Abstract
Stroke is a major cause of mortality and morbidity in the United States. The ideal therapeutic approach would minimize cell death and regenerate brain tissue. In order to investigate some questions that are related to such an approach, we have generated a mouse model in which we induce a stroke using the Middle Cerebral Artery Occlusion method. After 2h occlusion followed by reperfusion, 99% of mice died within 8 days of stroke. Total bone marrow cell transplantation by intravenous injection revealed an optimal timing of cell transfer in two doses on days 1 (same day of surgery) and 2 after surgery. Moreover, intravenous injection of Sca1+ bone marrow cells (enriched in hematopoietic stem cells) showed a dose-response effect on survival. Surviving mice also had no signs of apparent paralysis or weakness. Tracking analysis using donor stem cells expressing LacZ revealed only few donor cells in the brain. We conclude that hematopoietic stem cell-rich Sca1+ bone marrow cell transplantation after stroke protects the brain of a sizeable portion of mice subjected to stroke and alleviate remarkably the resulting neurological morbidity in surviving mice.
Keywords: Stroke, hematopoietic stem cells, transplantation, neuroprotection, survival
Introduction
Stroke is one of the most common causes of death and severe disabilities in developed countries (Davenport and Dennis, 2000). Stem cell therapy has been used with variable outcomes both in clinics (Bang et al., 2005; Savitz et al., 2004) and in experimental stroke (mice and rats) (Chen et al., 2003; Borlongan et al, 2004; Gage et al, 1995; Liu et al, 2006; Honna et al, 2006; Zin’Kova et al, 2007; Wei et al, 2007; Ukai et al, 2007; Yanagisawa et al, 2006; Hayashi et al, 2006; Tagushi et al, 2004; Nystedt et al, 2006; Willing et al, 2003; Jiang et al, 2006; Comi et al, 2008; Lee et al, 2007; Kelly et al, 2004; Guzman et al, 2008). Interestingly, the infarct size impact in the brain was quite variable between studies, ranging from <50% of the hemisphere (Hanna et al, 2006; Nystdet et al, 2006) to > 50% of the hemisphere (Ukai et al, 2003; Jiang et al, 2006), often decreasing after stem cell transplantation (Hanna et al, 2006; Ukai et al, 2003; Jiang et al, 2006), but sometimes remaining unchanged (Nystedt et al, 2006). The difference in the outcome could be related to the initial lesion, the modality of transplantation (intravenous vs intracranial injection) or type of transplanted cells (human CD34+ cells, murine hematopoietic, mesenchymal, neural, or embryonic stem cells). Indeed, some studies used neural stem cells (NSCs) from rodents (Jiang et al, 2006; Comi et al, 2008; Guzman et al, 2008; Gage et al, 1995) or human (Kelly et al, 2004; Lee et al, 2007), or primate origin (Hayashi et al, 2006), mesenchymal stem cells (MSC) from mouse (Veng et al, 2008; Zin’kova et al, 2007; Wei et al, 2007; Ukai et al, 2007) and human (Liu et al, 2006; Honna et al, 2006), mouse ES cells (Yanagisawa et al, 2006), and human hematopoietic stem cells (HSCs) (Nystedt et al, 2006; Willing et al, 2003; Tagushi et al, 2004). While the primary focus in previous studies ranged from elucidating the fate of donor cells and infarct size to the description of functional recovery, to our knowledge, the ability of transplanted stem cells to rescue mice from a lethal injury has never been reported. Furthermore, there has been no study evaluating murine HSCs in such a rescue experiment.
In this study, using the MCAO technique, we generated a mouse model for stroke with a large reproducible infarct size that resulted in the death of almost all mice within 8 days of surgery, and demonstrated that the intravenously (IV) administered murine Sca1+ stem and progenitor cells can rescue ~40% of animals and reduce infarct size by ≥50%. Our study provides the first evidence that murine HSC-rich Sca1+ bone marrow cell population plays a major role in protecting mice from death after induction by a stroke model. We also showed that the timing of the transplantation is important for the success of the therapy.
Materials and methods
Mouse strains
Mice used were obtained from the Jackson laboratories (Jackson Laboratories, Bar Harbor, ME, USA). Animals used for generating the stroke model were male C57BL/6J, 8–10 weeks of age. Mice used as stem cell donor were either male C57BL/6J, or male ROSA26 mice on a C57BL/6J background, 8–12 weeks of age. All mice were housed in a pathogen-free environment, and procedures were conducted in compliance with the Guidelines of the Institutional Animal Care and Use Committee (IACUC).
Generating the stroke model
The stroke model was generated using the middle cerebral artery occlusion (MCAO) method, as previously described (Hess et al, 2002). Briefly, mice were anesthetized and the right carotid arteries exposed. The common carotid artery (CCA) and external carotid artery (ECA) were ligated, and a small incision done in the CCA through which a silicon rubber-coated monofilament 6-0 was inserted (diameter with coating 0.31 ± 0.02mm, length of coating 5–6 mm, Docol Corporation) and pushed 11 mm inside of the internal carotid artery (ICA) to block the middle cerebral artery (MCA). The CCA and ICA were ligated to secure the filament in place and prevent bleeding. In permanent MCAO, the filament was kept in place, while in transient MCAO, it was withdrawn after variable occlusion time (2h, 4h) to re-establish blood flow through the MCA. The CCA was ligated afterwards to prevent bleeding. Mice were sutured and observed afterwards. Animal temperature was monitored throughout the procedure.
Detecting and quantifying the infarct
Mice were sacrificed by halothane inhalation and decapitated. Whole brains or 1 mm slices were stained with 2% 2,3,5, triphenyl tetrazolium chloride (TTC) for 30 min at 37°C, and then fixed with 4% paraformaldehyde, as previously described (Bederson et al, 1986; Yanamoto et al, 2003). Live tissue stains red while infarct areas appear white. The infarct volume was quantified using ImageJ software, and expressed as a percentage relative to the ipsilateral hemisphere.
