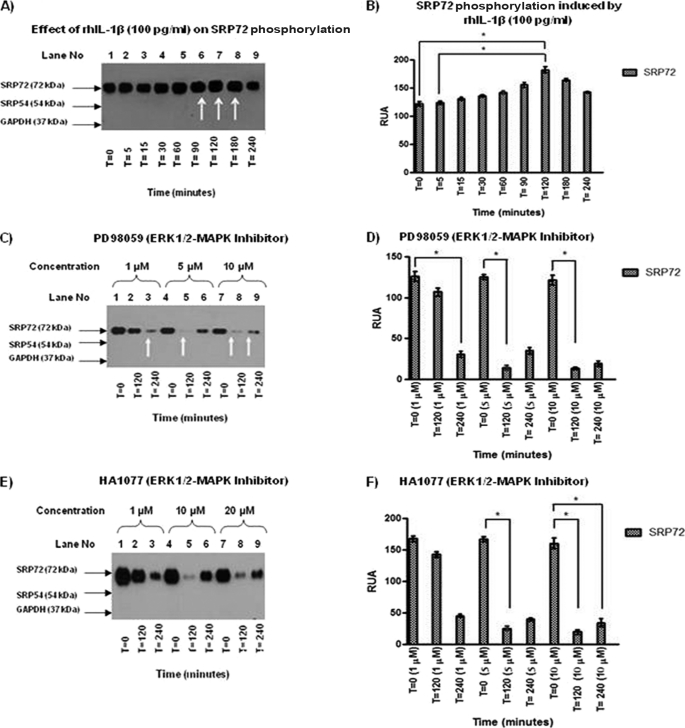
rhIL-1β Induces Serine Phosphorylation of SRP72 in Jurkat Cells
The effects of rhIL-1β on phosphorylation of SRP72 in Jurkat cells were evaluated by immunoprecipitating the SRP complex using anti-SRP19 antibodies followed by specific anti-phosphoserine antibodies in WB. An increased reactivity of SRP72 at 90–180 min after addition of rhIL-1β was observed, indicating rhIL-1β induced phosphorylation of SRP72 (Fig. 3A, lanes 6–8). RUA quantification of SRP72 band showed increased phosphorylation peaking at 120 (p < 0.05, Fig. 3B).
FIGURE 3.
A, IP of Jurkat cell lysates treated with rhIL-1β was carried out using anti-human SRP19 antibody because this is the only antibody able to immunoprecipitate the SRP complex. An anti-phosphoserine antibody was used to identify phosphorylated proteins. An increase of intensity in the SRP72 band at 90, 120, and 180 min (lanes 6–8) was found. B, results were analyzed and RUA illustrated with significant results at 120 versus 0 and versus 5 min, respectively, with p < 0.05. C, effect of ERK1/2 inhibitor PD98059 (1, 5, and 10 μm) was evaluated in SRP72-immunoprecipitated cell lysates. WB using anti-phosphoserine antibody showed a decreased SRP72 band when used as the inhibitor at concentrations of 1 μm at 240 min, 5 μm at 120 min, and 10 μm at 120 and 240 min (lanes 3, 5, 8, and 9). D, results were analyzed and RUA illustrated, finding significant results at 1 μm at 240 versus 0, 5 μm at 120 versus 0, and 10 μm at 120 versus 0 min. E, SRP72 immunoprecipitated from Jurkat cell lysate with anti-human SRP19 antibody was done to evaluate the effect of HA1077 ERK/12 inhibitor. WB using anti-phosphoserine antibody was done to identify phosphorylation status. We found a decreased intensity of SRP72 at concentration of 1 μm (120 min) and 10 and 20 μm (120 min). This finding was interesting because it suggests re-phosphorylation phenomena. F, RUA was illustrated, obtaining significant results at 5 μm at time 0 versus 120 min and 10 μm at 120 versus 0 and 240 versus 0 min (p < 0.05). * indicates p < 0.05. The experiments were repeated three times.
