Abstract
Serotonin (5-hydroxytryptamine, 5-HT) is mitogenic for several cell types including pulmonary arterial smooth muscle cells (PASMC), and is associated with the abnormal vascular smooth muscle remodeling that occurs in pulmonary arterial hypertension. RhoA/Rho kinase (ROCK) function is required for 5-HT-induced PASMC mitogenesis, and 5-HT activates RhoA; however, the signaling steps are poorly defined. Rho guanine nucleotide exchange factors (Rho GEFs) transduce extracellular signals to Rho, and we found that 5-HT treatment of PASMC led to increased membrane-associated Lbc Rho GEF, suggesting modulation by 5-HT. Lbc knockdown by siRNA attenuated 5-HT-induced thymidine uptake in PASMC, indicating a role in PASMC mitogenesis. 5-HT triggered Rho-dependent serum response factor-mediated reporter activation in PASMC, and this was reduced by Lbc depletion. Lbc knockdown reduced 5-HT-induced RhoA/ROCK activation, but not p42/44 ERK MAP kinase activation, suggesting that Lbc is an intermediary between 5-HT and RhoA/ROCK, but not ERK. 5-HT stimulation of PASMC led to increased association between Lbc, RhoA, and the α-catulin scaffold. Furthermore, α-catulin knockdown attenuated 5-HT-induced PASMC thymidine uptake. 5-HT-induced PASMC mitogenesis was reduced by dominant-negative Gq protein, suggesting cooperation with Lbc/α-catulin. These results for the first time define a Rho GEF involved in vascular smooth muscle cell growth and serotonin signaling, and suggest that Lbc Rho GEF family members play distinct roles. Thus, the Lbc/α-catulin axis participates in 5-HT-induced PASMC mitogenesis and RhoA/ROCK signaling, and may be an interventional target in diseases involving vascular smooth muscle remodeling.
Keywords: Cell/Mitogens, G Proteins/Low Molecular Weight, Growth Factors, RNA/RNAi, Signal Transduction/G-proteins, Tissue/Organ Systems/Muscle/Smooth
Introduction
Serotonin (5-hydroxytryptamine, 5-HT)2 is well recognized as an important neurotransmitter and vasoconstrictor; additionally, it regulates the proliferation and migration of a variety of cell types, including lung, kidney, prostate, and mast cells (1, 2). At near-physiologic concentrations, 5-HT is mitogenic for certain smooth muscle cell types including cultured pulmonary arterial smooth muscle cells (PASMC) (3). Extensive clinical and experimental studies link 5-HT to idiopathic pulmonary arterial hypertension (PAH) (4), a progressive disease that is ultimately fatal despite available therapies. 5-HT is implicated in the abnormal vascular smooth muscle remodeling leading to pulmonary arterial obstruction in PAH (5). In addition, 5-HT may be involved in cardiovascular disease (6). Thus, understanding the mechanisms of serotonin-mediated vascular smooth muscle responses is important, and may help define novel therapeutic targets in disease.
5-HT-induced PASMC mitogenesis requires RhoA/Rho kinase (ROCK) function (7), in addition to p42/44 ERK MAP kinase (8) and AKT pathways (9). RhoA mediates multiple cellular responses such as cell shape changes, contractility, migration, gene transcription, and growth via a distinct signaling pathway that regulates actomyosin filament assembly, serum response factor (SRF)-dependent gene transcription, and cell cycle progression (10). Defective RhoA/ROCK signaling is implicated in cancer and cardiovascular disease, including PAH (11, 12), however, its regulation by extracellular factors is only partially understood.
In PASMC, the mitogenic effect of 5-HT is initiated by binding to one or more of the 5-HT receptors or the 5-HT transporter (2), and there is evidence that multiple receptor systems are involved in 5-HT-induced RhoA activation (4). However, signaling intermediaries involved in 5-HT receptor-induced Rho signaling in pulmonary smooth muscle cells are poorly understood. Rho guanine nucleotide exchange factors (GEFs) transduce signals from receptors to Rho and catalyze GDP/GTP exchange leading to formation of active GTP-Rho (13). However, the role/identity of any Rho GEFs involved in 5-HT-induced Rho signaling in PASMCs is unknown. Of the family of Rho-specific GEFs, the Lbc Rho GEF family, comprising the Lbc and AKAP-Lbc (e.g. AKAP13, Brx, and Ht31) splice products (14–17), is structurally and functionally distinct from the RGS Rho GEFs (p115, LARG, and PDZ Rho GEF) (18), and Gq effector Rho GEFs (Trio, p63) (19). The 107-kDa Lbc splice form is widely expressed (heart, lung, blood, muscle, epithelia, ovary, and testes) (20, 21), and encodes the Rho GEF (DH domain)-pleckstrin homology domain cassette, and a C-terminal regulatory region (20). The Lbc C-terminal region associates with α-catulin, a distant relative of α-catenin that likely acts as a scaffold for the Lbc/Rho signaling complex (22–24). Lbc is linked with increased proliferative responses; for example, a truncated form (onco-Lbc) is tumorigenic in nude mice (20), ectopic Lbc expression induces cell cycle progression (25), and Lbc expression correlates with increased proliferation in hepatocellular carcinoma (26).
The role of endogenous Lbc in primary cell responses remains poorly understood. Lbc has been implicated in a number of signaling pathways (27–29), although these findings are based on overexpression studies in cell lines. Lbc is expressed in vascular cells including PASMC; given the role of the RhoA pathway in 5-HT-induced mitogenesis of PASMC, and the association of Lbc with Rho signaling and growth, we investigated the role of Lbc and its associated scaffold α-catulin in primary pulmonary vascular smooth muscle cell growth responses using short interfering RNA (siRNA) (30). Our results indicate that Lbc Rho GEF participates in serotonin-induced mitogenic and transcriptional responses in primary pulmonary vascular smooth muscle cells via RhoA/ROCK, in conjunction with the α-catulin scaffold.
EXPERIMENTAL PROCEDURES
Cell Culture
Primary bovine pulmonary arterial cells were isolated by a modification of the method of Ross (50) as described in Ref. 3 and maintained in RPMI 1640 medium with 10% fetal bovine serum (FBS), 100 units/ml of penicillin, and 100 μg/ml of streptomycin, and used between passages 3 and 8. Human embryonic kidney (HEK) 293T cells (ATCC) were grown in Dulbecco's modified essential medium (DMEM) (Invitrogen) with 10% FBS and 100 units/ml of penicillin and 100 μg/ml of streptomycin. Cells were maintained in a humidified, 5% CO2 incubator at 37 °C.