Assessing the mouse functional status
Neurological impairment was assessed 2h after recovering from anesthesia, then 7 days and 14 days later in surviving animals. The scoring system was used as previously described (Mc Cullough et al, 2005) with slight modifications: for each sign, the grade “0” was given if the sign is not detected, and “1, 2, 3, or 4” given respectively to each of the following signs: torso turned towards the right, circular motion towards the right, mouse can’t bare weight i.e. leaning down at the side of paralysis, barrel rolling precipitated by slightly lifting the mouse by the tail or placing it on its back. The final score was the sum of the grades of all 4 signs, ranging from 0 (normal) to 10 (highly impaired). In addition to this score, the righting reflex was tested by placing the mouse on its back: when the mouse displays a random motion before righting up it is given the sign “+” i.e. impaired; if it rights up normally it is given the sign “−” i.e. not impaired. Mice not showing neurological deficit (circular motion) 2h after recovery from anesthesia were excluded from the study.
Hematopoietic stem cell transplantation (HSCT)
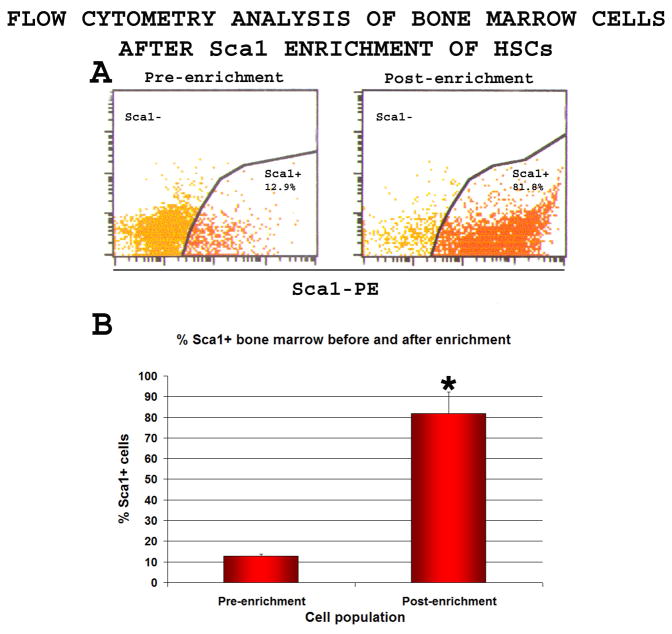
At various times after the surgery (2h, 1 day, 2 days, 3 days, 4 days) MCAO mice were subjected to intravenous injection of either total bone marrow cells or Sca1+-enriched bone marrow cells without prior immunosuppression. The Sca1+-enriched cell population includes hematopoietic stem and progenitor cells, excluding most differentiated cells and inflammatory cells (Wilson et al, 2007). Briefly, bone marrow was extracted from long bones of healthy donor mice (C57BL/6J or ROSA26 on C57BL/6J background, 8–12 weeks of age) and red blood cells were lysed with RBC lysis buffer (Sigma). Cells were then either directly used for bone marrow transplantation (BMT), or processed for the positive selection of Sca1+ cells using the EasySep Mouse Sca1 Selection Cocktail (Stem Cell Technologies, Vancouver, Canada), whereby the cells are labeled with anti-Sca1 antibody that is coupled to the fluorescent dye Phyco-Erythrin (PE). Samples of the cells before and after enrichment were passed through a flow cytometer to assess the enrichment using Phyco-Erythrin labeling, which showed ~6–7-fold enrichment of the stem and progenitor cells (Figure 2). Total bone marrow cells or Sca1+ cells were injected intravenously in 50–100 μl PBS in a lateral tail vein.
Figure 2. Sca1 positive selection of hematopoietic stem and progenitor cells.
Total bone marrow cells were labeled with Phycoerythrin-coupled anti-Sca1 antibody and processed for positive selection of Sca1+ cells. Samples taken before and after enrichment were analyzed.
A-The Sca1+ cell population increased from 12.9±1.1% before enrichment to 81.9±10.5% after enrichment (n=5)
B-A 6.4 ±0.7 fold increase in the percentage of Sca1+ cells was observed (n=5)
*T-test, % Sca1+ cells before enrichment vs. after enrichment, p<0.0001.
Tracing of donor cells
Sca1+ cells obtained from ROSA26 mice were used as donor cells. Mice were sacrificed with a lethal inhalation of halothane followed by decapitation. Brains were quickly extracted and fixed for 30 min at RT in a mix of 0.2% glutaraldehyde and 1.48% formaldehyde in PBS. Brains were then sliced into 1 mm-thick slices and β-galactosidase expressing cells were detected by incubating the sections at 37°C overnight with X-gal (final concentration of 1 mg/ml) in X-Gal developer mix (35mM K3Fe(CN)6/35mM K4Fe(CN)63H2O/2mM MgCl2 in PBS) to form a blue reaction product within the cell. Slices were then incubated in 20% sucrose overnight, then frozen and processed for cryosectioning. From each 1mm brain slice 10 μm sections separated by 50μm were obtained using a cryotome and counterstained with nuclear fast red resulting in a pink background. Cell counting was done with an automated Zeiss microscope and was fully computerized with the combined use of two advanced softwares that are directly connected to the microscope: the AxioVision Rel4.5 and the GSA Image Analyzer v3.5.6 softwares: the AxioVision Rel4.5 allowed precise and complete automated scan of consecutive and adjacent fields for full coverage of the slices and image acquisition; the acquired images were directly relayed to the GSA Image Analyzer v3.5.6 which counted the cells. The LacZ+ cells and total brain cells in each field were electronically counted based on morphological characteristics recognized by the software. The amount of LacZ+ cells was then corrected to the total number of cells on the slices used, and expressed as number of LacZ+ cells per 10000 total brain cells.
