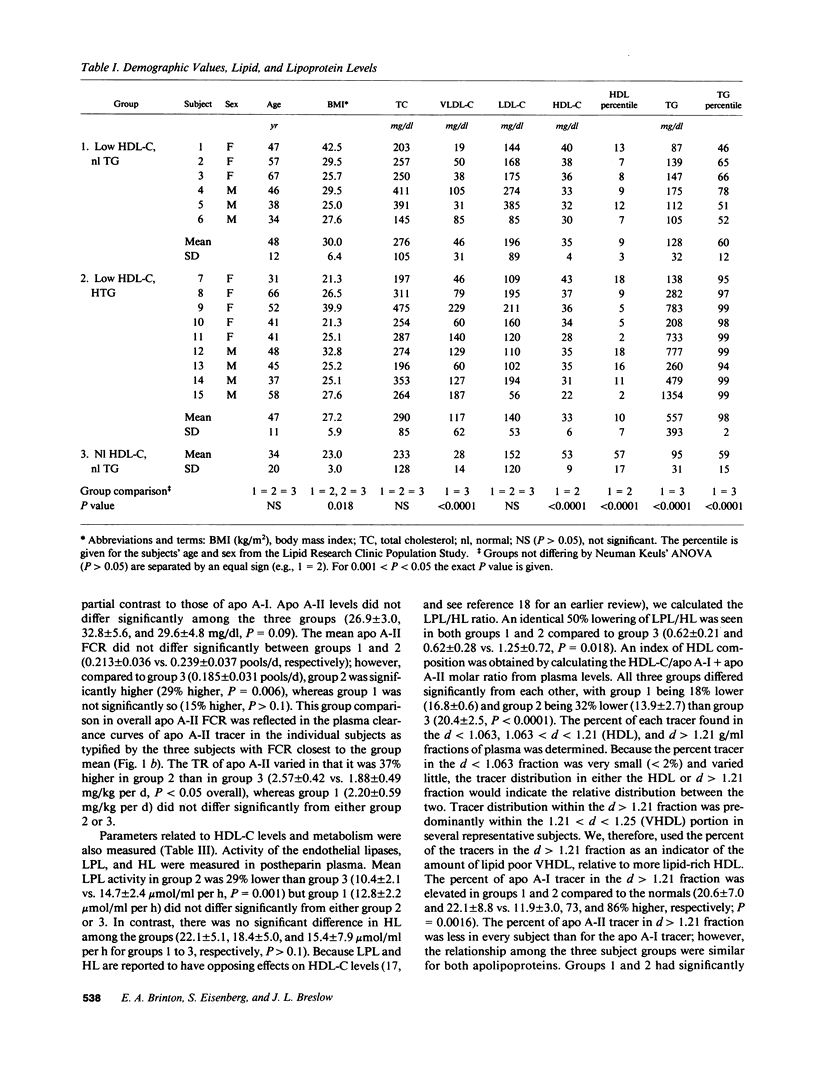
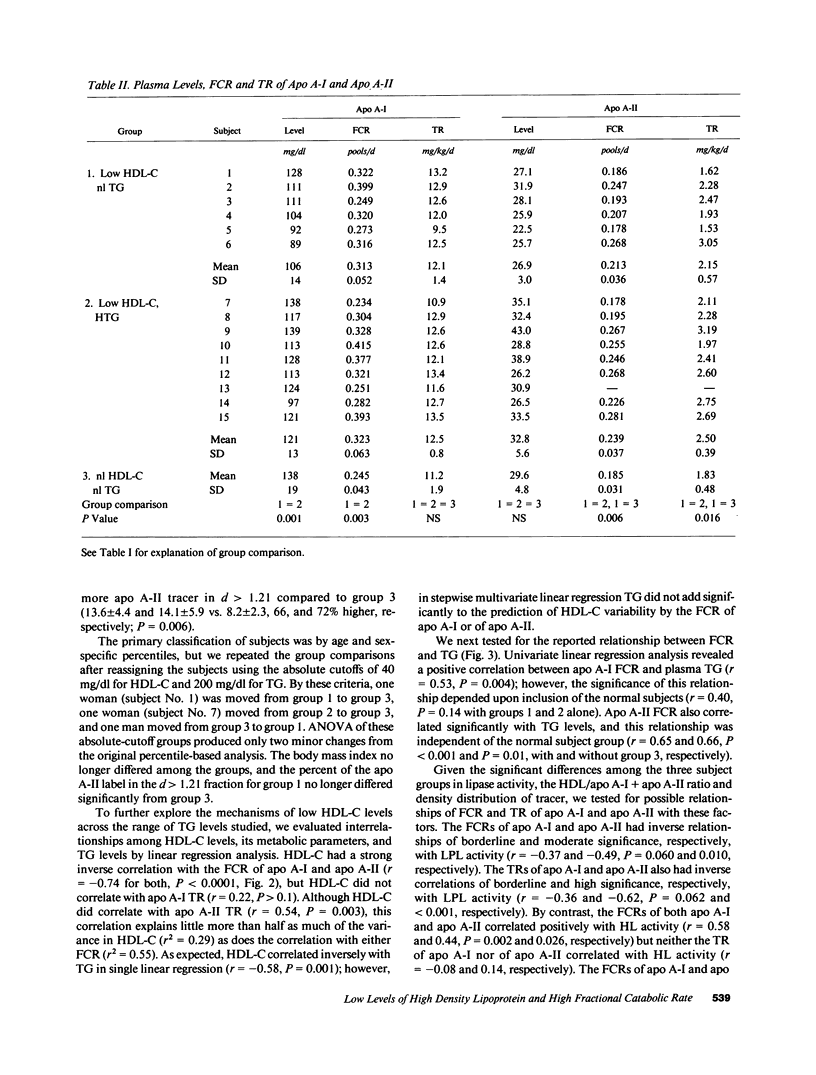
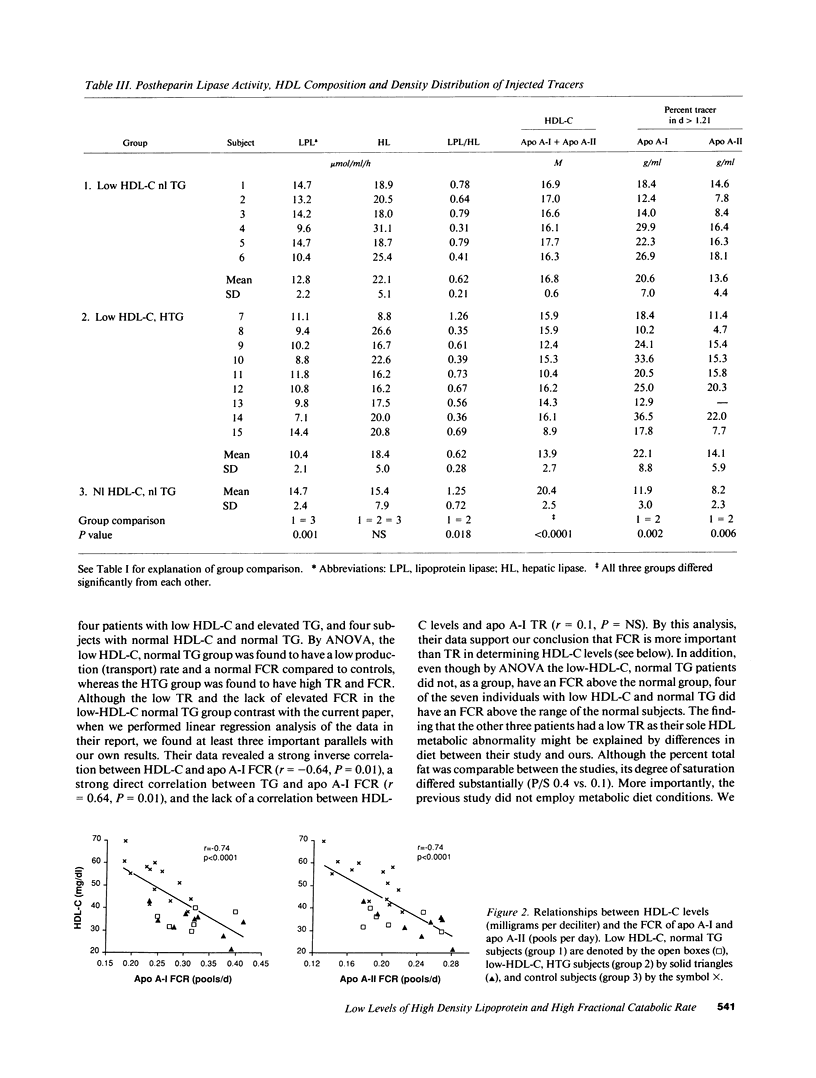
Abstract
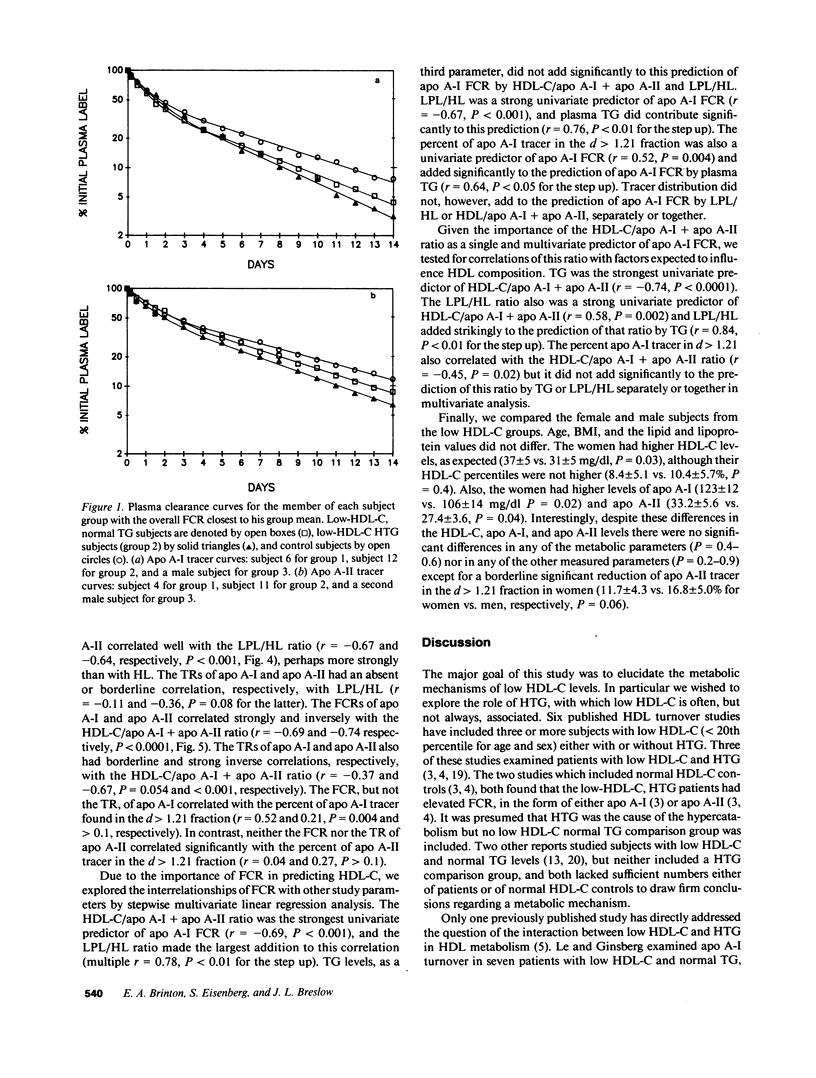
Low HDL-cholesterol (HDL-C) levels may elevate atherosclerosis risk, and often associate with hypertriglyceridemia (HTG); however, the metabolic causes of low HDL-C levels with or without HTG are poorly understood. We studied the turnover of radioiodinated HDL apolipoproteins, apo A-I and apo A-II, in 15 human subjects with low HDL-C, six with normal plasma TG levels (group 1) and nine with high TG (group 2), and compared them to 13 control subjects with normal HDL-C and TG levels (group 3). The fractional catabolic rate (FCR) was equally elevated in groups 1 and 2 vs. group 3 for both apo A-I (0.313 +/- 0.052 and 0.323 +/- 0.063 vs. 0.245 +/- 0.043 pools/d, P = 0.003) and apo A-II (0.213 +/- 0.036 and 0.239 +/- 0.037 vs. 0.185 +/- 0.031 pools/d, P = 0.006). Thus, high FCR characterized low HDL-C regardless of the presence or absence of HTG. In contrast, transport rate (TR) of apo A-I did not differ significantly among the groups and the apo A-II TR differed only between groups 2 and 3 (2.15 +/- 0.57, 2.50 +/- 0.39, and 1.83 +/- 0.48 mg/kg per d for groups 1 to 3, respectively, P = 0.016). Several HDL-related factors were similar in groups 1 and 2 but differed in group 3, as with FCR, including the ratio of lipoprotein lipase to hepatic lipase activity (LPL/HL) in post-heparin plasma, the ratio of the HDL-C to apo A-I plus apo A-II levels, and the percent of tracer in the d greater than 1.21 fraction. In linear regression analysis