Abstract
The mechanism by which regulatory T (Treg) cells suppress the immune response is not well defined. A recent study has shown that β-catenin prolongs Treg cell survival. Because β-catenin is regulated by glycogen synthase kinase 3β (GSK-3β)-directed phosphorylation, we focused on GSK-3β and the role it plays in Treg cell function. Inhibition of GSK-3β led to increased suppression activity by Treg cells. Inhibitor-treated Treg cells exhibited prolonged FoxP3 expression and increased levels of β-catenin and of the antiapoptotic protein Bcl-xL. Systemic administration of GSK-3β inhibitor resulted in prolonged islet survival in an allotransplant mouse model. Our data suggest that GSK-3β could be a useful target in developing strategies designed to increase the stability and function of Treg cells for inducing allotransplant tolerance or treating autoimmune conditions.
Keywords: Glycogen Synthase Kinase 3, Immunology, Immunosuppressor, Lymphocyte, Signal Transduction
Introduction
Regulatory T (Treg)5 cells are charged with the important task of maintaining homeostatic T cell reactivity and are thought to prevent autoimmunity by restraining self-reactive T cells that escape thymic deletion (1, 2). Moreover, Treg cells have been shown to down-regulate all T cell-driven immunity (3, 4), which is of increasing interest in the transplant community. However, expansion of Treg cells has proved challenging because of their anergic potential. Although some studies have shown induction of CD4+CD25+ Treg cells from CD4+CD25− cells with various exogenous cytokines or peptides (5–7), their functional profile may not be the same as that of naturally occurring CD4+CD25+ Treg cells (8). This difference has given impetus to those searching for a method to improve CD4+CD25+ Treg cell stability and activity.
Recent work shows that β-catenin may play a central role in Treg cell stabilization. Ding et al. (9) have shown that ectopic expression of a mutated form of β-catenin that lacks the classic Ser-33/Ser-37/Thr-41/Ser-45 N-terminal amino acids and is therefore nondegradable was able to enhance Treg cell survival in vitro. β-Catenin is best known for its role in Wnt signaling, a pathway that governs patterning and cell fate determination during embryogenesis (10, 11). β-Catenin is regulated by the serine/threonine kinase glycogen synthase kinase 3β (GSK-3β). Phosphorylation of β-catenin by GSK-3β targets β-catenin for ubiquitination and degradation, terminating the signal (11). In contrast, inhibiting GSK-3β activity with Li+ or other GSK-3β inhibitors or by expressing a dominant inhibitory mutant of GSK-3β leads to a significant increase in β-catenin levels (12). When cellular levels of β-catenin rise, β-catenin interacts with transcription factors of the high mobility group box family, LEF-1 and T cell factor; enters the nucleus; and regulates induction of genes believed to be involved in the proliferative response (13). It is this downstream activation of cellular growth that has made β-catenin especially interesting to immunologists searching for immunosuppressive targets. Recently, manipulation of the Wnt/β-catenin pathway has been shown to alter T cell expansion and their cytokine profile (14–17). This association between β-catenin and T cell proliferation has prompted strong query into the action of β-catenin in Treg cells.
By utilizing the GSK-3β inhibitor SB216763, we examined what role GSK-3β plays in Treg cell activity. We also suggest a possible mechanism by which SB216763 may act on increasing Treg cell suppressive function. Treg cells treated with SB216763 were found to maintain elevated levels of FoxP3 protein and the antiapoptotic protein Bcl-xL as compared with the untreated controls. Given the possible overt relationship between SB216763 and prevention of apoptosis in Treg cells, we also examined what effects DEVD-CHO, a caspase-3 inhibitor, had on Treg cell function. Caspase-3 inhibitor, when used alone, resulted in an increase in Treg cell suppression activity. When used in combination with GSK-3β inhibitor, we observed no further augmentation in this suppressive effect, suggesting that the two inhibitors may be acting on the same or parallel pathways. Additionally, when SB216763 was given systemically in an islet allotransplant mouse model, islet allograft survival was observed. Nevertheless, SB216763 and drugs similar in function provide obvious laboratory and clinical advantages in defining the role of Treg cell activity in models for autoimmunity and transplantation.
EXPERIMENTAL PROCEDURES
Animals
C57BL/6 (designated B6 for clarity) (H-2b), BALB/c (H-2d), and C3H (H-2k) mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). All mice were kept in pathogen-free, filter top isolator cages. All animals were cared for according to methods prescribed by the American Association for the Accreditation of Laboratory Animal Care. All protocols were approved by the Institutional Committee for Research Animal Care.
CD4+CD25− Effector T Cell and CD4+CD25+ Treg Cell Isolation
Single cell suspensions were prepared from B6 and BALB/c mice using standard methods. After a brief erythrocyte lysis using Red Blood Cell Lysing Buffer (Sigma-Aldrich), the cells were resuspended in degassed PBS with 0.5% BSA and 2 mm EDTA. Separation of CD4+CD25− and CD4+CD25+ cells was accomplished with magnetic labeling using a MACS CD4+CD25+ Treg Cell Isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). CD4+CD25− cells were negatively selected with the flow-through fraction, whereas the CD4+CD25+ cells were positively selected. The purity of CD4+CD25+ fractions was confirmed by fluorescence-activated cell sorting (FACS) analysis because anti-CD25-PE antibody was used for purification. The final purity of the cell population was 90–95% (data not shown).
