Abstract
Objective
To determine the ability of an intramuscular cobinamide sulfite injection to rapidly reverse the physiologic effects of cyanide toxicity.
Background
Exposure to cyanide in fires and industrial exposures and intentional cyanide poisoning by terrorists leading to mass casualties is an ongoing threat. Current treatments for cyanide poisoning must be administered intravenously, and no rapid treatment methods are available for mass casualty cyanide exposures. Cobinamide is a cobalamin (vitamin B12) analog with an extraordinarily high affinity for cyanide that is more water-soluble than cobalamin. We investigated the use of intramuscular cobinamide sulfite to reverse cyanide toxicity induced physiologic changes in a sublethal cyanide exposure animal model.
Methods
New Zealand white rabbits were given 10 mg sodium cyanide intravenously over 60 minutes. Quantitative diffuse optical spectroscopy and continuous wave near infrared spectroscopy monitoring of tissue oxy- and deoxyhemoglobin concentrations were performed concurrently with blood cyanide level measurements and cobinamide levels. Immediately after completion of the cyanide infusion, the rabbits were injected intramuscularly with cobinamide sulfite (n=6) or inactive vehicle (controls, n=5).
Results
Intramuscular administration led to rapid mobilization of cobinamide and was extremely effective at reversing the physiologic effects of cyanide on oxyhemoglobin and deoxyhemoglobin extraction. Recovery time to 63% of their baseline values in the central nervous system was in a mean of 1032 minutes in the control group and 9 minutes in the cobinamide group with a difference of 1023 minutes (95% confidence interval [CI] 116, 1874 minutes). In muscle tissue, recovery times were 76 and 24 minutes with a difference of 52 minutes (95% CI 7, 98min). Red blood cell cyanide levels returned towards normal significantly faster in cobinamide sulfite-treated animals than in control animals.
Conclusions
Intramuscular cobinamide sulfite rapidly and effectively reverses the physiologic effects of cyanide poisoning, suggesting that a compact cyanide antidote kit can be developed for mass casualty cyanide exposures.
Introduction
The development of cyanide toxicity may occur from smoke inhalation, industrial exposure, and acts of terrorism. (1-4). Doses of as little as 50 mg may be fatal to humans, with more than 5.2 billion pounds of cyanide produced annually worldwide (5). Lethal exposures can occur from cyanide ingestion or inhalation, and irreversible injury or death can occur within minutes of exposure. Mass casualty cyanide exposure from intentional terrorism acts is a major concern to civilian and military personnel. Terrorist plans to attack passengers in the NY subway system using cyanide were discovered by the United States intelligence authorities in 2003 (6).
Current treatments for cyanide poisoning include three general classes of agents: methemoglobin generators (sodium nitrite, amyl nitrite, and dimethyl aminophenol), sulfur donors (sodium thiosulfate and glutathione), and direct binding agents (hydroxocobalamin and dicobalt edetate) (4, 7). These drugs are effective for cyanide exposure of only a small number of victims concurrently, because they must be administered intravenously by skilled personnel. Given the need for immediate treatment of cyanide exposed-persons, and the ever present danger of mass casualties from cyanide poisoning, rapidly acting cyanide antidotes that can be administered simply are desperately needed. An ideal candidate for treating cyanide poisoning would have a long shelf-life, exhibit minimal toxicity, i.e., have a high therapeutic index, and could be administered by minimally trained individuals or by self-administration, e.g., intramuscular injection.