Plasmids
pSRFlag:proto-Lbc is described in Ref. 21, pcDNAmyc:α-catulin in Ref. 22, and ΔDHLbc and ΔNTLbc in Ref. 31. SRE.L luciferase reporter (Dr. Kozo Kaibuchi) and p115 Rho GEF (Dr. Matthew Hart) plasmids were gifts; pcDNAGq and G13 plasmids were from Guthrie cDNA (MO).
Antibodies
Anti-actin, anti-RhoA monoclonal, anti-p42/44 ERK, anti-phospho-ERK, anti-Gq, anti-G13, and anti-cytoplasmic dynein antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-MYPT-1, anti-phospho-MYPT1 (Thr696), and anti-PDGFR antibodies were from Upstate Inc. (NY). Murine anti-FLAG, rabbit anti-myc, Alexa- and Texas Red-conjugated murine and rabbit antibodies, and HRP-conjugated mouse and rabbit antibodies were from Cell Signaling Technology (MA). Anti-Lbc peptide rabbit antiserum is as described in Ref. 21, anti-α-catulin rabbit antiserum is as described in Ref. 22. Anti-α-catulin (anti-CTNNAL1) monoclonal antibody was from Sigma. Sepharose G-conjugated FLAG and myc antibodies were from Sigma.
Reagents
19-Mer ready-to-use duplex siRNA oligonucleotides for Lbc, LARG GEF, scrambled (scr), and α-catulin were purchased from Dharmacon or Ambion. siRNA sequences are included under supplemental “Experimental Procedures”. 5-HT and PDGF were purchased from Sigma.
Cell Transfection
Cells at 70–90% confluence were transfected with siRNA/plasmid DNA using Lipofectamine 2000 (Invitrogen) for 5 h as recommended.
[3H]Thymidine Incorporation
Smooth muscle cells were grown to 80% confluence in 96-well dishes, transfected, and growth arrested in 0.1% FBS for 48 h. Each group contained 6 wells. Cells were then incubated with 1 μm 5-HT or vehicle for 20 h, followed by labeling with [methyl-3H]thymidine (20 mCi/ml; PerkinElmer Life Sciences) for 4 h. After labeling, medium was removed and the cells were harvested into Unifilter 96-well microplates with a Packard harvester. Incorporation of [3H]thymidine was measured by a scintillation counter.
Dual Luciferase Transcriptional Reporter Assay
Cells were seeded into 48-well dishes, transfected with SRE.L luciferase reporter (32), and serum starved for 48 h, then stimulated with 5-HT for 4 h before lysis. Dual Luciferase Reporter Assay System (Promega) was used as described in Ref. 31. Points were in triplicate.
Immunoblotting
Cellular lysates were resolved by 10% SDS-PAGE, and immunoblotted as described in Ref. 22.
Confocal Immunofluorescence Imaging
Cells were fixed and immunostained with antibodies as described (22) followed by Alexa- or Texas Red-conjugated secondary antibodies. Images were obtained by a Leica TCS SP2 confocal microscope and associated software using 1.32 NA ×63 lens. Emission filters were 500–535 nm for the green signal, and 600–700 nm for the red channel. Filters were set to minimize cross-talk between the two channels.
Immunoprecipitation
Immunoprecipitation was carried out as described in Ref. 22.
RhoA-GTP Pulldown
In vivo GTP-Rho pulldown was carried out using the GST-Rhotekin Rho binding domain (RBD) fusion protein kit (Cytoskeleton) as recommended by the manufacturer.
Subcellular Fractionation
High speed (100,000 × g) fractionation of cells into cytosolic and membrane-rich fractions was performed as described in Ref. 22. Equal volume amounts of each fraction were analyzed by SDS-PAGE.
Statistical Analysis
Statistical analysis was performed by the paired Student's t test; differences were considered as significant where p < 0.05.
RESULTS
5-HT Induces Lbc Membrane Translocation in PASMC
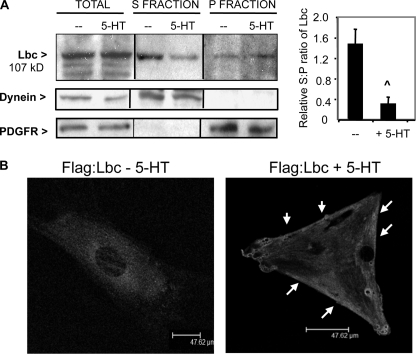
On the basis that 5-HT activates RhoA/ROCK in PASMC (7), we investigated whether 5-HT may modulate Lbc Rho GEF in primary cultured PASMC. Translocation of GEFs to a membrane-proximal site in response to cellular stimuli is an indication of GEF activation (33), and we initially tested the effect of 5-HT on Lbc subcellular localization in PASMC by high-speed fractionation. In serum-starved PASMC, most of the Lbc was present in the cytosolic (S) fraction (Fig. 1A), as was the control cytoplasmic protein dynein. Stimulation of growth-arrested PASMC with 5-HT led to a decrease in cytosolic Lbc, and a concomitant increase of Lbc levels in the membrane-rich fraction (P) (Fig. 1A) that also contains the PDGF receptor, suggesting that 5-HT signaling may regulate an Lbc fraction. In contrast, PDGF treatment did not induce changes in Lbc localization (not shown). Immunofluorescence confocal microscopy of serum-starved PASMC transfected with the FLAG:Lbc plasmid showed a mainly cytosolic, pericentral distribution of Lbc (Fig. 1B, left panel). In contrast, 5-HT-stimulated cells showed a redistribution of Lbc toward the periphery and along the cell edge (Fig. 1B, right panel); this is consistent with the biochemical data showing increased Lbc levels in the membrane-rich fraction of stimulated cells.
FIGURE 1.