ERK1/2 Inhibitors (PD98059 and HA1077) Diminish the SRP72 Phosphorylation
Effects of inhibitors of ERK1/2 (PD98059 (Fig. 3, C and D) and HA1077 (Fig. 3, E and F) on IL-1β-induced serine phosphorylation of SRP72 were examined using immunoprecipitates by anti-SRP19 antibodies. These inhibitors dramatically reduced the SRP72 phosphorylation in a concentration-dependent manner (Fig. 3, C and E, respectively). Quantification of RUA of the corresponding SRP72 band showed significant reduction. All of were statistically significant at p < 0.05 (Fig. 3, D and F). The experiments were repeated three times with similar results.
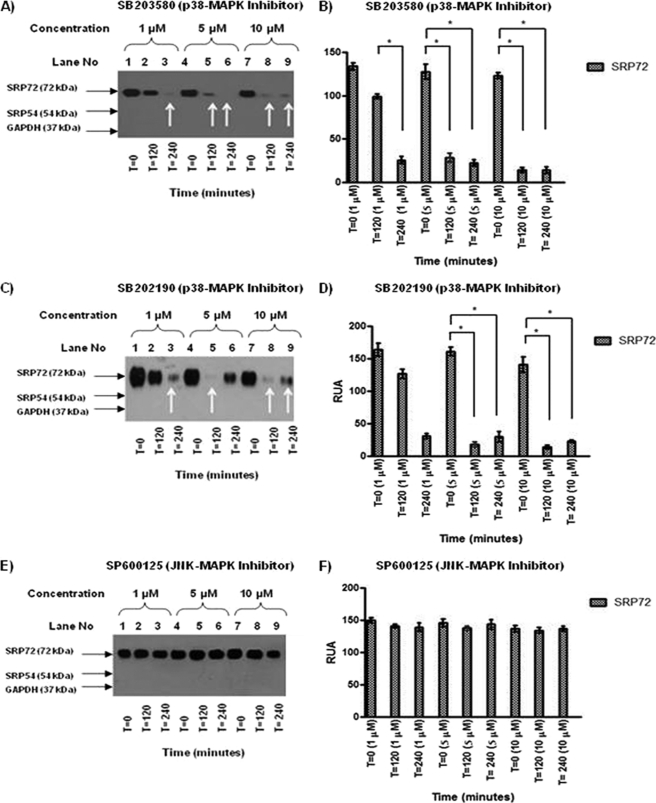
p38 MAPK Inhibitors Reduce SRP72 Phosphorylation
Effects of p38 MAPK inhibitors (SB203580 and SB202190) on serine phosphorylation of SRP72 were examined in Jurkat cells stimulated by rhIL-1β. Both inhibitors showed a concentration-dependent reduction of SRP72 phosphorylation (Fig. 4, A and C, *, p < 0.05). Quantification of the SRP72 phosphorylation confirmed statistically significant reduction of SRP72 serine phosphorylation (Fig. 4, B and D, *, p < 0.05). In contrast, phosphorylation of SRP54 was not observed, indicating an exclusive phosphorylation effect of SRP72 evaluated by anti-phosphoserine antibody.
FIGURE 4.
A, effect of p38 MAPK inhibitor SB203580 (1, 5, and 10 μm) on SRP72 phosphorylation was evaluated. Jurkat cells were stimulated with rhIL-1β, harvested at 0, 120, and 240 min, lysed, and immunoprecipitated using anti-human SRP19 and WB using anti-phosphoserine antibodies. A decreased intensity of SRP72 band was found when using the inhibitor at concentration of 1 μm at 240 min and 5 and 10 μm at 120 and 240 min. B, results were analyzed and RUA illustrated, finding significant results at 1 μm 240 versus 120, 5, and 10 μm at 120 versus 0 and 240 versus 0 min *, p < 0.05. C, effect of SB202190 (1, 5, and 10 μm) on SRP72 phosphorylation was tested. A decreased intensity of SRP72 expression when used at 1 μm (240 min) and 5 and 10 μm (120 and 240 min) was found. D, RUA illustrated obtaining significant results at concentration 5 and 10 μm at 120 versus 0 and 240 versus 0 min. * indicates p < 0.05. E, JNK inhibitor SP600125 (1, 5, and 10 μm) had no effect on the SRP72 intensity of bands by IP-WB using anti-phosphoserine antibody. F, results were analyzed and RUA illustrated confirming no effect of JNK inhibitors in the SRP72 expression. * indicates p < 0.05. The experiments were repeated three times.