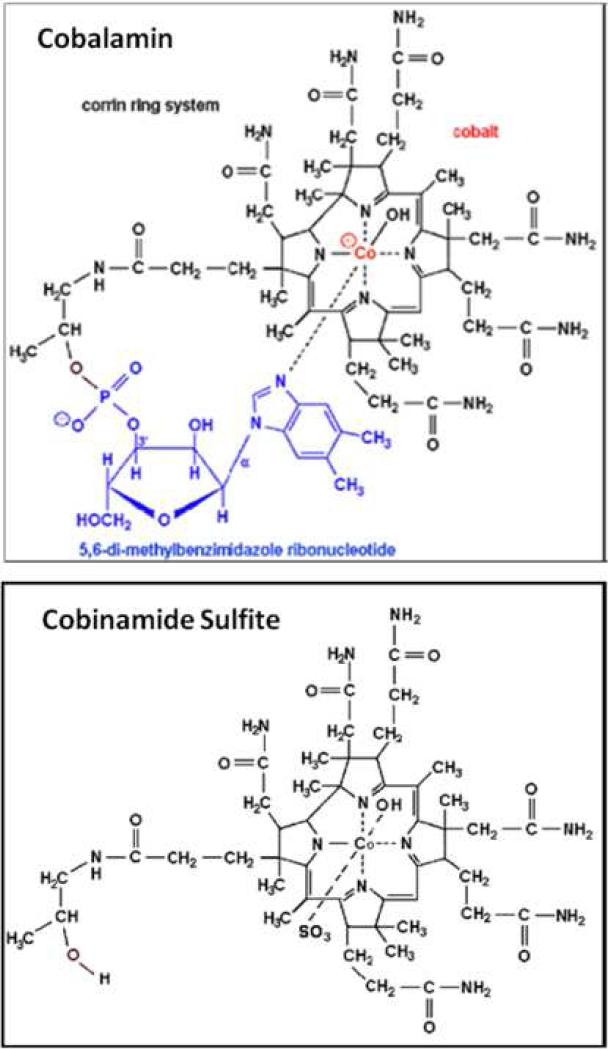
Cobinamide is a potential agent for treating cyanide poisoning (8) that may satisfy these criteria. It is the penultimate precursor in cobalamin biosynthesis, lacking the dimethyl-benzimidazole ribonucleotide tail coordinated to the cobalt atom in the lower axial position (Figure 1). Thus, whereas cobalamin has only an upper ligand binding site, cobinamide has both an upper and lower ligand binding site. Moreover, the dimethylbenzimidazole group has a negative trans-effect on the upper binding site, thereby reducing cobalamin's affinity for ligands (9). The combined effect is that each cobinamide molecule can bind two cyanide molecules, and that cobinamide has a much greater affinity for cyanide than cobalamin, with a KA overall of ≈1022 M-1 [binding affinity for first cyanide ion is 1014 M-1 and for second ion is 108 M-1] (10). This suggests that cobinamide should be a more effective cyanide detoxifying agent than cobalamin, as we found in previous work (8). In aqueous solution, cobinamide exists as aquohydroxocobinamide, which we show is at least five times more water soluble than cobalamin. The combination of cobinamide's high binding affinity for cyanide, binding of two cyanide molecules, and relatively high water solubility suggest it could be administered in concentrated enough solutions for intramuscular injection. In addition, cobinamide is stable at room temperature, and we provide evidence that cobinamide sulfite exhibits minimal toxicity to mice and rats at doses as high as 300-800 mg/kg. For these reasons, we assessed the feasibility of intramuscular injections of cobinamide sulfite in a cyanide toxic animal model (11).
Figure 1.
Comparison of molecular structure of cobalamin (top) to cobinamide sulfite (bottom). Cobinamide lacks the dimethyl-benzimidazole ribonucleotide tail coordinated to the cobalt atom in the lower axial position. Whereas cobalamin has only an upper ligand binding site, cobinamide has both an upper and lower ligand binding site. The dimethylbenzimidazole group on cobalamin has a negative trans-effect on the upper binding site, thereby reducing cobalamin's affinity for ligands (11). The combined effect is that each cobinamide molecule can bind two cyanide molecules, and that cobinamide has a much greater affinity for cyanide than cobalamin.