5-HT influences Lbc subcellular localization. A, serum-starved PASMC were stimulated with 1 μm 5-HT, then fractionated into cytosolic (S) or membrane-rich (P) fractions, and immunoblotted for 107-kDa Lbc, cytoplasmic dynein (cytosolic control), and PDGF receptor (PDGFR) for membrane control. A representative experiment of three is shown; the graph quantitates the S:P ratio of the relative densitometric values of Lbc in each fraction from three experiments. ∧, p < 0.05 for 5-HT-treated versus untreated cells. Bars = S.D. B, PASMC transfected with FLAG:proto-Lbc plasmid were serum starved, then stimulated with 1 μm 5-HT and stained with FLAG antibody followed by Alexa-conjugated secondary antibody for Lbc detection by confocal microscopy; the z-slice was 0.33 μm. Arrows indicate peripheral Lbc localization in 5-HT-treated cells.
Characterization of Lbc siRNA Activity and Specificity
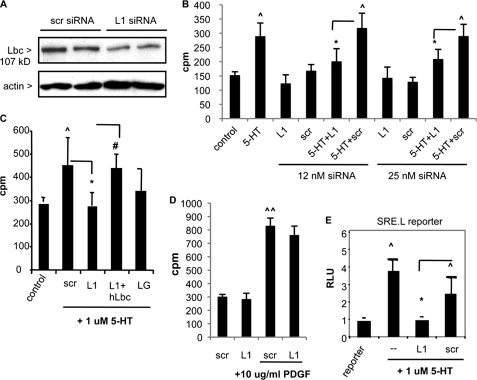
To investigate the potential role of Lbc in 5-HT-induced PASMC proliferation, we initially characterized the activity and specificity of Lbc siRNAs in HEK293 cells. Synthetic 21-bp duplex siRNAs representing three different Lbc sequences (L1–L3) were designed based on recommended criteria (30) (supplemental Fig. S1A). Knockdown activity of Lbc siRNAs on Lbc-induced signaling was evaluated by cotransfection of siRNAs with Lbc plasmid along with SRE.L luciferase transcriptional reporter that is Rho-dependent (32). Luciferase levels were measured by dual luciferase assay. Of the three siRNAs, L1 siRNA most effectively blocked Lbc-induced luciferase reporter activation to near background levels, whereas scr siRNA did not (supplemental Fig. S1B). L1 siRNA had no effect on p115 Rho GEF plasmid-induced reporter activation (supplemental Fig. S1C). These data demonstrate the knockdown activity and specificity of L1 Lbc siRNA. For subsequent studies in bovine PASMC, siRNA encoding the L1 homologous bovine sequence (one nucleotide difference between human:bovine) was used.
Lbc Knockdown Attenuates 5-HT-induced PASMC Mitogenesis
We examined the role of endogenous Lbc in 5-HT-induced cellular responses in PASMC using siRNAs. L1 siRNA transfection into PASMC followed by immunoblotting 48–72 h later confirmed knockdown of Lbc levels by ≥70% (Fig. 2A), in contrast to control (scr) siRNA. To determine the effect of Lbc knockdown on PASMC mitogenesis, cells were transfected with siRNAs for 5 h, switched to low serum (0.1%) overnight, then assayed for DNA synthesis by measuring [3H]thymidine uptake. Unstimulated siRNA-transfected cells showed background thymidine incorporation at comparable levels to mock-transfected cells. Stimulation with 1 μm 5-HT overnight led to a significant 2-fold increase in thymidine uptake (150 versus 300 mean cpm) (Fig. 2B). Transfection of control scr siRNA at either 12 or 25 nm yielded a comparable increase in 5-HT-induced thymidine uptake, indicating no effect. In contrast, Lbc L1 siRNA transfection significantly attenuated 5-HT-induced thymidine uptake by ∼70% at both siRNA doses (Fig. 2B). Similar data were obtained using L2 Lbc siRNA (not shown). Co-transfection of human Lbc plasmid with the bovine L1 siRNA reversed the knockdown effect (Fig. 2C), indicating the specificity of effect. We further tested the effect of LARG Rho GEF knockdown using LARG siRNA (LG); whereas some reduction in 5-HT-induced thymidine uptake was observed, it was not statistically significant (Fig. 2C). These findings were confirmed by cell count assays. Consistent with other reports (34), 5-HT treatment induced an up to 2-fold increase in PASMC number by 72 h in the presence of scr siRNA, which was significant; this was reduced by L1 siRNA (supplemental Fig. S2). In contrast to its effect on 5-HT-induced mitogenesis, L1 siRNA did not effectively inhibit PDGF-induced PASMC thymidine uptake (Fig. 2D), suggesting a selective effect on 5-HT-induced responses. These data indicate that Lbc participates in 5-HT-induced PASMC mitogenesis. To independently confirm the findings from siRNA studies, we utilized previously described dominant-negative Lbc mutants ΔDHLbc and ΔNTLbc that lack the DH GEF domain (supplemental Fig. S3A), and whose expressions have been previously verified (31). Comparable with siRNA knockdown, transfection of these mutants attenuated 5-HT-induced thymidine uptake (supplemental Fig. S3B).
FIGURE 2.
Attenuation of 5-HT-induced mitogenesis and SRE. L-mediated reporter activation in PASMC by Lbc knockdown. A, Lbc immunoblot of PASMC transfected with 25 nm scr or Lbc (L1) siRNA after 48 h. B and C, PASMC were either mock-transfected (control and 5-HT) or transfected with scr or L1 siRNA (12 and 25 nm) (B) or with 25 nm LARG GEF siRNA (LG) and L1 siRNA + human Lbc (hLbc) plasmid (C). After 48 h serum withdrawal, cells were treated with 5-HT overnight and assayed for [3H]thymidine uptake. n = 6, ∧, p < 0.05 for 5-HT + scr groups versus control; *, p < 0.05 for 5-HT + L1 versus 5-HT + scr groups; #, p < 0.05 for 5-HT + L1 + hLbc plasmid versus 5-HT + L1. D, PASMC transfected with scr or L1 siRNA were serum withdrawn, then treated with PDGF overnight and assayed for [3H]thymidine uptake. ∧∧, p < 0.05 for PDGF + scr versus scr. E, PASMC transfected with SRE.L luciferase reporter + siRNAs were serum-starved for 48 h, then treated with 5-HT for 5 h, and luciferase levels were measured. RLU, relative luciferase units. ∧, p < 0.05 for 5-HT group versus untreated control (reporter); *, p < 0.05 for L1 versus scr group, n = 3. Bars = S.D.