Opposite the p38 MAPK inhibitors, JNK-MAPK pathway inhibitor SP600125 showed no effect on SRP72 phosphorylation in rhIL-1β-stimulated Jurkat cells (Fig. 4E). Quantification of the SRP72 protein band confirmed the results of IP-WB (Fig. 4F) as expected, because JNK is involved in tyrosine phosphorylation and not in serine phosphorylation as it occurs in SRP72.
Effects of MAPK Inhibitors on Proliferation and Apoptosis of Jurkat Cells
Results shown above indicated reduced SRP72 protein expression as well as reduced serine phosphorylation by MAPK inhibitors in rhIL-1β-stimulated Jurkat cells. One possible explanation is that these effects might be related to toxic effects of the MAPK inhibitors and, more specifically, induction of apoptosis or inhibition of cell proliferation. Therefore, the effects of MAPK inhibitors on cell proliferation and apoptosis were evaluated by proliferation cell growth curve and by flow cytometry analysis of forward and side scatter pattern of the cells. Jurkat cells were cultured with the inhibitors at a concentration used in the above experiments, and the cell growth was counted at 0, 12, 24, 48, and 72 h. No effects of reduced Jurkat cell proliferation was observed in the presence of MAPK inhibitors (data not shown). In addition, forward and side scatter analysis of cells after culturing with these inhibitors showed a normal distribution pattern (data not shown). Thus, at least MAPK inhibitors do not appear to induce significant apoptosis or reduction of cell proliferation in the conditions used in this study.
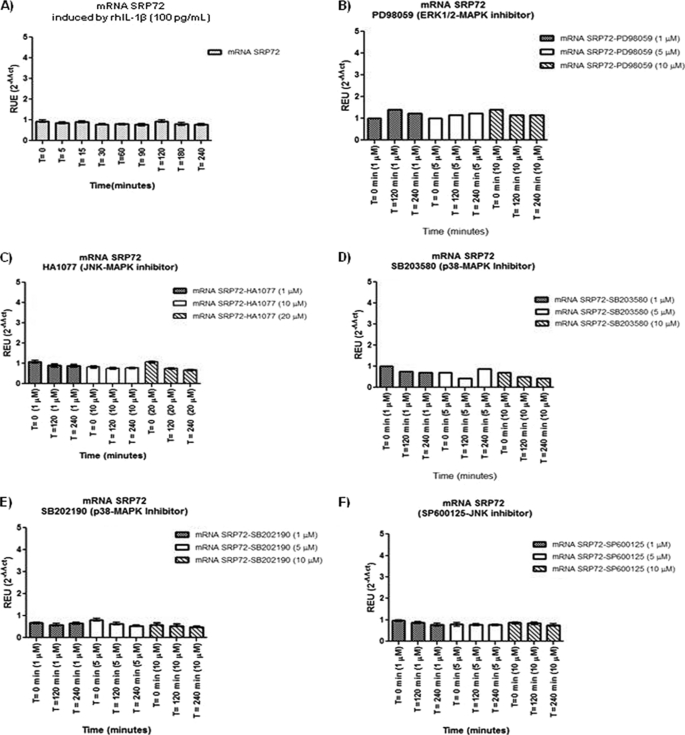
mRNA Expression of SRP72 Is Not Modified by Inhibitors of MAPK Pathway
Because ERK1/2 and p38 MAPK inhibitors reduced SRP72 protein expression in rhIL-1β-stimulated Jurkat cells, SRP72 mRNA was evaluated using quantitative RT-PCR. Jurkat cells were stimulated with rhIL-1β without inhibitor (Fig. 5A) or with ERK1/2 inhibitor PD98059 (Fig. 5B), JNK inhibitor HA1077 (Fig. 5C), p38 inhibitor SB203580 (Fig. 5D), p38 inhibitor SB202190 (Fig. 5E), or JNK inhibitor SP600125 (Fig. 5F). The results did not show significant changes on the mRNA expression of SRP72 mRNA after the treatment with these inhibitors, suggesting that the reduction of SRP72 protein expression is not mediated by down-regulation of SRP72 mRNA.