In Vitro Suppression Assay
CD4+CD25+ Treg cells were co-cultured with CD4+CD25− T cells stimulated with anti-CD3/CD28 Dynabeads (Invitrogen) with 5 μm SB216763 (Sigma-Aldrich) or with 0.05% DMSO (vehicle control). CD4+CD25+ Treg cells were added in increasing ratios to CD4+CD25− T cells for dose-response measurements. Proliferation was measured in triplicates by the incorporation of tritiated thymidine over the last 18–20 h of the co-culture. All cells were cultured in complete RPMI 1640 medium. The DEVD-CHO CPP32/apopain inhibitor (Calbiochem) was used at 10 and 100 μm.
Flow Cytometry
Flow cytometry was accomplished using a BD FACScan flow cytometer (BD Biosciences) and was performed on cells incubated with 0.5 μg of PE-Cy5-conjugated anti-mouse CD4 (L3T4) antibody (BD Pharmingen) for 30 min at 4 °C. Cells were then washed and permeabilized with 0.2% Igepal CA-630 (Sigma-Aldrich) and stained intracellularly with 0.5 μg of antibody for 30 min at room temperature with FITC-conjugated anti-mouse FoxP3 (FJK-16s) (eBioscience), anti-mouse GSK-3β (Tyr(P)-216) (Cell Signaling Technology), anti-mouse β-catenin (Ser(P)-33/Ser(P)-37/Thr(P)-41) (27C10) (Cell Signaling Technology), anti-mouse β-catenin (H-102) (Santa Cruz Biotechnology), or anti-mouse Bcl-xL (Santa Cruz Biotechnology). Secondary goat anti-rabbit Alexa Fluor 488-conjugated antibody was purchased from Invitrogen.
Western Blot Analysis
Column-purified CD4+CD25+ cells were plated at 400,000 cells/well in a 96-well plate and incubated in the absence or presence of 10 μm SB216763 (Sigma-Aldrich). Cells were counted and lysed in a volume of 1× SDS loading buffer (Bio-Rad), proportionate to the number of cells, at 42 and 66 h. To evaluate Bcl-2 levels, proportionate samples were loaded on a 10% sodium dodecyl sulfate-polyacrylamide gel, electrophoresed, and transferred onto Immobilon-P membrane (Millipore). Western blotting was performed as described previously (18) by using anti-Bcl-2 antibodies (1:1,000; Cell Signaling Technology). As a loading control, the immunoblot was stripped and reprobed with anti-β-actin antibody (1:1,000; Cell Signaling Technology).
Mouse Islet Isolation and Transplantation
In this in vivo model, B6 mice were used as recipients, and C3H mice were used as islet donors. Prior to islet transplantation, B6 mice were rendered diabetic by intraperitoneal injections of 200 mg/kg streptozotocin (Sigma-Aldrich). Diabetes was defined as blood glucose levels >200 mg/dl for at least 2 consecutive days. Islets from C3H mice were isolated by the standard technique of collagenase digestion and Ficoll purification. Following isolation, 500 fresh islets were transplanted under the kidney capsule of diabetic B6 mice treated with saline containing 1% DMSO in the control group or with daily intraperitoneal injections of SB216763 (100 μm) in the experimental group. Euglycemia was defined as a non-fasting blood glucose level <200 mg/dl. Rejection was diagnosed when animals became hyperglycemic again with blood glucose >200 mg/dl for at least 2 consecutive days. For Treg cell depletion studies, mice were injected intraperitoneally with 250 μg of PC61 anti-CD25 (BioXCell, Lebanon, NH) on days −8 and −3 prior to islet transplantation. On the day of transplant, peripheral blood was analyzed with GK1.5 anti-CD4 and 7D4 anti-CD25 (eBioscience) to demonstrate Treg cell depletion.
Statistical Analyses
Data are presented as means ± S.E. Where indicated, we determined the statistical significance between two groups by the log rank test (Mantel-Cox test). Values of p < 0.05 were considered statistically significant.
RESULTS
Naïve CD4+CD25+FoxP3+ Treg Cells Exhibit Elevated GSK-3β Activity and Increased β-Catenin Phosphorylation in Vitro
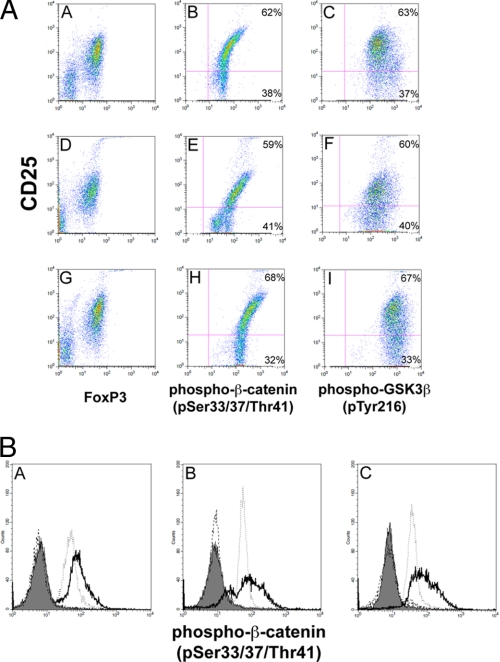
Activated GSK-3β is characterized by phosphorylation of Tyr-216. In Fig. 1, A and B, we analyzed by FACS isolated, column-purified, CD25-PE-labeled B6 splenic naïve CD4+CD25+ cells for the presence of phospho-Tyr-216 and showed that CD4+CD25+hi cells exhibited the greatest staining with phospho-Tyr-216 antibody (Fig. 1A, panels C, F, and I). GSK-3β phosphorylates β-catenin on serines 33/37 and threonine 41, resulting in the degradation of β-catenin. Using antibodies specific to phospho-β-catenin (Ser-33/37 and Thr-41), we show that CD4+CD25+hi cells also displayed the greatest phospho-β-catenin (Ser-33/37 and Thr-41) staining as revealed by FACS analysis (Fig. 1A, panel B). We obtained similar FACS analysis results with similarly purified CD4+CD25+ cells from B6 naïve lymph nodes (Fig. 1A, panel E) and from BALB/c naïve spleen (Fig. 1A, panel H). These results are important because they show that naïve CD4+CD25+hi cells, which also have the greatest FoxP3 levels, possess the highest GSK-3β activity as indicated by having the highest degree of phospho-β-catenin (Ser-33/37 and Thr-41) (60–70%) labeling when compared with CD4+CD25+med and CD4+CD25+lo cells. In addition, analysis of CD4+CD25−FoxP3− cells revealed a lower GSK-3β activity when compared with CD4+CD25+hi cells as ascertained by phospho-β-catenin (Ser-33/37 and Thr-41) labeling (Fig. 1B).