5-HT-induced SRF-mediated Transcription Is Attenuated by Lbc Knockdown
The transcription factor, SRF, binds to the serum response element (SRE) found in the promoters of many genes and regulates their transcription (32). Extracellular factor-stimulated SRF-induced gene transcription is Rho-dependent (32), and we found that in PASMC, 5-HT stimulates the SRF-dependent transcriptional reporter SRE.L (Fig. 2E). Moreover, Lbc siRNA reduced 5-HT-induced SRE.L activation in PASMC, whereas scr siRNA did not (Fig. 2E). These data suggest that Lbc is also required for Rho-dependent 5-HT-induced SRF-mediated transcriptional responses in PASMC.
Lbc Knockdown Inhibits 5-HT-induced RhoA Activation and RhoA Translocation in PASMC
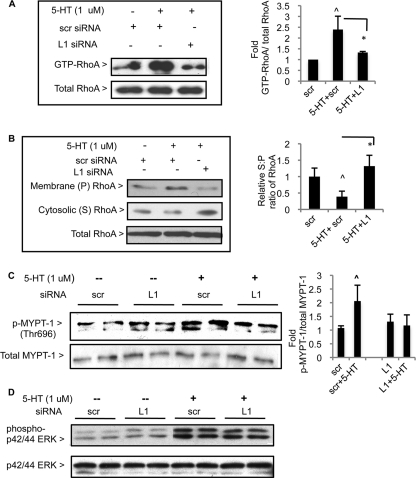
Because 5-HT activates RhoA/ROCK (7), we tested the effect of Lbc knockdown on 5-HT-induced RhoA activation. PASMC transfected with siRNAs were serum starved for 48 h. Following a 10-min stimulation with 1 μm 5-HT, cells were lysed and assayed for relative levels of active, GTP-bound RhoA by pulldown. In cells transfected with scr siRNA, 5-HT treatment led to a ∼2-fold increase in GTP-RhoA levels, whereas transfection with L1 siRNA did not result in significant activation (Fig. 3A and supplemental Fig. S4A). Another indicator of RhoA activation is redistribution of RhoA from the cytosol to the membrane (35), and to test this cells were treated as before and lysates were subjected to high-speed fractionation. As expected, 5-HT treatment of cells transfected with scr siRNA led to increased membrane-associated (P fraction) RhoA, and a concomitant decrease in cytosolic (S) RhoA (Fig. 3B). In contrast, cells transfected with L1 siRNA did not show significant membrane redistribution of RhoA in response to 5-HT relative to the scr group (Fig. 3B).
FIGURE 3.
Lbc knockdown inhibits 5-HT-induced RhoA and ROCK activation, but not ERK1/2 MAPK activation. PASMC were transfected with siRNAs, serum-starved, and treated with 5-HT for 10 min. A, relative GTP-RhoA levels were assayed by pulldown, and pulldown material and total lysates were immunoblotted for RhoA. ∧, p < 0.05 for 5-HT + scr group versus scr; *, p < 0.05 for 5-HT + L1 group versus 5-HT + scr. B, PASMC lysates were subjected to high-speed fractionation, and relative levels of membrane-associated (P) and cytosolic RhoA (S) were detected by immunoblotting for RhoA. The graph quantitates the S:P fraction ratio of the relative densitometric values of RhoA in each group from three experiments; ∧, p < 0.05 for 5-HT + scr group versus scr; *, p < 0.05 for 5-HT + L1 group versus 5-HT + scr. Bars = S.D. For A and B, a representative experiment of three is shown. Bars = S.D. C, duplicate groups of PASMC lysates were immunoblotted for total and phospho-(Thr696)-MYPT-1. The graph shows data from two independent experiments with duplicates, ∧, p < 0.05 for 5-HT + scr versus scr. D, duplicate groups of PASMC lysates were immunoblotted for total and phospho-p42/44 ERK. A representative experiment of three is shown.
Lbc Knockdown Attenuates 5-HT-induced ROCK Activity in PASMC
We next tested the effect of Lbc knockdown on 5-HT-induced ROCK activity. 1 μm 5-HT treatment of PASMC in the presence of scr siRNA led to a nearly 2-fold increase in phospho-(Thr696) MYPT-1 phosphatase, a key ROCK substrate whose phosphorylation leads to enhanced myosin phosphorylation and subsequent actomyosin contractility (36) (Fig. 3C). In contrast, cells transfected with L1 siRNA did not show a significant increase in phospho-MYPT-1 levels upon 5-HT stimulation (Fig. 3C). Levels of total MYPT-1 were not significantly altered by L1 compared with the respective control groups (supplemental Fig. S4B).
p42/44 ERK MAPK Activation Is Unaffected by Lbc Knockdown
5-HT induces p42/44 ERK activation in PASMC (8), and we found that 5-HT-induced p42/44 ERK activation was not affected by Lbc knockdown (Fig. 3D). This shows the specificity of Lbc siRNA action on the Rho signaling pathway.
5-HT Stimulates α-Catulin/Lbc Complex Formation in PASMC
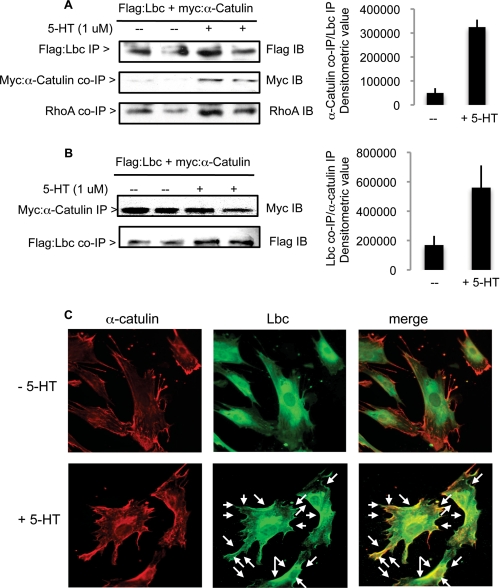
As a putative scaffold and Lbc interaction partner, we hypothesized that α-catulin may be involved in PASMC growth. Initially we assessed whether 5-HT signaling modulates α-catulin and Lbc complex formation by co-immunoprecipitation methods. 5-HT stimulation of serum-starved PASMC transfected with myc:α-catulin and FLAG:Lbc plasmids led to increased levels of α-catulin co-precipitation with Lbc (Fig. 4A). We further found that the level of RhoA associated with this complex was increased in stimulated cells (Fig. 4A). Reciprocally, levels of Lbc co-precipitating with α-catulin were increased in 5-HT-treated cells (Fig. 4B); this was also observed for endogenous proteins (supplemental Fig. S5A).