FIGURE 5.
We used real time RT-PCR to investigate the effects of rhIL-1β (A), PD98059 (B), HA1077 (C), SB203580 (D), SB202190 (E), and SP600125 (F) on SRP72 mRNA expression. All the samples were treated under the same experimental conditions used in protein tests done by WB, and phosphorylation was measured indirectly by IP-WB. The results did not show significant changes on the mRNA expression of SRP72 after the treatment with these inhibitors.
DISCUSSION
In Fig. 1, A and B, we showed induction of SRP72 by rhIL-1β after 90 min, peaking at 240 min. Interleukins are known to activate cells to exert many effects pro- or anti-inflammatory, angiogenesis, etc. MAPK kinase family is a key point on transcription factors such as NF-κβ (8, 9, 21, 22). All the pro-inflammatory factors mediated by this transcriptional factor will be translated through the rough ER (23), where SRP plays an important role (24). SRP72 is the only component in the SRP complex that can be phosphorylated. Our results confirmed that SRP72 was a phosphoprotein to which a serine residue is phosphorylated (25). The physiological and potential pathological implication of this phenomenon in production of autoantibodies to SRP72 is not known. We suggest a few possible applications of this reaction on the cell physiology as follows. 1) It has been described that a GTPase domain on the docking protein for SRP at the rough ER could be related to the release of the complex SRP-docking-ribosome-mRNA-translocon (5, 24, 26–28). 2) The phosphorylation of the SRP72 might increase the affinity of SRP for its receptor on the rough ER membrane and facilitate the rough ER translocation of protein (5, 23, 29–31). 3) The phosphorylation of SRP72 influence on the SRP complex activity in general because of the targeting of the protein by SRP does not requires GTPase activity (31–35).
A lot of work has to be done for the complete understanding of this phenomenon. The main function of the SRP complex is to attach the signal nascent polypeptide sequence, arrest the elongation, and finally, transport to the rough ER lumen the proteins that will be secreted to accomplish post-translational modifications. Indeed, the major role of SRP72 along SRP68 is to translocate proteins into the rough ER lumen (35, 36). Why, in inflammatory myopathies, SRP72 as well as the SRP54 subunits are the target of autoantibody production is not known. In 1998, it was reported that a serum from a patient with DM could immunoprecipitate the SRP complex from Jurkat cells metabolically labeled with [35S]methionine (36) or [32P]orthophosphate and that SRP72 is phosphorylated on a phosphoserine residue. This SRP72 subunit was the only phosphoprotein among the SRP components. A cleavage of SRP72 into a 66- and 6-kDa fragment after induction of apoptosis in Jurkat cells was shown. This apoptotic cleavage of autoantigens has been described as a possible mechanism of breaking tolerance against self-peptides on different autoimmune rheumatic diseases, so this is the first evidence related so far to an SRP72 subunit change once it is induced by apoptosis. We cannot assume that this might be the possible explanation as to why DM patients develop autoantibodies against SRP components.
The information about clinical characteristics of DM patients who are positive for antibodies against SRP54 is that they have an aggressive outcome with poor response to conventional therapies (11). The aim of this study was to evaluate the pathways of phosphorylation and overexpression of the SRP72 polypeptide, using an in vitro model of Jurkat cells stimulated by rhIL-1β in the presence of MAPK inhibitors. Our results showed that rhIL-1β causes up-regulation of protein expression and phosphorylation of SRP72 in Jurkat cells. Inhibitors of MAPK pathway ERK1/2 or p38α/β down-regulate the expression of SRP72 autoantigen in Jurkat cells stimulated by rhIL-1β. Studies in rheumatic diseases like rheumatoid arthritis and osteoarthritis have shown the important implication of IL-1β on the overexpression of cyclooxygenase-2 (COX-2), influencing the progression of the disease (30, 31). The increase of SRP72 influenced by IL1β might be involved in triggering tissue damage in the patients with polymyositis and DM (11).