FIGURE 1.
A, FACS analysis of purified CD4+CD25+ cells for FoxP3, active GSK-3β, and phosphorylated β-catenin. Single cell suspensions of isolated CD4+CD25+ cells from B6 and BALB/c spleen and lymph nodes demonstrate co-staining of FoxP3+, GSK-3β (Tyr(P)-216), and phosphorylated β-catenin (Ser(P)-33/Ser(P)-37/Thr(P)-41). This finding demonstrates that CD4+CD25+hiFoxP3+ cells exhibit high GSK-3β activity. Moreover, the signal overlaps with CD4+CD25+hi cells stained for β-catenin (Ser(P)-33/Ser(P)-37/Thr(P)-41), suggesting that CD4+CD25+hiFoxP3+ cells possess elevated GSK-3β activity, reflected in increased β-catenin phosphorylation. Panels A–C, B6 spleen; panels D–F, B6 lymph nodes; panels G–I, BALB/c spleen. Data are representative of at least three independent experiments, gating on the purified lymphocyte population. B, histogram analyses of purified CD4+CD25− and CD4+CD25+ cells for phosphorylated β-catenin (Ser(P)-33/Ser(P)-37/Thr(P)-41). This finding demonstrates that CD4+CD25+hi cells exhibit higher GSK-3β activity than CD4+CD25−FoxP3− cells. Panel A, B6 spleen; panel B, B6 lymph nodes; panel C, BALB/c spleen. Shaded area, CD4+CD25+hi cells plus Alexa Fluor 488-labeled secondary antibody alone; dashed line (---), CD4+CD25−FoxP3− cells plus Alexa Fluor 488-labeled secondary antibody alone; solid line (—), CD4+CD25+hi cells plus anti-phosphorylated β-catenin (Ser(P)-33/Ser(P)-37/Thr(P)-41) antibody; dotted line (···), CD4+CD25−FoxP3− cells plus anti-phosphorylated β-catenin (Ser(P)-33/Ser(P)-37/Thr(P)-41) antibody. Data are representative of at least three independent experiments, gating on the purified, lymphocyte population.
Inhibition of GSK-3β with SB216763 Increased Suppressive Activity of CD4+CD25+FoxP3+ Cells
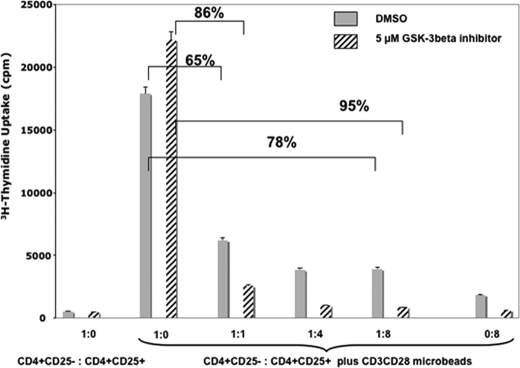
To assess the effect of GSK-3β inhibition on the functional properties of Treg cells, we performed an in vitro suppression assay in the absence or presence of SB216763, a specific GSK-3β inhibitor (Fig. 2). We isolated CD4+CD25− effector T cells and CD4+CD25+ Treg cells using magnetic separation. CD4+CD25− effector T cells were incubated in the presence or absence of anti-CD3/CD28 microbeads with increasing amounts of CD4+CD25+ Treg cells with or without SB216763. Proliferation was assessed by [3H]thymidine uptake. Notably, CD4+CD25− T effector cells incubated with an equal number of anti-CD3/CD28 microbeads and in the absence of Treg cells showed a 25% increase in [3H]thymidine uptake with the addition of SB216763 when compared with vehicle-treated control. This is not unprecedented because Ohteki et al. (19) have demonstrated previously that inhibition of T cells with lithium, an inhibitor of GSK-3β, resulted in increased T cell proliferation. More importantly, there was a marked difference in suppression of CD4+CD25− T cells cultured with CD4+CD25+ Treg cells with and without SB216763. At a 1:1 ratio of CD4+CD25− T cells and CD4+CD25+ Treg cells, the suppression was 65% as measured by [3H]thymidine uptake. Although this was not unexpected, we observed an increase in suppression by CD4+CD25+ Treg cells in cultures supplemented with SB216763. Moreover, this enhanced suppression was titratable with increasing numbers of SB216763-treated CD4+CD25+ Treg cells, yielding better suppression than with control CD4+CD25+ Treg cells. Namely, 95% suppression was seen when CD4+CD25− T cells were combined in a 1:8 ratio with CD4+CD25+ Treg cells and SB216763, whereas CD4+CD25− T cells without the GSK-3β inhibitor yielded 78% inhibition.
FIGURE 2.