FIGURE 4.
5-HT stimulates α-catulin/Lbc/RhoA complex formation and α-catulin/Lbc peripheral colocalization in PASMC. Duplicate groups cotransfected with equimolar amounts of FLAG:Lbc and myc:α-catulin plasmids were serum-starved, then stimulated with 5-HT for 10 min. Cell lysates were immunoprecipitated (IP) with (A) bead-conjugated anti-FLAG (for Lbc) or (B) bead-conjugated anti-myc (for α-catulin) antibodies, and immunoblotted (IB) for FLAG, myc, and RhoA. The graphs show the mean densitometric values of duplicate co-precipitated material levels normalized to precipitated reciprocal protein; bars = range. C, immunofluorescence imaging of untreated (top panels) or 5-HT-treated (bottom panels) cells costained for endogenous Lbc and α-catulin using rabbit anti-Lbc and mouse α-catulin, followed by Alexa-conjugated rabbit secondary antibody for Lbc, and Texas red-conjugated mouse secondary antibody for α-catulin. Arrows in the merged image of stimulated cells show yellow areas indicative of co-localization.
PASMC were also analyzed for Lbc and α-catulin colocalization by immunofluorescence microscopy after double staining with anti-Lbc and anti-α-catulin antisera. Both unstimulated and stimulated cells showed a distinct continuous α-catulin distribution along the cell periphery, and some cytosolic localization (Fig. 4C, left top and bottom panels); there was no notable difference between the two groups. This was corroborated by subcellular fractionation showing the presence of α-catulin in both cytosolic and membrane-rich fractions, with comparable levels in both unstimulated and stimulated cells (supplemental Fig. S5B). As shown in Fig. 1B, unstimulated cells showed a pericentral distribution for Lbc with little or no peripheral Lbc localization (Fig. 4C, middle top panel); consequently, the merged image of untreated cells did not show noticeable Lbc/α-catulin colocalization (Fig. 4C, right top panel). In contrast, as observed in Fig. 1B, 5-HT treatment resulted in increased peripheral Lbc distribution (Fig. 4C, middle bottom panel, see arrows). Furthermore, the merged image of stimulated cells showed considerable Lbc/α-catulin peripheral colocalization, as shown by the yellow areas along the cell edges (Fig. 4C, right bottom panel; see arrows). These data suggest that 5-HT promotes increased Lbc/α-catulin complex formation, likely localized at the cell periphery.
α-Catulin Knockdown Attenuates 5-HT-induced PASMC Thymidine Uptake and SRF Reporter Activation
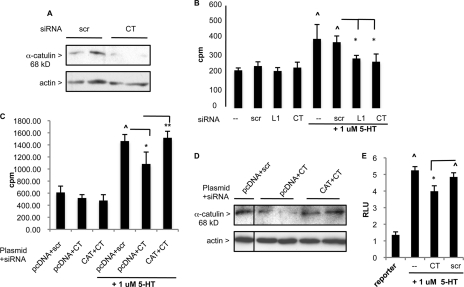
The role of α-catulin was further studied using siRNA. PASMC transfected with α-catulin CT siRNA showed knockdown of α-catulin levels of >50% (Fig. 5A). Transfection of PASMC with CT siRNA significantly attenuated 5-HT-induced PASMC thymidine uptake (∼50%), whereas scr siRNA did not (Fig. 5B). To confirm the target specificity of CT siRNA, the α-catulin plasmid was co-expressed to rescue the inhibition by CT siRNA, based on the report that the RISC complex responsible for siRNA degradation can be saturated and protein levels restored (37). Fig. 5C shows that pcDNA:α-catulin plasmid (CAT) overexpression rescued the inhibitory effect of CT siRNA. Fig. 5D confirms the restored expression of catulin in the CAT + CT siRNA group compared with the vector + CT siRNA group. Furthermore, transfection of CT, but not scr siRNA, led to reduced activation of 5-HT-induced SRF-mediated SRE.L reporter (Fig. 5E).
FIGURE 5.
α-Catulin knockdown attenuates 5-HT-induced PASMC mitogenesis and SRF-mediated reporter activation. A, α-catulin immunoblot of PASMC transfected with 50 nm scr or α-catulin (CT) siRNA after 48 h. B, [3H]thymidine uptake by PASMC transfected with 50 nm scr, L1, or CT siRNA after 48 h, then stimulated with 5-HT. n = 6; ∧, p < 0.05 for 5-HT-treated control and scr groups versus untreated control and scr, respectively; *, p < 0.05 for 5-HT + L1 or 5-HT + CT groups versus 5-HT + scr, bars = S.D. C, [3H]thymidine uptake by PASMC transfected with siRNA (50 nm) + plasmid (60 ng/96-well pcDNA or pcDNA:α-catulin (CAT)) after 48 h, then stimulated with 5-HT. ∧, p < 0.05 for pcDNA + scr +5-HT group versus pcDNA + scr; *, p < 0.05 for 5-HT + pcDNA + CT group versus 5-HT + pcDNA + scr; **, p < 0.05 for 5-HT + CAT + CT versus 5-HT + pcDNA + CT. Bars = S.D. D, cells seeded into 6-well dishes were transfected with pcDNA + scr, pcDNA + CT, or pcDNA:myc:catulin (CAT) plasmid and immunoblotted for α-catulin after 48 h serum starvation. E, cells transfected with SRE.L luciferase reporter and siRNAs were treated with 5-HT, and luciferase levels were measured. RLU, relative luciferase units. ∧, p < 0.05 for 5-HT-treated control and scr groups versus untreated control (reporter); *, p < 0.05 for CT + 5-HT versus scr + 5-HT group, n = 3. Bars = S.D.