Post-translational modifications such as hyperphosphorylation of proteins have been proposed as one of the mechanisms of breaking tolerance to some specific epitopes of autoantigens such as La/SSB in patients with systemic lupus erythematosus and Sjögren syndrome (37–39). Our results show that phosphorylation of SRP72 is increased by rhIL-1β. In summary, changes in phosphorylation status of some proteins that are potential autoantigens may unmask cryptic epitopes. Once the antigens in unconventional form are processed and presented on antigen presenting cells, an autoimmune response may be triggered (36). Previous studies reported that IL-1β is able of activate the MAPK pathway (39–43).
Our hypothesis is that MAPK inhibitors of p38α/β and ERK1/2, might influence the SRP72 autoantigen expression in Jurkat cells challenged by rhIL-1β. To validate our hypothesis, we included SRP54 antibody testing in the same conditions as we tested SRP72 subunit along with JNK inhibitors, showing that both SRP polypeptides and the GAPDH constitutive protein did not suffer any change when they were evaluated under the same experimental conditions (11). Additionally, we were able to show by cell counting for up to 72 h in a time course experiment that the exponential growth of Jurkat cells was not affected by MAPK inhibitors used in this study (data not shown). Lack of increased apoptosis by MAPK inhibitors was also confirmed by flow cytometry.
This demonstrates that our results are valid using the appropriate controls (SRP54 and GAPDH), and the results are reproducible. One caveat of our study is that rhIL-1β was not able to show an increase of SRP72 expression at 120 and 240 min in the presence of MAPK inhibitors.
Real time RT-PCR was done to measure the autoantigen of interest induced by rhIL-β in Jurkat cells tested with MAPK inhibitors, showing no effect on mRNA expression of the SRP72 gene, which could explain that the SRP72 and all the components of the SRP complex are synthesized and assembled inside the nucleus to do their functions in the cytoplasm (2, 35). Our results are similar to reports that have described that the levels of mRNA are not a reflection of the cellular protein activity in several physiological situations (43). SRP72 is a constitutive protein present in all eukaryotic organisms that is essential for survival; it is the unique organism that can survive after deletion of this SRP72 protein or other polypeptides of the SRP complex such as Saccharomyces cerevisiae (23), which demonstrates the importance of this protein inside the cellular mechanisms, mainly the translocation of secreted proteins.
Some studies show the use of mRNA expression patterns by themselves, however, is insufficient for understanding the expression of protein products, as additional post-transcriptional mechanisms, including protein translation, post-translational modification, and degradation, may influence the level of a protein present in a given cell or tissue (44, 45). These cases probably represent a situation where, in the cell, having significantly controlled the mRNA expression to produce a specific level of protein, the mechanisms to control the translation will probably not be employed. Alternatively, those proteins that have very low occupancy rates have uncorrelated mRNA and protein expression; thus, given that the cell has not tightly controlled the mRNA expression, it will dictate the resulting protein levels through rigorous controls of its translation (46). A second option for a general lack of correlation between mRNA and protein abundance may be that proteins have very different half-lives as the result of varied protein synthesis and degradation. Protein turnover can vary significantly depending on a number of different conditions; the cell can control the rates of degradation or synthesis for a given protein, and there is significant heterogeneity even within proteins that have similar functions (47, 48).
Experimental therapy using inhibitors of the MAPK pathway for the treatment of rheumatoid arthritis has been under development; the anti-inflammatory and anti-erosive effects in experimental models of arthritis, beside the relief of inflammatory pain, are shown (41, 49). Our results highlight the importance of studying the pathways of activation and overexpression of autoantigens. It will be necessary to perform careful research on various kinase pathways, including MAPK in DM and other rheumatic diseases, to help explain the routes of activation and inhibition of autoantigens. The understanding of this process may help develop the design of new therapies to prevent and control the loss of the tolerance toward our own proteins.