Immunohistochemistry
Frozen 10μm brain sections were processed for the detection of neurons and astrocytes using the NeuN and GFAP markers, respectively. Briefly, cells were permeabilized with 0.2% Triton X-100 for 30 minutes at room temperature (RT), washed with PBS and blocked with 3% Normal Goat Serum (Vector Laboratories), then incubated with primary antibodies at 4°C overnight (mouse anti-NeuN, 1:200, Chemicon; rabbit anti-GFAP, 1:200, Santa Cruz). Sections were then washed with 0.1% Triton X-100 and incubated with secondary antibodies (goat anti-mouse Fluorescein, 1:200; goat anti-rabbit Texas Red 1:200, both from Chemicon) for 2.5h at RT, washed with 0.1% Triton X-100, then DAPI was added. Sections were examined using a Zeiss fluorescent microscope, and pictures were acquired using AxioCan M Rm camera (Zeiss) and AxioVision Rel4.5 software. The quantification of the cells was done as described above for LacZ cells, and the relative proportion of NeuN+ cells and GFAP+ cells were expressed as percentage relative to the total number of DAPI+ cells counted.
Statistical analysis
Statistical analysis was performed using the Student t-test available in Microsoft Excel Software, and values were considered significant when p<0.05.
Results
Generating the MCAO model: infarct size, survival, and behavior
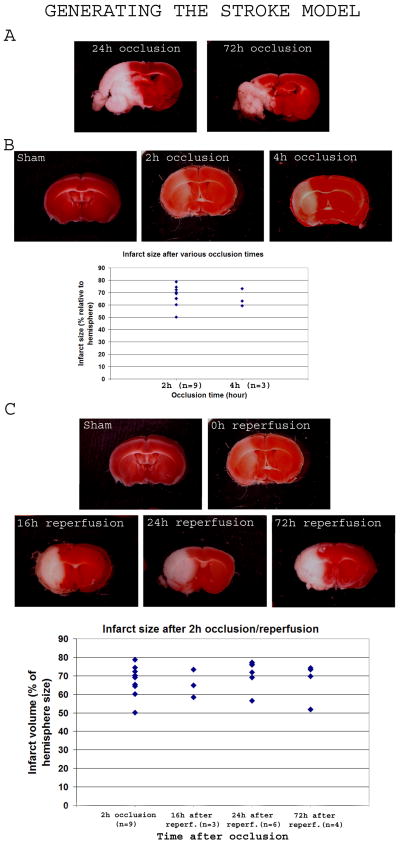
Permanent occlusion of the middle cerebral artery resulted in death of the mice within 4 days (n=10; Table 1). The infarct was substantial and occupied most of the affected hemisphere after 24h and 72h occlusion (Figure 1A). Occlusion for 2h (n = 12) and 4h (n = 7) resulted in the reproducible formation of a large infarct (infarct volume occupied 67.3 ± 8.4% and 65.2 ±7.1% of the ipsilateral hemisphere after 2h- and 4h-occlusion, respectively) that increased in intensity with the occlusion time when brains were stained immediately after reperfusion (Figure 1B). When mice were subjected to a two-hour occlusion followed by reperfusion (n=115), the survival time was < 2 weeks (only one mouse survived past 2 weeks) (Table 2), and TTC staining of their brains at various times after reperfusion (16h, 24h, 72h) revealed complete tissue death by 16h, while the infarct size remained comparable overtime (Infarct volume relative to the ipsilateral hemisphere, respectively: 65.56 ±7.57%, n=3; 71.15 ±7.82%, n=6; 67.34 ±9.13%, n=4) (Figure 1C).
Table 1.
Survival time of mice after permanent Middle Cerebral Artery Occlusion
| Survival time after MCAO | n |
|---|---|
| 16h | 1 |
| 1 day | 3 |
| 2 days | 1 |
| 3 days | 3 |
| 4 days | 2 |
A total of 10 mice subjected to permanent MCAO were observed until death occurred, and the time elapsed between the surgery and death was recorded (left column); the “n” above represents the number of mice among the 10 observed that died at each time point indicated in the left column.
FIGURE 1. Generating the stroke model.
Brains of control mice and mice subjected to stroke were sectioned and stained with 2, 3, 5 triphenyl tetrazolium chroride (TTC) to detect the infarct area. Intact tissue stained red while infarcted tissue appears white.
1A-After permanent occlusion, TTC-stained brains after 24h and 72h of MCAO revealed a major infarct area occupying most of the hemisphere, and liquefying at 72h.
1B-Permanent occlusion of only 2h or 4h resulted in a clear infarct, more severe with a longer occlusion time, and respectively occupying 69.7 ±6.5% and 65.2 ±7.1% of the ipsilateral hemisphere as shown in the histogram.
1C-Mice were subjected to 2h MCAO followed by reperfusion and TTC staining at variable times afterwards (16h, 24h, 72h). The infarct area appeared a mix of white and pink in color just after 2h. Overtime, the color became fully white in the affected area suggesting complete death of the tissue in the affected area. The scatter graphic shows a comparative quantification of the infarct size just after 2h occlusion (left bar), then at various times after reperfusion of a 2h-occlusion MCAO (16h, 24h and 72h after reperfusion, respectively second, third and fourth cluster).
Table 2.
Survival time of mice after transient middle cerebral artery occlusion followed by reperfusion
| Occlusion time | n | Survival |
|---|---|---|
| 2h | 115 | 16h – 12 days* |
n, number of mice; h, hours
one mouse remained alive all through follow-up
Mice subjected to 2h-occlusion followed by reperfusion were further characterized to assess the neurological impairment (Table 4). They all displayed a circular clockwise motion, torso turned towards the right, inability to bear their weight on the side of paralysis, impaired righting reflex. 29% of them displayed barrel rolling precipitated by either partially lifting the mouse by the tail, or placing it on its back. Due to the reproducibility of the phenotype it yielded, a 2h occlusion followed by reperfusion was adopted as our model in this study.
Table 4.