HDL-C levels correlated inversely with the FCR of apo A-I and apo A-II (r = -0.74, P less than 0.0001 for both). Major correlates of FCR were HDL-C/apo A-I + apo A-II, LPL/HL, and plasma TG levels. We hypothesize that lipase activity and plasma TG affect HDL composition which modulates FCR, which in turn regulates HDL-C. Thus, HTG is only one of several factors which may contribute to elevated FCR and low HDL-C. Given the relationship of altered HDL composition with high FCR and low HDL-C levels, factors affecting HDL composition may increase atherosclerosis susceptibility.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blum C. B., Levy R. I., Eisenberg S., Hall M., 3rd, Goebel R. H., Berman M. High density lipoprotein metabolism in man. J Clin Invest. 1977 Oct;60(4):795–807. doi: 10.1172/JCI108833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton E. A., Eisenberg S., Breslow J. L. A low-fat diet decreases high density lipoprotein (HDL) cholesterol levels by decreasing HDL apolipoprotein transport rates. J Clin Invest. 1990 Jan;85(1):144–151. doi: 10.1172/JCI114405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton E. A., Eisenberg S., Breslow J. L. Elevated high density lipoprotein cholesterol levels correlate with decreased apolipoprotein A-I and A-II fractional catabolic rate in women. J Clin Invest. 1989 Jul;84(1):262–269. doi: 10.1172/JCI114149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung M. C., Albers J. J. Distribution of cholesterol and apolipoprotein A-I and A-II in human high density lipoprotein subfractions separated by CsCl equilibrium gradient centrifugation: evidence for HDL subpopulations with differing A-I/A-II molar ratios. J Lipid Res. 1979 Feb;20(2):200–207. [PubMed] [Google Scholar]