This work was supported by COECyTJAL Grant PS-2009-420 (to M. V.-D. M.).
- ER
- endoplasmic reticulum
- RUA
- relative units of area
- IP-WB
- immunoprecipitation-Western blot
- rhIL
- recombinant human IL
- DM
- dermatomyositis
- SRP
- signal recognition particle.
REFERENCES
- 1.Bui N., Strub K. (1999) Biol. Chem. 380, 135–145 [DOI] [PubMed] [Google Scholar]
- 2.Nagai K., Oubridge C., Kuglstatter A., Menichelli E., Isel C., Jovine L. (2003) EMBO J. 22, 3479–3485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buskiewicz I. A., Jöckel J., Rodnina M. V., Wintermeyer W. (2009) RNA 15, 44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fulga T. A., Sinning I., Dobberstein B., Pool M. R. (2001) EMBO J. 20, 2338–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang Y., Cheng Z., Mandon E. C., Gilmore R. (2008) J. Cell Biol. 180, 1149–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter T. (1995) Cell 80, 225–236 [DOI] [PubMed] [Google Scholar]
- 7.Manning G., Plowman G. D., Hunter T., Sudarsanam S. (2002) Biochem. Sci. 27, 514–520 [DOI] [PubMed] [Google Scholar]
- 8.Seger R., Krebs E. G. (1995) FASEB J. 9, 726–735 [PubMed] [Google Scholar]
- 9.Chen R. E., Thorner J. (2007) Biochem. Biophys. Acta 1773, 1311–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao Y., Xu Q., Kwon M. J., Matta R., Liu Y., Hong S. C., Chang C. H. (2006) J. Immunol. 177, 70–76 [DOI] [PubMed] [Google Scholar]
- 11.Kamachi M., Le T. M., Kim S. J., Geiger M. E., Anderson P., Utz P. J. (2002) J. Exp. Med. 196, 1213–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imajo M., Tsuchiya Y., Nishida E. (2006) IUBMB Life 58, 312–317 [DOI] [PubMed] [Google Scholar]
- 13.Casciola-Rosen L., Andrade F., Ulanet D., Wong W. B., Rosen A. (1999) J. Exp. Med. 190, 815–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Paepe B., Creus K. K., De Bleecker J. L. (2008) Front. Biosci. 13, 2548–2577 [DOI] [PubMed] [Google Scholar]
- 15.Liang K. C., Lee C. W., Lin W. N., Lin C. C., Wu C. B., Luo S. F., Yang C. M. (2007) J. Cell. Physiol. 211, 759–770 [DOI] [PubMed] [Google Scholar]
- 16.Sheryanna A., Bhangal G., McDaid J., Smith J., Manning A., Foxwell B. M., Feldmann M., Cook H. T., Pusey C. D., Tam F. W. (2007) J. Am. Soc. Nephrol. 18, 1167–1179 [DOI] [PubMed] [Google Scholar]
- 17.Arana-Argaez V. E., Martínez-Garía E. A., Martín-Marquez B. T., Muñoz-Valle J. F., Garcia-Iglesias T., Delgado-Rizo V., Vazquez-Del Mercado M. (2009) Bioquimia 34, 77–82 [Google Scholar]
- 18.De Paepe B., Creus K. K., De Bleecker J. L. (2009) Curr. Opin. Rheumatol. 21, 610–616 [DOI] [PubMed] [Google Scholar]
- 19.Yang H. T., Cohen P., Rousseau S. (2008) Cell. Signal. 20, 375–380 [DOI] [PubMed] [Google Scholar]
- 20.Bradford M. M. (1976) Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 21.Patel D. N., King C. A., Bailey S. R., Holt J. W., Venkatachalam K., Agrawal A., Valente A. J., Chandrasekar B. (2007) J. Biol. Chem. 282, 27229–27238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dodeller F., Schulze-Koops H. (2006) Arthritis Res. Ther. 8, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lakkaraju A. K., Mary C., Scherrer A., Johnson A. E., Strub K. (2008) Cell 133, 440–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lingappa V. R., Katz F. N., Lodish H. F., Blobel G. (1978) J. Biol. Chem. 253, 8667–8670 [PubMed] [Google Scholar]