Neurological scoring of MCAO mice
| Mouse groups | Pre-HSCT (2h after MCAO) |
Post-HSCT (7 days after transplantation) |
14 d |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Tors Tilt 1 | Circul motion2 | Can’t bare weight | Barrel rolling | Right reflex | Score | n (%) | Tors tilt | Circul motion | Can’t bare weight | Barrel rolling | Right reflex | Score | Score | |
| MCAO cont (A) | 15 (71%) | 1 | 2 | 3 | 0 | + | 6 | 4 (67%) | 1 | 2 | 3 | 0 | + | 6 | n/a |
| MCAO cont (B) | 6 (29%) | 1 | 2 | 3 | 4 | + | 10 | 2 (33%) | 1 | 2 | 3 | 4 | + | 10 | n/a |
| MCAO + 6 × 106 | 17 (59%) | 1 | 2 | 3 | 0 | + | 6 | 5 (42%) | 1* | 2** | 0 | 0 | − | 3a,c | 0 |
| HSCs (C) | 4 (33%) | 0 | 0 | 0 | 0 | − | 0a,c | 0 | |||||||
| MCAO + 6 × 106 | 12 (42%) | 1 | 2 | 3 | 4 | + | 10 | 2 (17%) | 1* | 2** | 0 | 0 | − | 3b,d | 0 |
| HSCs (D) | 1 (8%) | 0 | 0 | 0 | 0 | − | 0b,d | 0 | |||||||
| MCAO + 12 × 106 | 7 (70%) | 1 | 2 | 3 | 0 | + | 6 | 3 (60%) | 1* | 2** | 0 | 0 | − | 3e,f | 0 |
| HSCs (E) | 1 (20%) | 0 | 0 | 0 | 0 | − | 0 | 0 | |||||||
| MCAO + 12 × 106 | 3 (30%) | 1 | 2 | 3 | 4 | + | 10 | 1 (20%) | 0 | 0 | 0 | 0 | − | 0 | 0 |
| HSCs (F) | |||||||||||||||
n, number of mice; %, percentage of mice; MCAO, middle cerebral artery occlusion; HSCT, hematopoietic stem cell transplantation; HSCs, hematopoietic stem cells; Naïve cont., control mice that were not subjected to MCAO; MCAO cont., control mice subjected to MCAO only; Tors tilt, torso turned towards the right; circul motion, circular motion towards the right; n/a, not applicable (mice were dead by that time); 14d, 14 days post HSCT.
Scoring: all signs but the righting reflex were given a score as previously described with slight modification (McCullogh et al, 2005). Different signs were given a different “grading” number and the total score was the sum of the grades of all the graded signs; 0, sign not detected; 1, 2, 3, 4, sign detected; Righting reflex was noted as impaired (+, i.e. mouse shakes/moves randomly before righting up), or normal (−, mouse right up immediately without any random movement).
Circular motion: mouse turns in a circular fashion instead of walking straight; Can’t bare weight, mouse is leaning towards the paralyzed side and cannot hold up its body weight on that side; Barrel rolling; when placed on their back or slightly lifted by the tail, mice roll once or twice like a barrel; righting reflex; mice are placed on their back.
the torso is turned to the right in a pronounced way;
the torso is slightly turned to the right;
circular motion 10–21 times per minute;
circular motion ≤ 5 times per minute.
t-test:
group C 2h vs. 7 days, p<4 × 10-8;
group D, 2h vs. 7 days, p<0.02;
group A vs. C, 7 days, p<0.004;
group D vs. B, 7 days, p<0.02;
groups E & F, 2h 7 days, p<0.0003;
groups E & F, 7 days vs. groups A & B, 7 days, p<0.0008
Bone marrow transplantation (BMT) strategy and findings
In order to test the effect of cellular therapy on the phenotype of our stroke model, we first used total bone marrow cell infusion, with bulk unpurified BM cells as donor, by exploring three experimental parameters: number of total bone marrow (BM) cells injected (7.0 × 107, 1.5 × 108, 2.0 × 108, 5.0 × 108), number of injections (1, 2 or 3), and timing of cell administration (same day of surgery or day 1, following days: day 2, 3, 4). Animals subjected to MCAO and injected with PBS were used as controls (n=13). They all showed the same phenotype and survival as the non-injected MCAO mice (data not shown).
Animals subjected to MCAO received total bone marrow cells in one injection on day 1 (7.0 × 107 cells; n=3) or day 2 (7.0 × 107 cells, n=6; 1.5 × 108 cells, n=6), in 2 equal injections on days 1 and 2 (total 2.0 × 108 cells; n=3), or in 3 equal injections on days 2, 3, 4 (5.0 × 108 cells; n=6). (Table 3). Mice transplanted on day 1 all died within the following 24h while mice transplanted on day 2 with an equal number of cells showed a slightly better pattern with one mouse remaining alive for over two weeks and two surviving 4 days. Injection of twice more cells on day 2 allowed few mice to survive 5 or 7 days while the remaining died within 24h (Table 3). When cells were injected in two equal doses on day 1 & 2, none of them died in 24h, and two of three mice survived 6 and 7 days respectively. BMT in three injections at days 2, 3 & 4 of 5.0 × 108 cells did not show any improvement in survival compared to mice transplanted on days 1 & 2 (Table 3). Although most of these mice died within the range of non-transplanted controls, there was a clear shift towards longer survival when more cells were used (7.0 × 107 vs. 1.5 × 108 & 5.0 × 108). The timing of cell injection also seemed to be important: three consecutive injections on days 2, 3 & 4 were not more efficient than two injections on days 1 & 2. We therefore hypothesized that the best BMT strategy would be two injections on days 1 (day of surgery) & 2 (one day after surgery). The data also suggested that a very large amount of cells does not seem necessary or beneficial. Furthermore, most mice which were transplanted with total unpurified BM cells died in less than 2 weeks even with higher amounts of cells, suggesting the possibility that inflammatory cells present in the total BM might be having a negative effect. Therefore we decided to use an enriched population of HSC where differentiated “lineage” cells would be removed.
Table 3.
Transplantations with total bone marrow cells: strategies and outcomes
| Transplantation time | Total No. of cells | No. of injections | No. of recipients | No. of mice surviving > 2 weeks | Survival time of individual mice dying in < 2 weeks |
|---|---|---|---|---|---|
| Day 1* | 7.0 × 107 | 1 | 3 | 0 | < 24h |
| Day 2 | 7.0 × 107 | 1 | 7 | 1 | < 24h (n=4); 4d (n=2) |
| Day 2 | 1.5 × 108 | 1 | 7 | 0 | 2d (n=4); 5d (n=2); 7d (n=1) |
| Days 1, 2 | 2.0 × 108 | 2 | 3 | 0 | 3d (n=1); 6d (n=1); 7d (n=1) |
| Days 2, 3, 4 | 5.0 × 108 | 3 | 6 | 0 | 2d (n=1); 5d (n=3); 6d (n=2) |
No., number; n, number of mice; d, days;
day 1 is the same day of the MCAO surgery.