Effect of GSK-3β inhibition, using SB216763, on Treg cell suppression. Performing a classical suppression assay, CD4+CD25− T cells and CD4+CD25+ Treg cells were isolated using magnetic separation and co-cultured in a 1:1 ratio with anti-CD3/CD28 microbeads with and without SB216763. Cells were pulsed with tritiated thymidine after 18–20 h, and proliferation was measured. Using this assay, we show that SB216763 potentiates Treg cell function in vitro as judged by increased suppression of T effector CD4+CD25− cell proliferation when T effector CD4+CD25− cells were incubated at a 1:1 ratio with CD4+CD25+ Treg cells in 5 μm SB216763 (86% inhibition versus 65% without the drug (DMSO vehicle control)). Moreover, cells incubated in a 1:8 ratio, respectively, with Treg cells again in 5 μm SB216763 demonstrate 95% inhibition versus 78% without the drug, suggesting that GSK-3β inhibition can significantly enhance Treg cell suppression. Data are represented as means ± S.E. of triplicate samples. All data are representative of at least two independently performed experiments.
Inhibition of GSK-3β with SB216763 Results in Stabilization of β-Catenin in CD4+CD25+hiFoxP3+ Cells
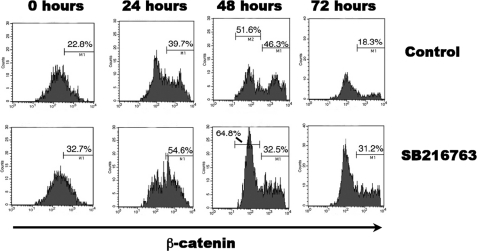
Ding et al. (9) have shown that the introduction of a stable mutant form of β-catenin leads to the increased survival of Treg cells. GSK-3β has been shown to phosphorylate β-catenin, targeting it for degradation via ubiquitination. Therefore, we analyzed what effect inhibition of GSK-3β by SB216763 would have on β-catenin levels in our experimental system. We co-cultured CD4+CD25− effector T cells and CD4+CD25+ Treg cells with anti-CD3/CD28 microbeads in the presence or absence of SB216763. Using FACS analysis, we assessed untreated and SB216763-treated cultures for β-catenin while gating on the CD4+CD25+hiFoxP3+ Treg cells (Fig. 3). In this experimental design, β-catenin was elevated in Treg cells during all time points. CD4+CD25− cells also showed increased β-catenin levels (data not shown), which would explain why they exhibited greater proliferation in the presence of SB216763 in the absence of Treg cells (Fig. 2). This is unsurprising given that β-catenin is implicated in cellular proliferation with its heightened presence due to GSK-3β inhibition. Interestingly, although there were increased β-catenin levels in treated cells, we also observed β-catenin protein stabilization in untreated CD4+CD25+hiFoxP3+ cells. This stabilization of β-catenin was observed in CD4+CD25+hiFoxP3+ cells only when they were co-cultured with CD4+CD25− cells (data not shown).
FIGURE 3.
FACS analysis of total β-catenin protein levels in presence or absence of SB216763. To assess β-catenin levels in active Treg cells, we elected to mimic a suppression assay without radioactive isotopes. A time course was performed to trend the β-catenin intensity in Treg cells in intervals of 0, 24, 48, and 72 h. Given that GSK-3β targets β-catenin for degradation, the effect of GSK-3β inhibition on β-catenin was measured using flow cytometry with targeting FITC antibodies. Unsurprisingly, an increase in total β-catenin protein was observed in FoxP3+ Treg cells co-cultured with CD4+CD25− cells and treated with SB216763. All data are representative of at least two independently performed experiments, gating on CD4+CD25+hiFoxP3+ cells.
CD4+CD25+FoxP3+ Treg Cells Exhibit Less FoxP3 Protein Turnover in Presence of SB216763
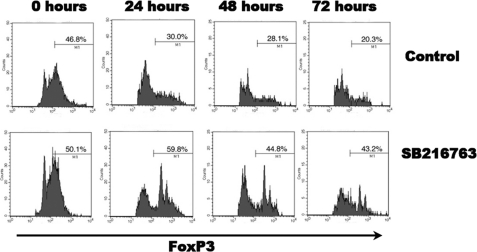
We noticed that CD4+CD25+hiFoxP3+ cells, when grown in vitro, exhibited rapid loss of FoxP3 protein (data not shown). Given that inhibition of GSK-3β enhanced Treg cell activity, we wanted to assess whether SB216763 treatment influenced FoxP3 turnover.
CD4+CD25− T cells and CD4+CD25+ Treg cells were isolated using magnetic separation and co-cultured in a 1:1 ratio with anti-CD3/CD28 microbeads with and without SB216763. The collective grouping of T effector and Treg cells was fixed and stained with anti-CD4-PE-Cy5 and -FoxP3-FITC at intervals of 0, 24, 48, and 72 h (Fig. 4). CD4+CD25− T cells alone incubated with anti-CD3/CD28 microbeads with and without SB216763 served as controls for this experiment. Using FACS analysis and gating on FoxP3+ cells, we found that CD4+CD25+ Treg cells co-cultured with CD4+CD25− T cells and treated with SB216763 maintained their FoxP3+ signature. FoxP3 expression in untreated CD4+CD25+ Treg cells was detected in 39.7% of cells at 24 h, in 51.6% at 48 h, and in 18.3% at 72 h. Contrastingly, SB216763-treated CD4+CD25+ Treg cells displayed a slower pace of loss of FoxP3 protein. These cells had a FoxP3 signal of 54.6% at 24 h, 32.5% at 48 h, and 31.2% at 72 h. This finding suggests that treatment with SB216763 slows FoxP3 cell turnover in Treg cells in vitro. When CD4+CD25− effector T cells that had proliferated in the presence of anti-CD3/CD28 microbeads were examined, they were found to display minimal to no FoxP3 expression (data not shown).
FIGURE 4.