- 25.Utz P. J., Hottelet M., Le T. M., Kim S. J., Geiger M. E., van Venrooij W. J., Anderson P. (1998) J. Biol. Chem. 273, 35362–35370 [DOI] [PubMed] [Google Scholar]
- 26.Connolly T., Gilmore R. (1993) J. Cell Biol. 123, 799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Römisch K., Miller F. W., Dobberstein B., High S. (2006) Arthritis Res. Ther. 8, R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shan S. O., Chandrasekar S., Walter P. (2007) J. Cell Biol. 178, 611–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rapiejko P. J., Gilmore R. (1992) J. Cell Biol. 117, 493–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Migliaccio G., Nicchitta C. V., Blobel G. (1992) J. Cell Biol. 117, 15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rapiejko P. J., Gilmore R. (1994) Mol. Biol. Cell. 5, 887–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siu F. Y., Spanggord R. J., Doudna J. A. (2007) RNA 13, 240–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lütcke H. (1995) Eur. J. Biochem. 228, 531–550 [DOI] [PubMed] [Google Scholar]
- 34.Shyu A. B., Wilkinson M. F., van Hoof A. (2008) EMBO J. 27, 471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel V., Walter P. (1988) Cell 52, 39–49 [DOI] [PubMed] [Google Scholar]
- 36.Utz P. J., Anderson P. (1998) Arthritis Rheum. 41, 1152–1160 [DOI] [PubMed] [Google Scholar]
- 37.Rutjes S. A., Utz P. J., van der Heijden A., Broekhuis C., van Venrooij W. J., Pruijn G. J. (1999) Cell Death Differ. 6, 976–986 [DOI] [PubMed] [Google Scholar]
- 38.Utz P. J., Gensler T. J., Anderson P. (2000) Arthritis Res. 2, 101–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell M. D., Laird R. E., Brown R. D., Long C. S. (2007) Am. J. Physiol. Heart Circ. Physiol. 292, H1139–H1147 [DOI] [PubMed] [Google Scholar]
- 40.Inoue T., Boyle D. L., Corr M., Hammaker D., Davis R. J., Flavell R. A., Firestein G. S. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 5484–5489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barksby H. E., Lea S. R., Preshaw P. M., Taylor J. J. (2007) Clin. Exp. Immunol. 149, 217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies S. P., Reddy H., Caivano M., Cohen P. (2000) Biochem J. 351, 95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson P. (2008) Nat. Immunol. 9, 353–359 [DOI] [PubMed] [Google Scholar]
- 44.Giordano T. J., Shedden K. A., Schwartz D. R., Kuick R., Taylor J. M., Lee N., Misek D. E., Greenson J. K., Kardia S. L., Beer D. G., Rennert G., Cho K. R., Gruber S. B., Fearon E. R., Hanash S. (2001) Am. J. Pathol. 159, 1231–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barksby H. E., Hui W., Wappler I., Peters H. H., Milner J. M., Richards C. D., Cawston T. E., Rowan A. D. (2006) Arthritis Rheum. 54, 540–550 [DOI] [PubMed] [Google Scholar]
- 46.Glickman M. H., Ciechanover A. (2002) Physiol. Rev. 82, 373–428 [DOI] [PubMed] [Google Scholar]
- 47.Pratt J. M., Petty J., Riba-Garcia I., Robertson D. H., Gaskell S. J., Oliver S. G., Beynon R. J. (2002) Mol. Cell. Proteomics 1, 579–591 [DOI] [PubMed] [Google Scholar]
- 48.Gerner C., Vejda S., Gelbmann D., Bayer E., Gotzmann J., Schulte-Hermann R., Mikulits W. (2002) Mol. Cell. Proteomics 1, 528–537 [DOI] [PubMed] [Google Scholar]
- 49.Saklatvala J. (2004) Curr. Opin. Pharmacol. 4, 372–377 [DOI] [PubMed] [Google Scholar]