Sca1+ selection provides over 6-fold enrichment of stem and progenitor cells
Total mouse bone marrow cells obtained from healthy donors were subjected to lineage depletion through Sca1+-selection with Phycoerythrin-labelled Sca1 antibody.
Flow cytometry analysis of bone marrow cells before and after enrichment showed that the percentage of Sca1+ cells increased from 12.9±1.1% before selection to 81.9±10.5% after selection (n=5) (Figure 2A), a 6.4 ±0.7 fold enrichment (Figure 2B). These data provided direct evidence for elimination of a major part of lineage (differentiated and inflammatory) cells.
Sca1+ bone marrow cell transplantation rescues mice after MCAO
In order to test if HSCT can rescue the phenotype of mice in our model, Sca-1+-enriched bone marrow cell population were collected from healthy C57BL/6 and intravenously administered to MCAO mice ~2h after the reperfusion. Increasing number of cells (5.0 × 105, n=7; 1.0 × 106, n=3; 1.5 × 106, n=4; 2.0 × 106, n=4; 3.0 × 106, n=16) resulted in a modest increase in survival (Figure 3A). However, the majority of mice died. When mice were transplanted with two doses of 3.0 × 106 cells with the second dose 24h later (totaling 6.0 × 106 cells, n=29), ~40% of mice survived >2 months (Figure 3A). A similar result was obtained after doubling the number of cells per injection to 6.0 × 106, totaling 12 × 106 cells in two doses (n=11) (Figure 3A). Kaplan-Meier survival curves illustrate the improvement of survival for these mice (Figure 3B).
Figure 3. Survival rate of MCAO mice after Sca1+ bone marrow cell transplantation.
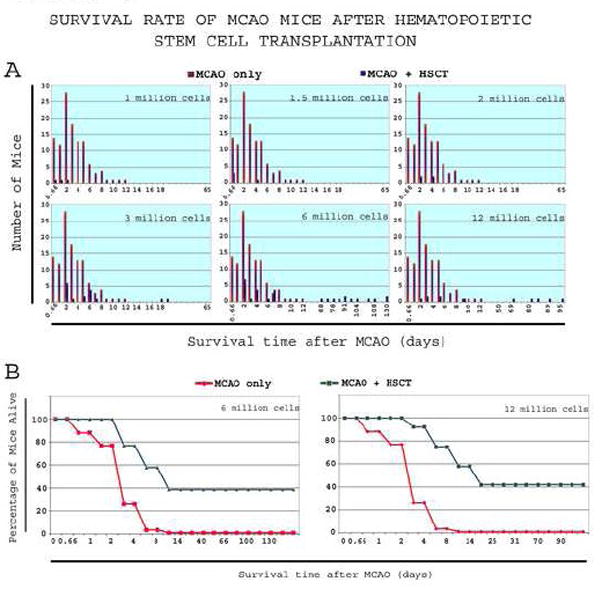
Mice subjected to MCAO followed by Sca1+ cell transplantation, as well as non-transplanted control, had their lifespan measured
3A-Over 60% of the controls died within 3 days. After Sca1+ cell transplantation, a progressive shift-to-the-right in survival was observed with increasing number of cells. Starting with 2 million cells, no mice died in <24h, and with 3 million cells, the average survival was clearly shifted towards longer lifespan, with few mice remaining alive after 2 months. Starting 6 million cells, ~40% of mice survived past 2 months.
3B-Kaplan-Meier curves of MCAO controls and transplant-recipient of 3 and 6 million cells shows long-term survival of a relatively large proportion of mice compared to controls.
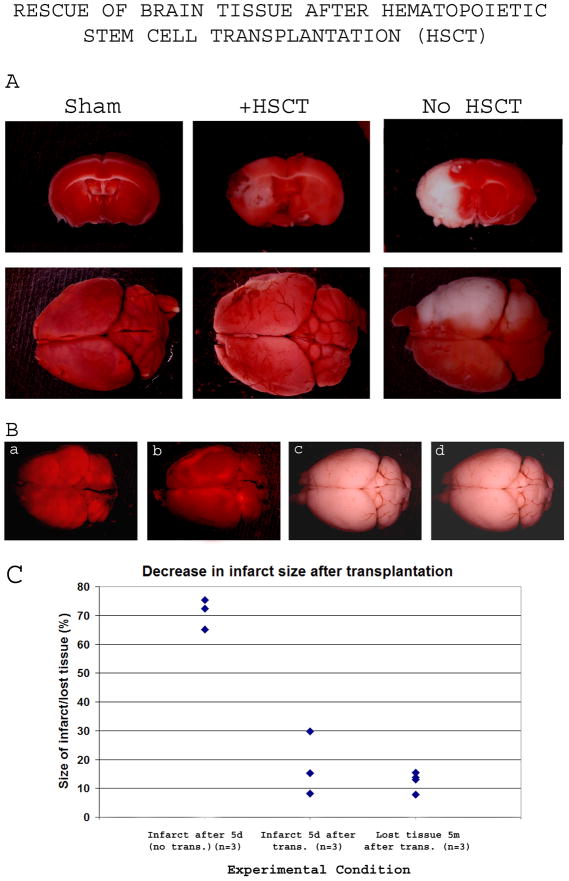
Surviving mice had smaller infarct and improved behavior
Surviving mice exhibited a rapid improvement in the behavioral phenotype, with the righting reflex, barrel rolling and ability to bare weight corrected within a week after the transplantation, and the circular motion and the torso turning to the right substantially reduced after a week and fully corrected by 14 days after the transplantation (Table 4). When the brains of transplant-recipient mice were dissected at day 5 after HSCT and compared to age-matched controls, the infarct size was much smaller in animals receiving transplantation (infarct volume relative to the ipsilateral hemisphere ranged from 8.22% to 29.79% in transplant-recipient mice, compared to 65.07% to 75.33% in non-transplanted MCAO controls) (Figure 4A, 4C), indicating an effect of donor HSC on brain tissue recovery.