Assessment of FoxP3 protein levels in presence or absence of SB216763. Again a co-culture of isolated Treg cells and T effector cells in a 1:1 ratio with anti-CD3/CD28 microbeads with and without 5 μm SB216763 was performed. The collective grouping of T effector and Treg cells was fixed and stained with FoxP3-FITC in intervals of 0, 24, 48, and 72 h. Using FACS analysis, we clearly show that CD4+CD25+ Treg cells co-cultured with CD4+CD25− T cells and treated with SB216763 maintain their FoxP3+ signature. This striking finding suggests that SB216763 slows FoxP3 cell turnover in vitro. All data are representative of at least two independently performed experiments, gating on CD4+CD25+hiFoxP3+ cells.
Increase in Levels of Antiapoptotic Proteins Bcl-xL and Bcl-2 in Treg Cells following GSK-3β Inhibition
Previous studies have shown that inhibition of GSK-3β results in increased cell survival by the stabilization of β-catenin (9). Our data suggest that SB216763 treatment results in increased Bcl-xL protein expression in Treg cells. Bcl-xL is an antiapoptotic protein that works to counteract cellular death through inhibitory binding of apoptotic mediators (9). Interestingly, we demonstrated heightened Bcl-xL protein levels in CD4+CD25+ Treg cells when they were co-cultured with CD4+CD25− T cells and with anti-CD3/CD28 microbeads. FACS analysis on gated CD4+CD25+hi cells demonstrated that untreated cells exhibited a 19% increase in Bcl-xL signal, whereas SB216763-treated cells exhibited a 31% increase in Bcl-xL within 24 h (Fig. 5a). By 48 h, untreated cells returned to their original Bcl-xL levels, whereas treated cells maintained 13% higher levels of Bcl-xL protein even after 72 h. Western blot analysis of purified CD4+CD25− cells treated in vitro with SB216763 showed that Bcl-2 levels were maintained at least up to 66 h following GSK-3β inhibition versus untreated cells (Fig. 5b). The increase in Bcl-xL and Bcl-2 protein levels correlated with increased cell survival of CD4+CD25+ cells where SB216763-treated cells exhibited 20% more cells at 66 h versus controls (data not shown). Collectively, these findings suggest that the GSK-3β inhibitor SB216763 may work through Bcl-xL and Bcl-2 to protect CD4+CD25+ Treg cells from apoptosis in vitro.
FIGURE 5.
a, FACS analysis of the antiapoptotic protein Bcl-xL following SB216763 treatment. Our co-cultured group of cells treated with SB216763 demonstrates a higher intensity of Bcl-xL after 24 h than untreated cells. Moreover, the kinetics of Bcl-xL loss is significantly slower temporally in the treated group. Collectively, these findings suggest that the GSK-3β inhibitor SB216763 may work through Bcl-xL to protect Treg cells from apoptosis in vitro. All data are representative of at least two independently performed experiments, gating on CD4+CD25+hiFoxP3+ cells. B, Western blot analysis of CD4+CD25+ cells treated in vitro with SB216763. Cells treated with SB216763 maintain increased Bcl-2 levels up to 66 h versus untreated cells. The Western blot was stripped and reprobed with anti-β-actin antibody as a loading control. inh., inhibitor. C, effect of caspase-3 inhibition, using DEVD-CHO, on Treg cell suppression. Performing a classical suppression assay, the use of 100 μm DEVD-CHO, a caspase-3 inhibitor (Casp. Inh), resulted in a 20% increase in suppression activity. When caspase-3 inhibitor was used with GSK-3β inhibitor (GSK-3 Inh), we observed no additive affect, suggesting that the two inhibitors may be acting on the same or parallel pathways. Data are represented as means ± S.E. of triplicate samples. All data are representative of at least two independently performed experiments.
Caspase-3 Inhibition with CPP32/Apopain Results in Increased Suppression Activity in Treg Cells
GSK-3β has been shown to play a role in apoptosis in part by destabilizing Bcl-2 (20, 21). Because we observed a stabilization of the antiapoptotic proteins Bcl-xL and Bcl-2 following GSK-3β inhibition, we next examined whether SB216763 inhibition acted concordantly with inhibition of apoptosis. To explore this, we made use of a caspase-3 inhibitor, DEVD-CHO. In Fig. 5c, we show that addition of 100 μm DEVD-CHO resulted in a 30% increase in suppressive activity at a 1:1 ratio of CD4+CD25− T cells to CD4+CD25+ Treg cells in the presence of anti-CD3/CD28 microbeads. There was no additive effect when cells were co-treated with SB216763 and DEVD-CHO. These results suggest that DEVD-CHO and SB216763 may act through similar and/or parallel pathways to lessen Treg cell apoptosis and enhance suppressive activity.
Prolonged Survival of Islet Allotransplants following SB216763 Treatment
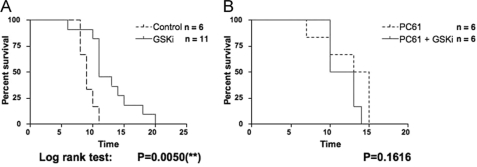
In an attempt to extrapolate our in vitro work to an in vivo system, we used an allograft islet transplantation system to assess any potential effects on islet allograft survival in the presence of GSK-3β inhibition. Systemic inhibition of GSK-3β has been used in animal models ranging from inflammation to Alzheimer disease to type II diabetes (22–27), but the effect of inhibiting GSK-3β in animal models for organ transplantation has not yet been reported. In an islet transplant model where C3H islets were transplanted into B6 mice, we showed that there was at least a modest prolongation of allograft islet survival in mice that were treated daily with 100 μm SB216763 beginning 1 day post-transplantation (Fig. 6A). We next depleted Treg cells using anti-CD25 antibody (PC61) and assessed whether the SB216763 effect was negated. The two-dose PC61 treatment of mice prior to transplantation resulted in an 87–90% depletion of CD4+FoxP3+ cells in peripheral blood lymphocytes as determined by FACS analysis (data not shown). In Fig. 6B, we show that prolongation of allograft islet survival was not seen if Treg cells were depleted.