- Cheung M. C., Albers J. J. The measurement of apolipoprotein A-I and A-II levels in men and women by immunoassay. J Clin Invest. 1977 Jul;60(1):43–50. doi: 10.1172/JCI108767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S. High density lipoprotein metabolism. J Lipid Res. 1984 Oct;25(10):1017–1058. [PubMed] [Google Scholar]
- Kuusi T., Ehnholm C., Viikari J., Härkönen R., Vartiainen E., Puska P., Taskinen M. R. Postheparin plasma lipoprotein and hepatic lipase are determinants of hypo- and hyperalphalipoproteinemia. J Lipid Res. 1989 Aug;30(8):1117–1126. [PubMed] [Google Scholar]
- Le N. A., Ginsberg H. N. Heterogeneity of apolipoprotein A-I turnover in subjects with reduced concentrations of plasma high density lipoprotein cholesterol. Metabolism. 1988 Jul;37(7):614–617. doi: 10.1016/0026-0495(88)90077-7. [DOI] [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- Mahalko J. R., Johnson L. K. Accuracy of predictions of long-term energy needs. J Am Diet Assoc. 1980 Nov;77(5):557–561. [PubMed] [Google Scholar]
- Malmendier C. L., Delcroix C., Ameryckx J. P. In vivo metabolism of human apoprotein A-I-phospholipid complexes. Comparison with human high density lipoprotein-apoprotein A-I metabolism. Clin Chim Acta. 1983 Jul 15;131(3):201–210. doi: 10.1016/0009-8981(83)90089-x. [DOI] [PubMed] [Google Scholar]
- Melchior G. W., Castle C. K., Vidmar T. J., Polites H. G., Marotti K. R. Apo A-I metabolism in cynomolgus monkeys: male-female differences. Biochim Biophys Acta. 1990 Mar 12;1043(1):97–105. doi: 10.1016/0005-2760(90)90115-e. [DOI] [PubMed] [Google Scholar]