Figure 4. Rescue of brain tissue after Sca1+ bone marrow cell transplantation (HSCT).
At various times after Sca1+ cell transplantation, MCAO mice were sacrificed and their TTC-stained brains were used to evaluate the infarct.
4A-5 days after 2h transient occlusion, the infarct had a volume of 68.7% ±5.15% of the affected hemisphere (right column) and became fully white, while transplant-recipient mice (middle column) showed a clear regression of the infarct size to almost one-half to one-fourth (17.73% ±11.00%).
4B-Transplant-recipient MCAO mice surviving past 2 months after the MCAO were sacrificed at 5 months after the transplantation. TTC Staining did not reveal any infarcted region, suggesting an elimination of the dead tissue overtime. The gross morphology of the brains revealed shrinkage of the affected hemisphere, clearly noticeable in 50% of animals analyzed, as shown in panels a and b. The amount of brain tissue lost ranged from 7.76% to 15.38% of ipsilateral hemisphere.
4C-Quantification of the infarct size shown in panels 4A and 4B. The left cluster represents the infarct size in MCAO controls 5 days after 2h occlusion, the middle cluster represents the infarct size in transplant-recipient (6 million Sca1+ cells) MCAO mice 5 days after a 2h occlusion, and the right cluster represents the percentage of lost brain tissue from the ipsilateral hemisphere 5 months after Sca1+ transplantation into 2h-occlusion MCAO mice. Abbreviations: d, day; m, month; trans., transplantation
When brains of transplant-recipient mice were analyzed 5 months after the transplantation, no infarct was detected using TTC staining (Figure 4B, panels a, b) demonstrating complete clearance of the dead tissue. Shrinkage of the affected hemisphere was observed (Figure 4B) but the missing tissue portion (7.76% to 15.38% of the ipsilateral hemisphere) was much smaller than the original infarct size that averaged 69.7% (Figure 4B panels a, b; Figure 4C), rendering difficult even to notice the difference in brain size in certain animals (Figure 4B, panels c, d). Such observation strongly suggests the presence of a protective effect from loss of tissue.
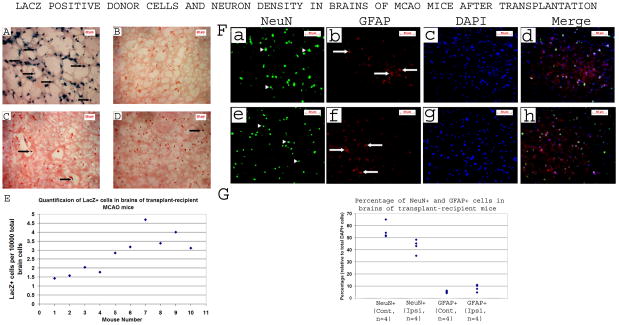
Tracking analysis revealed little donor cells in recipient brains and differences between various recipients
To verify whether donor cells have reached the brain following IV injection, Sca1+ cell transplantations were performed with donor cells obtained from ROSA26 on C57BL/6J background. Analysis of recipient brains (n=10) revealed the presence of LacZ+ cells (Figure 5), suggesting that donor cells have crossed the blood-brain barrier (BBB). These cells were observed in a narrow zone of cortical and subcortical areas, which roughly correspond to the peripheral part of the infarct. However, their density was very low (Figure 5C & D), and quantifications showed ~ 1.4–4.7 LacZ+ cells per 10 000 brain cells (Figure 5E), suggesting that their effect is unlikely to be through cell replacement/supplementation.
Figure 5. LacZ positive donor cells and neuron density in brains of MCAO mice after transplantation.
HSCs from ROSA26 mice were transplanted into MCAO mice in 2 IV injections 1h and 18h after MCAO. 3h after the second injection, mice were sacrificed and brain slices were stained for LacZ (in blue, arrows) and counterstained with nuclear fast red (in red). Only very few donor cells were detected.
A-Positive control for X-Gal staining (ROSA26 mouse brain): LacZ+ cells are readily detectable as blue/black in color (arrows point to some of them).
B-Negative control for X-Gal staining (C57BL/6J mouse): no detectable LacZ+ cells (absence of blue/black cells)
C, D-Brain sections of two separate transplant-recipient MCAO mice: very few LacZ+ cells are detectable (arrows)
E-Quantification of the number of LacZ+ cells in brains of recipient mice: each point represents an individual mouse. Values are expressed as number of LacZ+ cells per 10 000 brain cells.
F-Immunofluorescence staining of brain slices of transplant-recipient MCAO mice 5 months after the stroke/HSCT, both in the ipsilateral (a–d) and contra-lateral (e–h) hemispheres, detecting neurons (in green) using anti-NeuN antibody, and astrocytes (in red) using anti-GFAP antibody. DAPI was used to stain the nuclei (in blue).
G-Quantification of NeuN+ and GFAP+ cells of mice represented in section “F” above. Cont, contra-lateral; Ipsi, ipsilateral. The NeuN+ cell density was lower and the GFAP+ cell density was higher in the ipsilateral hemisphere.
Long term examination of brains revealed neuronal loss and increased astrocytes
Five months after the MCAO, transplant-recipient mice were sacrificed and brains processed for the detection of neurons using NeuN marker. Our data showed 23% less neurons in the ipsilateral hemisphere relative to the contra-lateral hemisphere. There was also a parallel increase in the proportion of GFAP+ cells, which, on average, were ~61% higher in the ipsilateral hemisphere (Figure 5, F & G). These data suggest long term loss of some neurons and an astrocytosis in surviving mice.