FIGURE 6.
Increased survival of allograft islet transplantation following SB216763 treatment. A, diabetogenic B6 recipients of C3H islets were injected daily with 100 μm SB216763 postoperatively. Interestingly, these islets had a 2-fold slower rejection time than untreated B6 recipients as demonstrated by prolonged euglycemia. p = 0.0056 (log rank test). B, diabetogenic B6 recipients of C3H islets, depleted of CD25+ cells prior to transplantation, were injected daily with 100 μm SB216763 postoperatively. Depletion of Treg cells resulted in the elimination of SB216763 effect. This demonstration of GSK-3β inhibition (GSKi) in an in vivo model hints at the powerful possibilities of potential Treg cell therapy in transplantation. p = 0.1616 (log rank test). Casp., caspase-3; Inh, inhibitor.
DISCUSSION
A recent study has shown that introduction of a stable form of β-catenin results in increased Treg cell survivability. This paralleled the need for fewer modified Treg cells in the alleviation of inflammatory bowel disease in a mouse model (9). In this study, we analyzed which upstream components of β-catenin regulation may play a role in Treg cell function. We focused on the serine/threonine kinase GSK-3β, which phosphorylates β-catenin, marking it for ubiquitination, resulting in its degradation. Our results show that the inhibition of GSK-3β potentiates Treg cell suppressive activity and that it does so by increasing Treg cell survivability, consistent with observations by Ding et al. (9).
To address the issue of the role of GSK-3β in Treg cell function, we isolated CD4+CD25+ cells and by FACS analysis showed that the highest portion of activated GSK-3β, as characterized by phosphorylation of tyrosine 216, was observed in CD4+CD25+hi cells. These cells also exhibited the greatest level of phospho-β-catenin (Ser-33/37 and Thr-41), indicative of greater GSK-3β activity. Inhibition of GSK-3β using SB216763, a specific GSK-3β inhibitor, resulted in increased Treg cell activity as exhibited by at least 20% greater suppression in cells treated with SB216763. This enhanced suppression was not a result of direct inhibition of CD4+CD25− cell proliferation by SB216763 because treatment of CD4+CD25− cells alone with inhibitor resulted in increased cellular proliferation. This is consistent with previous observations by Ohteki et al. (19) who demonstrated that inhibition of T cells with lithium, an inhibitor of GSK-3β, resulted in increased T cell proliferation and IL-2 production. But this is in contrast with what was observed by Ding et al. (9) who found that ectopic expression of stable β-catenin in CD4+CD25− effector T cells results in anergy. This could be simply due to the fact that mutant β-catenin in CD4+CD25− cells is competing with the endogenous wild type form by competing for binding to transcriptional factors and/or regulatory molecules, such as GSK-3β complex, resulting in the alteration in proliferation of T effector cells.
Using the Miltenyi Biotec kit for the isolation of CD4+CD25+ cells gives the added advantage that the cells are prestained with anti-CD25-PE antibody, and therefore phenotypic changes can be assessed in mixed cultures with unlabeled CD4+CD25− cells by following the labeled cells. Analysis of β-catenin protein showed that there was an increase in total β-catenin protein in CD4+CD25+hiFoxP3+ cells treated with GSK-3β inhibitor versus untreated cells. But although there was an increased level of β-catenin protein in treated cells, β-catenin protein stabilization also occurred in untreated CD4+CD25+hiFoxP3+ cells. This was only observed in CD4+CD25+hiFoxP3+ cells co-cultured with CD4+CD25− cells. This could be attributable to the fact that β-catenin stabilization may be a molecular event that occurs in Treg cells during the process of suppression and that what Ding et al. (9) and our laboratory have shown is an enhancement of this process either through the introduction of non-hydrolyzable β-catenin or through the inhibition of GSK-3β, respectively. It is possible that co-staining for FoxP3 and β-catenin could serve as identifying markers for assessing activated Treg cells in vivo.
We also looked at the status of FoxP3 expression in cells treated with or without SB216763 and found that treated CD4+CD25+hiFoxP3+ cells exhibited prolonged FoxP3 expression as compared with untreated cells. A possible explanation for this is that active GSK-3β has been shown to phosphorylate the transcriptional factor NFAT, resulting in its exit from the nucleus, negatively regulating its NFAT activity (28). NFAT is important in the transcriptional activation of FoxP3 expression (29). Therefore, inhibition of GSK-3β may result in the stabilization of NFAT activity, resulting in increased FoxP3 expression. In addition, it is well documented that TGF-β treatment results in increased FoxP3 expression (5, 30–33) in part by activating the Smad3 transcriptional factor (29), and it also can result in the inhibition of GSK-3β (34–38).
Ding et al. (9) have shown that messenger RNA levels of the antiapoptotic protein Bcl-xL are increased in Treg cells that ectopically express stable β-catenin as assessed by RT-PCR. Using FACS analysis and gating on FoxP3+ cells, we found that Bcl-xL protein levels were increased in SB216763-treated CD4+CD25+hiFoxP3+ cells versus untreated cells. This is in agreement with Ding et al. (9) and suggests that increased Treg cell suppressive function following GSK-3β inhibition may be due in part to increased Treg cell survival. To further validate that inhibition of apoptosis increases Treg cell function, we treated cells with the caspase inhibitor DEVD-CHO and observed about a 30% increase in the suppressive activity of Treg cells. When cells were treated with DEVD-CHO together with SB216763, we observed no additive effect, suggesting that GSK-3β inhibition may affect pathways similar or parallel to those inhibited by DEVD-CHO.