- Miller G. J., Miller N. E. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. 1975 Jan 4;1(7897):16–19. doi: 10.1016/s0140-6736(75)92376-4. [DOI] [PubMed] [Google Scholar]
- Packard C. J., Stewart J. M., Third J. L., Morgan H. G., Lawrie T. D., Shepherd J. Effects of nicotinic acid therapy on high-density lipoprotein metabolism in type II and type IV hyperlipoproteinaemia. Biochim Biophys Acta. 1980 Apr 18;618(1):53–62. doi: 10.1016/0005-2760(80)90053-3. [DOI] [PubMed] [Google Scholar]
- Rao S. N., Magill P. J., Miller N. E., Lewis B. Plasma high-density lipoprotein metabolism in subjects with primary hypertriglyceridaemia: altered metabolism of apoproteins AI and AII. Clin Sci (Lond) 1980 Nov;59(5):359–367. doi: 10.1042/cs0590359. [DOI] [PubMed] [Google Scholar]
- Saku K., Gartside P. S., Hynd B. A., Mendoza S. G., Kashyap M. L. Apolipoprotein AI and AII metabolism in patients with primary high-density lipoprotein deficiency associated with familial hypertriglyceridemia. Metabolism. 1985 Aug;34(8):754–764. doi: 10.1016/0026-0495(85)90027-7. [DOI] [PubMed] [Google Scholar]
- Schaefer E. J., Zech L. A., Jenkins L. L., Bronzert T. J., Rubalcaba E. A., Lindgren F. T., Aamodt R. L., Brewer H. B., Jr Human apolipoprotein A-I and A-II metabolism. J Lipid Res. 1982 Aug;23(6):850–862. [PubMed] [Google Scholar]
- Zech L. A., Schaefer E. J., Bronzert T. J., Aamodt R. L., Brewer H. B., Jr Metabolism of human apolipoproteins A-I and A-II: compartmental models. J Lipid Res. 1983 Jan;24(1):60–71. [PubMed] [Google Scholar]