Discussion
Stroke often results in high morbidity and mortality. To better understand the deleterious effects of blood vessel occlusion and improve potential stem cell treatment in humans, we have used a mouse stroke model. With permanent occlusion, a large brain lesion was detected (Figure 1A), but all mice died within 4 days (Table 1). We then reasoned that a transient occlusion would be a better model. We therefore performed MCAO with reperfusion and stained the brains with TTC either immediately after reperfusion or at various times afterwards. We found that after 2h occlusion, the infarct size was reproducible and large enough to be used for assessing any transplantation induced recovery (Figure 1B, 1C). These results suggested that reperfusion after 2h occlusion results in a slower propagation of the brain damage, allowing the mice to live longer, working as reproducible model for future therapeutic protocols.
To determine whether stem cell transplantation can improve infarct size and survival of mice, we first chose murine total bone marrow cells to conduct the experiments. These cells contain at least three types of stem cells: hematopoietic, mesenchymal and endothelial, which could provide a combinatory effect. We reasoned that injecting cells intravenously will pose no additional damage to the brain tissue, contrary to intra-brain injection. The survival rate was slightly prolonged when larger amounts of cells where administered. Two injections on days 1 and 2 had a better survival profile than a single injection on either day 1 or 2. Three injections on days 2, 3 & 4 did not improve survival protection. Altogether, these data suggested that two injections would be more efficient than a single one. However, the fact that even large amounts of cells failed to prolong the survival of mice beyond 2 weeks suggested a possible deleterious effect of inflammatory cells contained in the total bone marrow cell mix. Therefore we chose to use a cell population that is enriched in murine hematopoietic stem and progenitor cells, obtained by a positive selection using the Sca1 cell surface marker (Sca1+-population). Sca1 is expressed on the surface of hematopoietic stem cells and early progenitor cells but not on lineage positive cells (Wilson et al, 2007). The selection for Sca1+ cells provides a substantial enrichment in stem cells (Figure 2). This cell population is also depleted of lineage (differentiated) cells which could potentially be harmful. The enriched population of stem and respective progenitors can be safely administered and are readily available. Sca1+-enriched cells have not been previously tested in this particular setting. Starting with a low cell dose (5 × 105 cells) and progressively increasing the dose increased the survival rate of transplant-recipient mice. This data suggests a positive effect of donor cells on animal protection and culminates in ~40% survival with 6 million Sca1+ cells in two injections (Figures 3A, 3B). Since mice did not survive after receiving only one injection at either day 1 or day 2, but survived after 2 consecutive injections at days 1 & 2, we hypothesize that the first injection partially stabilizes the mice while the second functions as a booster, providing additional protection resulting in the observed recovery. The finding that the amount of brain tissue lost (at 5 months post-transplantation) was much smaller than the infarct size generated by the MCAO procedure used, (Figure 4A) implies that donor stem cells rescued the mice by protecting the brain tissue.
Tracking analysis revealed only very few donor cells in the brains of the recipients indicating that cell supplementation of ischemic brain by donor cells is unlikely to account for the recovery observed (Figure 5). The quantification of these cells helped us divide the recipient mice into two groups one with lower number (<2/10 000) and one with higher number (3–5/10 000) of LacZ+ cells (Figure 5E). Although in general the number of LacZ+ cells detected was low, these results suggested that the contribution of donor cells to the recovery might have depended, at least in part, on the number reaching the brain, with higher numbers providing more efficient protection, and could explain, partially, why recovery was observed in some but not all mice. The long-term (5 months) examination of brains from MCAO mice that received Sca1+ cells (Figure 4B) revealed little shrinkage of the affected hemisphere relative to the original infarct size (12.5% ±3.3%) (Figure 1C) suggestive of some protection of brain tissue. Our immunohistochemistry examination of neurons in surviving mice 5 months after HSCT showed that they were significantly (p<0.03) lower than controls in parallel to an increased astrocyte proportion, suggesting that the neuroprotective effect of HSCT that rescued these mice did not save the entire neuronal population. Given the shrinkage of the ipsilateral hemisphere and the loss of almost one fifth of the neuronal population, it is likely that the tissue loss was mostly due to the neuronal loss. The increase in the non-neuron cells might have prevented major tissue loss and could have contributed to the protection as well. Since the amount of brain tissue lost in transplant-recipient mice was much smaller than the size of the infarct observed after the stroke alone, the HSCT is very likely to have contributed to protecting a major proportion of brain cells. Although the molecular mechanism of this rescue remains to be elucidated, a previous study had demonstrated that ischemic brain extracts can induce growth factor secretion by human BM mesenchymal stem cells (Chen et al, 2002). It is tempting to speculate that a similar effect might also occur with murine HSC. Donor cells might secrete soluble protective factors not only in the brain, but also while in the circulation within the first few hours after injection. Since the blood-brain barrier (BBB) is impaired after stroke (Dijkhuizer et al, 2002; Montaner et al, 2003), some of these factors might cross the BBB and contribute to the recovery by helping endogenous cells survive. In addition, hMSC have been shown to modulate the inflammatory reaction in the brain (Ohtaki et al, 2008). Altogether, these data suggest that a reciprocal interaction between donor cells and the ischemic brain tissue might play a role in the recovery. We speculate that this induction could follow the trend of a positive feed-back loop between donor and recipient cells, whereby the ischemic tissue releases cytokines that stimulates donor cells to secrete growth and neurotrophic factors which in turn protect recipient cells. Future studies will help shedding the light on various aspects of the mechanism by which transplanted Sca1+ cells provide their neuroprotective effect.
In summary, we have demonstrated for the first time the ability of murine Sca1+ bone marrow cells to rescue 40% of mice subjected to lethal stroke through HSCT, reducing the infarct size and neurological deficit. We also showed that timing of cell injection has important effects on the above outcome. These results may have important implications on future human stroke, using a stem cell therapy.
Acknowledgments
This work was supported by the National Institutes of Health [P01HD032573] to GGH.