Because GSK-3β inhibition potentiates the suppressive activity of Treg cells in vitro, we have begun to study the effect of inhibition of GSK-3β in vivo using an islet transplant model where C3H islets are transplanted into B6 mice. Moreover, although we cannot draw definitive inferences on the effect of Treg cells in SB216763-treated animals, it is notable that euglycemia was prolonged in grafted animals in the treated group. Interestingly, although explanted islet grafts from both the control and experimental groups displayed lymphocytic infiltration suggestive of rejection (data not shown), the time to rejection was significantly different in animals treated with SB216763. Although we cannot exclude a myriad of variables involved, it is striking to note that islet grafts survived twice as long with GSK-3β inhibition than in the control. Depletion of Treg cells by PC61 resulted in the elimination of the SB216763 effect on prolonging allograft islet survival. Although the imprecise nature of these experiments cannot be understated, this disparity coupled with our in vitro work does raise the possibility that enhanced Treg cells, due to GSK-3β inhibition, contribute in promoting tolerance. Preliminary data suggest that naïve mice treated daily for 7 days with 100 μm SB216763 show a decrease in the number of FoxP3 positive cells in the spleen with an increase in the number of FoxP3-positive cells in the peripheral blood without a change in the overall number of FoxP3+ cells in treated versus untreated naïve mice (data not shown).
In summary, we have shown that inhibition of GSK-3β resulted in increased suppression activity by Treg cells. Analysis of SB216763-treated Treg cells showed that there was a stabilization of β-catenin and that this in part may account for the increased functionality of Treg cells. This is consistent with the findings of Ding et al. (9) when they ectopically expressed a non-hydrolyzable mutant form of β-catenin in Treg cells. We further show that GSK-3β inhibition was accompanied by increased stabilization of FoxP3 protein in Treg cells as well as an increase in Bcl-xL protein levels. There was no additive effect when cells were co-treated with SB216763 and DEVD-CHO, suggesting that the inhibitors are acting on similar and/or parallel pathways. When SB216763 was given systemically to recipients of transplanted islets, these allospecific cells exhibited prolonged survival. Knowledge of how GSK-3β affects FoxP3 stability and function and what role this plays in increasing Treg cell suppressive activity is timely, especially in light of the recent study by Zhou et al. (39) showing that the loss of FoxP3 expression in Treg cells results in the generation of “exFoxP3” T cells that have a potent, pathogenic memory T cell phenotype. Therefore, understanding the important molecular mechanism(s) underlying this form of immune regulation could aid in the development of therapeutics for managing allotransplantation rejection and autoimmunity.
Acknowledgments
We thank Drs. Henry Winn and Andrew Wells for critical and constructive comments regarding the manuscript and Susan Shea for technical expertise and assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants K01 DK079207-02 (to J. K.), K02 AI53103 and R01 HD050484 (to G. B.), R01 HL071932 (to J. C. M.), R01 AI048820-09 (to J. M.), 5P01 HL18646 and U19 DK080652 (to A. B. C.), and R01 AI081734-01 (to R. B. C.). This work was also supported by a Surgical Research Council junior faculty award (to A. A.).
- Treg
- regulatory T
- GSK-3β
- glycogen synthase kinase 3β
- DEVD-CHO
- N-acetyl-l-α-aspartyl-l-α-glutamyl-N-(2-carboxy-1-formylethyl)-l-valinamide
- PE
- phycoerythrin
- NFAT
- nuclear factor of activated T cells.
REFERENCES
- 1.Sakaguchi S., Sakaguchi N. (2005) Int. Rev. Immunol. 24, 211–226 [DOI] [PubMed] [Google Scholar]
- 2.Kim J. M., Rasmussen J. P., Rudensky A. Y. (2007) Nat. Immunol. 8, 191–197 [DOI] [PubMed] [Google Scholar]
- 3.Gavin M., Rudensky A. (2003) Curr. Opin. Immunol. 15, 690–696 [DOI] [PubMed] [Google Scholar]
- 4.Bluestone J. A., Abbas A. K. (2003) Nat. Rev. Immunol. 3, 253–257 [DOI] [PubMed] [Google Scholar]
- 5.Chen W., Jin W., Hardegen N., Lei K. J., Li L., Marinos N., McGrady G., Wahl S. M. (2003) J. Exp. Med. 198, 1875–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curotto de Lafaille M. A., Lino A. C., Kutchukhidze N., Lafaille J. J. (2004) J. Immunol. 173, 7259–7268 [DOI] [PubMed] [Google Scholar]
- 7.Kretschmer K., Apostolou I., Hawiger D., Khazaie K., Nussenzweig M. C., von Boehmer H. (2005) Nat. Immunol. 6, 1219–1227 [DOI] [PubMed] [Google Scholar]