5-HT-induced PASMC Mitogenesis Is Attenuated by Dominant-negative Gq
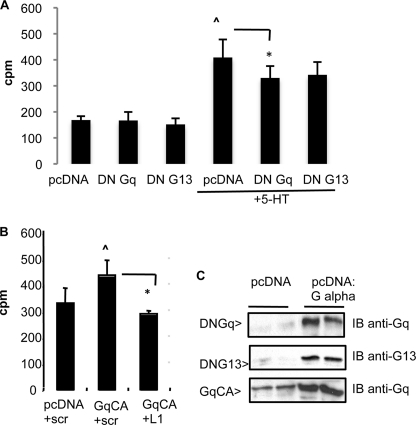
To examine the potential role of G proteins in 5-HT-induced mitogenesis, the effect of dominant-negative (DN) G proteins was tested by transfection. We found that expression of DN Gαq, but not DN Gα13, significantly diminished 5-HT-induced thymidine uptake (Fig. 6A). Furthermore, constitutively active Gαq expression stimulated PASMC mitogenesis, and this was reduced by L1 siRNA (Fig. 6B). Exogenous expression of these G proteins following plasmid transfection of PASMC was confirmed by immunoblot (Fig. 6C).
FIGURE 6.
Dominant-negative Gαq reduces 5-HT-induced PASMC mitogenesis. A, [3H]thymidine uptake by PASMC transfected with DN Gαq or 13 plasmids, then treated with 5-HT. ∧, p < 0.05 for pcDNA + 5-HT versus pcDNA; *, p < 0.05 for 5-HT + DN Gq versus 5-HT + pcDNA. B, [3H]thymidine uptake by cells transfected with constitutively activated (CA) Gαq plasmid and siRNAs. ∧, p < 0.05 for Gq CA + scr versus pcDNA + scr; *, p < 0.05 for Gq CA + L1 versus Gq CA + scr. Bars = S.D. C, immunoblots (IB) showing transfected Gα protein expression at 48 h.
DISCUSSION
The links of serotonin and Rho signaling with the abnormal smooth muscle remodeling observed in clinical and experimental PAH led us to further investigate serotonin/Rho signaling in the context of PASMC mitogenesis. Because 5-HT-induced RhoA/ROCK signaling in part involves 5-HT receptor(s) (4, 7), we reasoned that a Rho GEF(s) may participate in this process. Our observation that 5-HT treatment of PASMC induces Lbc Rho GEF translocation to a membrane-associated fraction (Fig. 1A) is the first demonstration of endogenous Rho GEF modulation by 5-HT, to our knowledge, and in particular in primary cells. This finding is supported by confocal microscopy imaging showing a redistribution of Lbc to the cell periphery in 5-HT-stimulated cells (Fig. 1B). This is compatible with increased RhoA membrane translocation induced by 5-HT, as we previously reported (38) and as shown in Fig. 3B.
Our finding that Lbc knockdown attenuates 5-HT-induced thymidine uptake (Fig. 2B) and cell proliferation (Fig. S2) indicates a role for Lbc in 5-HT-induced mitogenesis of primary PASMC, consistent with earlier studies showing that Lbc expression induces cell cycle progression (25). The lack of effect of Lbc knockdown on PDGF-induced mitogenesis (Fig. 2D) suggests a selective effect of Lbc for 5-HT-induced mitogenesis. Comparison of Lbc versus LARG Rho GEF knockdown (Fig. 2C) suggests that in the context of 5-HT and vascular smooth muscle mitogenesis, Lbc plays a more active role. This supports the concept that Rho GEFs exhibit functional specificity; e.g. LARG GEF transduces thrombin signals, whereas PDZ GEF transduces LPA signals (39). Furthermore, the embryonic cardiac defect phenotype of Brx Rho GEF knock-out mice demonstrates the distinct function of the Lbc family that is not shared by other Rho GEFs (40). Lbc likely plays a divergent physiologic role from its splice relatives, as it lacks the AKAP (protein kinase A anchoring) domain, and is not involved in PKA signaling. In this context, AKAP-Lbc is reported to function in α1-adrenergic receptor-induced cardiomyocyte hypertrophy (41). Thus our findings here on the role of the 107-kDa Lbc form in vascular smooth muscle mitogenesis suggest distinct roles for Lbc family members, consistent with their distinct structural motifs and tissue expression patterns.
Pulmonary arterial smooth muscle remodeling likely involves an altered transcriptional response of growth-related genes. SRF regulates the transcription of immediate-early genes and vascular smooth muscle-specific genes (42), and our finding that serotonin stimulates SRF-mediated transcription in PASMC (Fig. 2E) has not been previously reported to our knowledge. SRF-mediated transcription is Rho-dependent (32), and our demonstration that Lbc depletion inhibits 5-HT-induced SRF-mediated reporter activation (Fig. 2E) suggests that Lbc may be a novel intermediary in 5-HT-induced SRF activation via RhoA, and also indicates a shared role for Lbc in transcriptional and mitogenic responses. The subsequent mechanisms of Rho-mediated SRF activation via the SRF cofactor MAL have been extensively detailed (42), and this mechanism likely also operates in PASMC. The observed inhibition of 5-HT-induced RhoA/ROCK activation by Lbc depletion (Fig. 3) provides a mechanistic basis for the modulation of mitogenesis and transcription, because RhoA/ROCK are required for both responses (7), and implicates Lbc in 5-HT pathways to RhoA/ROCK.
Our finding from co-precipitation studies that 5-HT treatment increased α-catulin/Lbc complex formation in PASMC (Fig. 4, A and B) suggests that α-catulin also contributes to 5-HT-induced responses. This was corroborated by imaging analyses showing increased peripheral redistribution of Lbc induced by 5-HT (Figs. 1B and 4C), leading to increased peripheral colocalization of α-catulin/Lbc (Fig. 4C). Thus whereas the biochemical data provide physical evidence of this complex, the imaging analyses provide evidence for the peripheral colocalization in 5-HT-stimulated cells that is mainly determined by Lbc redistribution. α-Catulin likely facilitates complex formation between Lbc, RhoA, and other moieties at the cell periphery, thus promoting early signaling events triggered by 5-HT, and it will be of interest to further determine α-catulin function in this context. Our finding that α-catulin knockdown led to reduced 5-HT-induced PASMC mitogenesis and SRF reporter activation (Fig. 5, B and D) is compatible with its role as an Lbc/Rho scaffold. Moreover, the magnitude of inhibition was comparable with that observed by Lbc siRNA, consistent with α-catulin and Lbc cooperation in 5-HT-mediated mitogenesis. α-Catulin is distantly related to the cytoskeletal linkers α-catenin/vinculin (43, 44), and has been linked to epithelial proliferation (45). Its cell biologic function is only partially understood, and whether α-catulin has additional roles that influence mitogenesis remains to be determined.