We are thankful to Shirley Reynolds for excellent animal care and assistance, and to Juan Wang for great technical assistance. We also thank Dr. Yi Yang from University of New Mexico, Department of Neurology for helping on MCAO mouse model.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bang OY, Lee JS, Lee PH, Lee G. Autologous Mesenchymal Stem Cell Transplantation in Stroke Patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Lind JG, Dillon-Carter O, Yu G, Hadman M, Cheng C, Carroll J, et al. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain Research. 2004;1010:108–116. doi: 10.1016/j.brainres.2004.02.072. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu X, Gautam SC, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circulation Research. 2003;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J, et al. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22:275–279. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- Comi AM, Cho E, Mulholland JD, Hooper A, Li Q, Qu Y, et al. Neural stem cells reduce brain injury after unilateral carotid ligation. Pediatric Neurology. 2008;38:86–92. doi: 10.1016/j.pediatrneurol.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Davenport R, Dennis M. Neurological emergencies: acute stroke. J Neurol Neurosurg Psychiatry. 2000;68:277–288. doi: 10.1136/jnnp.68.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkhuizen RM, Asahi M, Wu O, Rosen BR, Lo EH. Rapid breakdown of microvascular barriers and subsequent hemorrhagic transformation after delayed recombinant tissue plasminogen activator treatment in a rat embolic stroke model. Stroke. 2002;33:2100–2104. doi: 10.1161/01.str.0000023534.37670.f7. [DOI] [PubMed] [Google Scholar]
- Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, et al. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci USA. 1995;92:11879–118. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman R, De Los Angeles A, Cheshier S, Choi R, Hoang S, Liauw J, et al. Intracarotid injection of fluorescence activated cell-sorted CD49d-positive neural stem cells improves targeted cell delivery and behavior after stroke in a mouse stroke model. Stroke. 2008;39:1300–1306. doi: 10.1161/STROKEAHA.107.500470. [DOI] [PubMed] [Google Scholar]
- Hayashi J, Takagi Y, Fukuda H, Imazato T, Nishimura M, Fujimoto M, et al. Primate embryonic stem cell-derived neuronal progenitors transplanted into ischemic brain. J Cereb Blood Flow Metab. 2006;26:906–914. doi: 10.1038/sj.jcbfm.9600247. [DOI] [PubMed] [Google Scholar]
- Hess DC, Hill WD, Martin-Studdard A, Carroll J, Brailer J, Carothers J. Bone marrow as a source of endothelial cells and NeuN-expressing cells after stroke. Stroke. 2002;33:1362–1368. doi: 10.1161/01.str.0000014925.09415.c3. [DOI] [PubMed] [Google Scholar]
- Honna T, Honmou O, Lihoshi S, Harada K, Houkin K, Hamada H, et al. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Experimental Neurology. 2006;199:56–66. doi: 10.1016/j.expneurol.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Zhang ZG, Ding GL, Silver B, Zhang L, Meng H, et al. MRI detects white matter reorganization after neural progenitor cell treatment of stroke. Neuroimage. 2006;32:1080–1089. doi: 10.1016/j.neuroimage.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel, et al. Transplanted human fetal neural stem cells survive, migrate and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH, Park IH, et al. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracranial hemorrhage stroke model. Stem Cells. 2007;25:1204–1212. doi: 10.1634/stemcells.2006-0409. [DOI] [PubMed] [Google Scholar]
- Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, et al. Neuroprotection by PIGF gene0modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006;129:2734–2745. doi: 10.1093/brain/awl207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. Journal of Cerebral Blood Flow and Metabolism. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- Montaner J, Molina CA, Monasterio J, Abilleria S, Arenillas JF, Ribo M, et al. Matrix metalloproteinase 9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107:598–603. doi: 10.1161/01.cir.0000046451.38849.90. [DOI] [PubMed] [Google Scholar]
- Nystedt J, Mäkinen S, Laine J, Jolkkonen J. Human cord blood CD34+ cells and behavioral recovery following cerebral ischemia in rats. Acta Neurobiol Exp (Wars) 2006;66:293–300. doi: 10.55782/ane-2006-1618. [DOI] [PubMed] [Google Scholar]
- Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci USA. 2008;105:14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz SI, Dinsmore JH, Wechsler LR, Rosenbaum DM, Caplan LR. Cell therapy for stroke. NeuroRx. 2004;1:406–414. doi: 10.1602/neurorx.1.4.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesisin a mouse model. Journal of Clinical Investigation. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukai R, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Mesenchymal stem cells derived from peripheral blood protects against ischemia. J Neurotrauma. 2007;24:508–520. doi: 10.1089/neu.2006.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei JJ, Zeng LF, Fan XT, Wang Y, Ma WB, Li GL, et al. Treatment of stroke in rats with bone marrow mesenchymal stem cells. Zhonghua Yi Xue Za Zhi. 2007;87:184–189. [PubMed] [Google Scholar]
- Willing AE, Vendrame M, Mallery J, Cassady CJ, Davis CD, Sanchez-Ramos J, et al. Mobilized peripheral blood cells administered intravenously produce functional recovery in stroke. Cell Transplant. 2003;12:449–454. doi: 10.3727/000000003108746885. [DOI] [PubMed] [Google Scholar]
- Wilson A, Oser GM, Jaworski M, Blanco-Bose WE, Laurenti E, Adolphe C, et al. Dormant and self-renewing hematopoietic stem cells and their niches. Ann NY Acad Sci. 2007;1106:64–75. doi: 10.1196/annals.1392.021. [DOI] [PubMed] [Google Scholar]
- Yanagisawa D, Qi M, Kim DH, Kitamura Y, Inden M, Tsuchiya D, et al. Improvement of focal ischemia-induced rat dopaminergic dysfunction by striatal transplantation of mouse embryonic stem cells. Neuroscience Letters. 2006;407:74–79. doi: 10.1016/j.neulet.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Yanamoto H, Nagata I, Niitsu Y, Xue JH, Zhang Z, Kikuchi H. Evaluation of MCAO stroke models in normotensive rats: standardized neocortical infarction by the 3VO technique. Experimental Neurology. 2003;182:261–274. doi: 10.1016/s0014-4886(03)00116-x. [DOI] [PubMed] [Google Scholar]
- Zin’kova NN, Gilerovich EG, Sokolova IB, Shvedova EV, Bilibina AA, Krugliakov PV, et al. Mesenchymal stem cells transplantation influences upon dynamics of morphological changes in rat brain after stroke. Tsitologiia. 2007;49:923–932. [PubMed] [Google Scholar]