- 8.Horwitz D. A., Zheng S. G., Gray J. D. (2008) Trends Immunol. 29, 429–435 [DOI] [PubMed] [Google Scholar]
- 9.Ding Y., Shen S., Lino A. C., Curotto de Lafaille M. A., Lafaille J. J. (2008) Nat. Med. 14, 162–169 [DOI] [PubMed] [Google Scholar]
- 10.He X., Saint-Jeannet J. P., Woodgett J. R., Varmus H. E., Dawid I. B. (1995) Nature 374, 617–622 [DOI] [PubMed] [Google Scholar]
- 11.Miller J. R., Moon R. T. (1996) Genes Dev. 10, 2527–2539 [DOI] [PubMed] [Google Scholar]
- 12.Stambolic V., Ruel L., Woodgett J. R. (1996) Curr. Biol. 6, 1664–1668 [DOI] [PubMed] [Google Scholar]
- 13.Papkoff J., Aikawa M. (1998) Biochem. Biophys. Res. Commun. 247, 851–858 [DOI] [PubMed] [Google Scholar]
- 14.Ioannidis V., Beermann F., Clevers H., Held W. (2001) Nat. Immunol. 2, 691–697 [DOI] [PubMed] [Google Scholar]
- 15.Staal F. J., Weerkamp F., Baert M. R., van den Burg C. M., van Noort M., de Haas E. F., van Dongen J. J. (2004) J. Immunol. 172, 1099–1108 [DOI] [PubMed] [Google Scholar]
- 16.Xu Y., Banerjee D., Huelsken J., Birchmeier W., Sen J. M. (2003) Nat. Immunol. 4, 1177–1182 [DOI] [PubMed] [Google Scholar]
- 17.Xu Y., Sen J. (2003) Eur. J. Immunol. 33, 12–18 [DOI] [PubMed] [Google Scholar]
- 18.Alessandrini A., Namura S., Moskowitz M. A., Bonventre J. V. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 12866–12869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohteki T., Parsons M., Zakarian A., Jones R. G., Nguyen L. T., Woodgett J. R., Ohashi P. S. (2000) J. Exp. Med. 192, 99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Letai A. (2006) Mol. Cell 21, 728–730 [DOI] [PubMed] [Google Scholar]
- 21.Maurer U., Charvet C., Wagman A. S., Dejardin E., Green D. R. (2006) Mol. Cell 21, 749–760 [DOI] [PubMed] [Google Scholar]
- 22.Cline G. W., Johnson K., Regittnig W., Perret P., Tozzo E., Xiao L., Damico C., Shulman G. I. (2002) Diabetes 51, 2903–2910 [DOI] [PubMed] [Google Scholar]
- 23.Dugo L., Abdelrahman M., Murch O., Mazzon E., Cuzzocrea S., Thiemermann C. (2006) Shock 25, 485–491 [DOI] [PubMed] [Google Scholar]
- 24.Dugo L., Collin M., Allen D. A., Patel N. S., Bauer I., Mervaala E. M., Louhelainen M., Foster S. J., Yaqoob M. M., Thiemermann C. (2005) Crit. Care Med. 33, 1903–1912 [DOI] [PubMed] [Google Scholar]
- 25.Martin M., Rehani K., Jope R. S., Michalek S. M. (2005) Nat. Immunol. 6, 777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakashima H., Ishihara T., Suguimoto P., Yokota O., Oshima E., Kugo A., Terada S., Hamamura T., Trojanowski J. Q., Lee V. M., Kuroda S. (2005) Acta Neuropathol. 110, 547–556 [DOI] [PubMed] [Google Scholar]
- 27.Ring D. B., Johnson K. W., Henriksen E. J., Nuss J. M., Goff D., Kinnick T. R., Ma S. T., Reeder J. W., Samuels I., Slabiak T., Wagman A. S., Hammond M. E., Harrison S. D. (2003) Diabetes 52, 588–595 [DOI] [PubMed] [Google Scholar]
- 28.Neal J. W., Clipstone N. A. (2001) J. Biol. Chem. 276, 3666–3673 [DOI] [PubMed] [Google Scholar]
- 29.Tone Y., Furuuchi K., Kojima Y., Tykocinski M. L., Greene M. I., Tone M. (2008) Nat. Immunol. 9, 194–202 [DOI] [PubMed] [Google Scholar]
- 30.Fantini M. C., Becker C., Monteleone G., Pallone F., Galle P. R., Neurath M. F. (2004) J. Immunol. 172, 5149–5153 [DOI] [PubMed] [Google Scholar]
- 31.Park H. B., Paik D. J., Jang E., Hong S., Youn J. (2004) Int. Immunol. 16, 1203–1213 [DOI] [PubMed] [Google Scholar]
- 32.Zheng S. G., Wang J. H., Gray J. D., Soucier H., Horwitz D. A. (2004) J. Immunol. 172, 5213–5221 [DOI] [PubMed] [Google Scholar]
- 33.Zheng S. G., Wang J. H., Koss M. N., Quismorio F., Jr., Gray J. D., Horwitz D. A. (2004) J. Immunol. 172, 1531–1539 [DOI] [PubMed] [Google Scholar]
- 34.Clifford R. L., Deacon K., Knox A. J. (2008) J. Biol. Chem. 283, 35337–35353 [DOI] [PubMed] [Google Scholar]
- 35.Ding Q., Xia W., Liu J. C., Yang J. Y., Lee D. F., Xia J., Bartholomeusz G., Li Y., Pan Y., Li Z., Bargou R. C., Qin J., Lai C. C., Tsai F. J., Tsai C. H., Hung M. C. (2005) Mol. Cell 19, 159–170 [DOI] [PubMed] [Google Scholar]
- 36.Guo X., Ramirez A., Waddell D. S., Li Z., Liu X., Wang X. F. (2008) Genes Dev. 22, 106–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo X., Wang X. F. (2009) Cell Res. 19, 71–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchetti A., Colletti M., Cozzolino A. M., Steindler C., Lunadei M., Mancone C., Tripodi M. (2008) Cell. Signal. 20, 2113–2118 [DOI] [PubMed] [Google Scholar]
- 39.Zhou X., Bailey-Bucktrout S. L., Jeker L. T., Penaranda C., Martínez-Llordella M., Ashby M., Nakayama M., Rosenthal W., Bluestone J. A. (2009) Nat. Immunol. 10, 1000–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]