5-HT-induced p42/44 ERK MAP kinase activation was unaffected by Lbc knockdown (Fig. 3D), indicating that Lbc lies downstream or parallel to ERK, consistent with the report that RhoA/ROCK inhibition does not affect 5-HT-induced ERK activation in PASMC (7). As a Rho GEF lacking a PDZ domain, Lbc presumably would not directly complex with receptors, and is likely downstream of early signaling intermediaries. PASMC express multiple 5-HT receptors, several of which are G protein-coupled receptors (5-HT GPCR 1a/1b and 3) (46), and the G proteins linked to 5-HT GPCRs in these cells are unknown. Our finding that dominant-negative Gαq attenuates 5-HT-induced PASMC mitogenesis (Fig. 6) presents the possibility that Gq signals cooperate with Lbc/α-catulin, as previously suggested (47). The effect of DN Gq was not as strong as that of Lbc knockdown; this may reflect the fact that the multiple 5-HT receptor isotypes in PASMC likely couple to more than one G protein type, and blocking a single G protein may only have a limited effect. In addition, Lbc may couple to alternative upstream signaling moieties. Overall, our studies for the first time demonstrate a requirement for a Rho GEF in primary vascular smooth muscle cell growth induced by serotonin, in conjunction with the α-catulin scaffold. Recent reports showing that ROCK is activated in pulmonary hypertension patients (48, 49) further implicate the Rho/ROCK pathway in this disease, and presents the possibility that the Lbc Rho GEF/α-catulin axis may be an interventional target in diseases involving vascular smooth muscle remodeling that includes PAH.
Supplementary Material
Acknowledgments
We thank Kozo Kaibuchi and Matthew Hart for plasmids, and Alenka Lovy-Wheeler for assistance in the Tufts Imaging Center for Neuroscience Research, Tufts University of Medicine, supported by Grant P30 NS047243 (Jackson).
This work was supported, in whole or in part, by National Institutes of Health Grant HL085260 (to B. L. F.) and American Heart Association Grant 0756021T (to D. T.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
- 5-HT
- 5-hydroxytryptamine
- PASMC
- pulmonary arterial smooth muscle cells
- PAH
- pulmonary arterial hypertension
- ROCK
- Rho kinase
- GEF
- guanine nucleotide exchange factor
- scr
- scrambled
- SRF
- serum response factor
- SRE
- serum response element
- DN
- dominant negative
- AKAP
- protein kinase A anchoring
- CT
- α-catulin.
REFERENCES
- 1.Fanburg B. L., Lee S. L. (1997) Am. J. Physiol. Lung Cell. Mol. Physiol. 272, L795–806 [DOI] [PubMed] [Google Scholar]
- 2.Azmitia E. C. (2001) Brain Res. Bull. 56, 413–424 [DOI] [PubMed] [Google Scholar]
- 3.Lee S. L., Wang W. W., Moore B. J., Fanburg B. L. (1991) Circ. Res. 68, 1362–13688 [DOI] [PubMed] [Google Scholar]
- 4.MacLean M. R., Dempsie Y. (2009) Curr. Opin. Pharmacol. 9, 281–286 [DOI] [PubMed] [Google Scholar]
- 5.Esteve J. M., Launay J. M., Kellermann O., Maroteaux L. (2007) Cell. Biochem. Biophys. 47, 33–44 [DOI] [PubMed] [Google Scholar]
- 6.Ramage A. G., Villalón C. M. (2008) Trends Pharmacol. Sci. 29, 472–481 [DOI] [PubMed] [Google Scholar]
- 7.Liu Y., Suzuki Y. J., Day R. M., Fanburg B. L. (2004) Circ. Res. 95, 579–586 [DOI] [PubMed] [Google Scholar]
- 8.Lee S. L., Wang W. W., Finlay G. A., Fanburg B. L. (1999) Am. J. Physiol. Lung Cell. Mol. Physiol. 277, L282–L291 [DOI] [PubMed] [Google Scholar]
- 9.Liu Y., Fanburg B. L. (2006) Am. J. Respir. Cell Mol. Biol. 34, 182–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaffe A. B., Hall A. (2005) Annu. Rev. Cell Dev. Biol. 21, 247–269 [DOI] [PubMed] [Google Scholar]
- 11.Toksoz D., Merdek K. D. (2002) Histol. Histopathol. 17, 915–927 [DOI] [PubMed] [Google Scholar]
- 12.Loirand G., Guérin P., Pacaud P. (2006) Circ. Res. 98, 322–334 [DOI] [PubMed] [Google Scholar]
- 13.Rossman K. L., Der C. J., Sondek J. (2005) Nat. Rev. Mol. Cell Biol. 6, 167–180 [DOI] [PubMed] [Google Scholar]
- 14.Zheng Y., Olson M. F., Hall A., Cerione R. A., Toksoz D. (1995) J. Biol. Chem. 270, 9031–9034 [DOI] [PubMed] [Google Scholar]
- 15.Diviani D., Soderling J., Scott J. D. (2001) J. Biol. Chem. 276, 44247–44257 [DOI] [PubMed] [Google Scholar]
- 16.Rubino D., Driggers P., Arbit D., Kemp L., Miller B., Coso O., Pagliai K., Gray K., Gutkind S., Segars J. (1998) Oncogene 16, 2513–2526 [DOI] [PubMed] [Google Scholar]
- 17.Klussmann E., Edemir B., Pepperle B., Tamma G., Henn V., Klauschenz E., Hundsrucker C., Maric K., Rosenthal W. (2001) FEBS Lett. 507, 264–268 [DOI] [PubMed] [Google Scholar]
- 18.Fukuhara S., Chikumi H., Gutkind J. S. (2001) Oncogene. 20, 1661–1668 [DOI] [PubMed] [Google Scholar]
- 19.Lutz S., Shankaranarayanan A., Coco C., Ridilla M., Nance M. R., Vettel C., Baltus D., Evelyn C. R., Neubig R. R., Wieland T., Tesmer J. J. (2007) Science 318, 1923–1937 [DOI] [PubMed] [Google Scholar]
- 20.Toksoz D., Williams D. A. (1994) Oncogene. 9, 621–628 [PubMed] [Google Scholar]
- 21.Sterpetti P., Hack A. A., Bashar M. P., Park B., Cheng S. D., Knoll J. H., Urano T., Feig L. A., Toksoz D. (1999) Mol. Cell. Biol. 19, 1334–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park B., Nguyen N. T., Dutt P., Merdek K. D., Bashar M., Sterpetti P., Tosolini A., Testa J. R., Toksoz D. (2002) J. Biol. Chem. 277, 45361–45370 [DOI] [PubMed] [Google Scholar]
- 23.Wiesner C., Winsauer G., Resch U., Hoeth M., Schmid J. A., van Hengel J., van Roy F., Binder B. R., de Martin R. (2008) Oncogene 27, 2159–2169 [DOI] [PubMed] [Google Scholar]
- 24.Kino T., Takatori H., Manoli I., Wang Y., Tiulpakov A., Blackman M. R., Su Y. A., Chrousos G. P., DeCherney A. H., Segars J. H. (2009) Sci. Signal. 2, ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olson M. F., Sterpetti P., Nagata K., Toksoz D., Hall A. (1997) Oncogene 15, 2827–2831 [DOI] [PubMed] [Google Scholar]
- 26.Sterpetti P., Marucci L., Candelaresi C., Toksoz D., Alpini G., Ugili L., Baroni G. S., Macarri G., Benedetti A. (2006) Am. J. Physiol. Gastroentest. Liver Physiol. 290, G624–G632 [DOI] [PubMed] [Google Scholar]
- 27.Majumdar M., Seasholtz T. M., Buckmaster C., Toksoz D., Brown J. H. (1999) J. Biol. Chem. 274, 26815–26821 [DOI] [PubMed] [Google Scholar]
- 28.Chen L. Y., Zuraw B. L., Ye R. D., Pan Z. K. (2004) J. Biol. Chem. 279, 7208–7212 [DOI] [PubMed] [Google Scholar]
- 29.Pi M., Spurney R. F., Tu Q., Hinson T., Quarles L. D. (2002) Endocrinology 143, 3830–3838 [DOI] [PubMed] [Google Scholar]
- 30.Elbashir S. M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. (2001) Nature 411, 494–498 [DOI] [PubMed] [Google Scholar]
- 31.Dutt P., Nguyen N., Toksoz D. (2004) Cell. Signal. 16, 201–209 [DOI] [PubMed] [Google Scholar]
- 32.Hill C. S., Wynne J., Treisman R. (1995) Cell 81, 1159–1170 [DOI] [PubMed] [Google Scholar]
- 33.Meyer B. H., Freuler F., Guerini D., Siehler S. (2008) J. Cell. Biochem. 104, 1660–1670 [DOI] [PubMed] [Google Scholar]
- 34.Long L., MacLean M. R., Jeffery T. K., Morecroft I., Yang X., Rudarakanchana N., Southwood M., James V., Trembath R. C., Morrell N. W. (2006) Circ. Res. 98, 818–827 [DOI] [PubMed] [Google Scholar]
- 35.Adamson P., Paterson H. F., Hall A. (1992) J. Cell Biol. 119, 617–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Somlyo A. P., Somlyo A. V. (2000) J. Physiol. 522, 177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dykxhoorn D. M., Novina C. D., Sharp P. A. (2003) Nat. Rev. Mol. Cell Biol. 4, 457–467 [DOI] [PubMed] [Google Scholar]
- 38.Li M., Liu Y., Dutt P., Fanburg B. L., Toksoz D. (2007) Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L463–471 [DOI] [PubMed] [Google Scholar]
- 39.Wang Q., Liu M., Kozasa T., Rothstein J. D., Sternweis P. C., Neubig R. R. (2004) J. Biol. Chem. 279, 28831–28834 [DOI] [PubMed] [Google Scholar]
- 40.Mayers C. M., Wadell J., McLean K., Venere M., Malik M., Shibata T., Driggers P. H., Kino T., Guo X. C., Koide H., Gorivodsky M., Grinberg A., Mukhopadhyay M., Abu-Asab M., Westphal H., Segars J. H. (2010) J. Biol. Chem. 285, 12344–12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Appert-Collin A., Cotecchia S., Nenniger-Tosato M., Pedrazzini T., Diviani D. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 10140–10145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Posern G., Treisman R. (2006) Trends Cell Biol. 16, 588–596 [DOI] [PubMed] [Google Scholar]
- 43.Zhang J. S., Nelson M., Wang L., Liu W., Qian C. P., Shridhar V., Urrutia R., Smith D. I. (1998) Genomics 54, 149–154 [DOI] [PubMed] [Google Scholar]
- 44.Janssens B., Staes K., van Roy F. (1999) Biochim. Biophys. Acta 1447, 341–347 [DOI] [PubMed] [Google Scholar]
- 45.Xiang Y., Tan Y. R., Zhang J. S., Qin X. Q., Hu B. B., Wang Y., Qu F., Liu H. J. (2008) J. Cell. Biochem. 103, 920–930 [DOI] [PubMed] [Google Scholar]
- 46.Day R. M., Agyeman A. S., Segel M. J., Chévere R. D., Angelosanto J. M., Suzuki Y. J., Fanburg B. L. (2006) Biochem. Pharmacol. 71, 386–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sagi S. A., Seasholtz T. M., Kobiashvili M., Wilson B. A., Toksoz D., Brown J. H. (2001) J. Biol. Chem. 276, 15445–15452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Do e Z., Fukumoto Y., Takaki A., Tawara S., Ohashi J., Nakano M., Tada T., Saji K., Sugimura K., Fujita H., Hoshikawa Y., Nawata J., Kondo T., Shimokawa H. (2009) Circ. J. 73, 1731–1739 [DOI] [PubMed] [Google Scholar]
- 49.Guilluy C., Eddahibi S., Agard C., Guignabert C., Izikki M., Tu L., Savale L., Humbert M., Fadel E., Adnot S., Loirand G., Pacaud P. (2009) Am. J. Respir. Crit. Care Med. 179, 1151–1158 [DOI] [PubMed] [Google Scholar]
- 50.Ross R. (1971) J. Cell Biol. 50, 172–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.