In addition to developing cyanide countermeasures, new methods to rapidly recognize and monitor cyanide exposure are essential to optimize treatment and determine therapeutic efficacy (12, 13). Optical technologies such as diffuse optical spectroscopy (DOS) and continuous wave near infrared spectroscopy (CWNIRS) may provide these capabilities. The cyanide anion binds to the iron in cytochrome oxidase, blocking electron transport and, thereby, interrupting cellular respiration (4, 7, 14-17). Because cytochrome oxidase accounts for > 90% of cellular oxygen consumption, oxygen is no longer consumed by cyanide-poisoned tissues, and the oxygen content increases in both arterial and venous blood (18). DOS can simultaneously measure tissue optical scattering and absorption, providing accurate quantitative measures of tissue oxy- and deoxyhemoglobin (11). We have used DOS to noninvasively measure the physiologic effects of cyanide poisoning in a non-lethal rabbit model of cyanide poisoning(11).
We hypothesized that intramuscular injection of cobinamide will rapidly reverse the physiologic effects of cyanide toxicity in the rabbit model and suggest that cobinamide sulfite may be a promising candidate agent for treating mass casualties from cyanide poisoning.
Methods
General preparation
Pathogen-free New Zealand White rabbits weighing 3.5-4.5 kg (Western Oregon Rabbit Supply, Philomath, Oregon), were used, and all procedures were reviewed and approved by the University of California, Irvine, Institutional Animal Care and Use Committee (IACUC). The methods for cyanide induction and DOS monitoring have been described previously and are summarized here (11).
Animals were anesthetized with an intramuscular injection of a 2:1 ratio of ketamine HCl (100 mg/ml, Ketaject, Phoenix Pharmaceutical Inc., St. Joseph, MI): xylazine (20 mg/ml, Anased, Lloyd Laboratories, Shenandoah, IA) at a dose of 0.75 cc/kg using a 23 gauge 5/8 inch needle. After the intramuscular injection, a 23 gauge 1 inch catheter was placed in the animals’ marginal ear vein to administer continuous IV anesthesia. Torbutrol, 0.1-0.5mg/kg , was given subcutaneously, and then the animals were intubated with a 3.0 cuffed endotracheal tube secured by a gauze tie; they were mechanically ventilated (dual phase control respirator, model 32A4BEPM-5R, Harvard Apparatus, Chicago, IL) at a respiration rate of 32/minutes, a tidal volume of 50 cc, and FiO2 of 100%. A pulse oximeter (Biox 3700 Pulse Oximeter, Ohmeda, Boulder, CO) with a probe was placed on the tongue to measure SpO2 and heart rate. Blunt dissection was performed to isolate the femoral artery and vein on the left thigh for blood sampling, cyanide infusion, and systemic pressure monitoring. Sodium cyanide (10 mg) dissolved in 60 ml of phosphate-buffered saline was given intravenously over 60 minutes; this dose of cyanide (~2.5 mg/kg) is non-lethal to rabbits, but does cause marked changes in tissue oxy- and deoxyhemoglobin concentrations (11). On completion of the experiment, the animals were euthanized with an intravenous injection of 1 ml Euthasol (1.0cc, Euthasol, Virbac AH, Inc. Fort Worth, Texas. 390 mg pentobarbital sodium, 50 mg phenytoin sodium/ml) administered through the marginal ear vein.
Venous blood cyanide levels were measured at baseline, 5 minutes prior to completion and at the end of the cyanide infusion, and at 2.5, 5, 7.5, 10, 15, 30, 45, and 60 minutes after injecting cobinamide sulfite. During this time, DOS and CWNIR measurements were taken continuously, with each measurement set requiring an average of 30 sec to complete.
Study and control groups
A total of 11 animals were studied: five animals (control group) received either no intramuscular injection (four animals) or an intramuscular injection of 12.5 mg sodium sulfite dissolved in 1 ml phosphate-buffered saline (one animal), and six animals received an intramuscular injection of 83.5 mg cobinamide (0.082 millimole) mixed with 12.5 mg sodium sulfite in 1 ml phosphate-buffered saline immediately following completion of the cyanide infusion and DOS measurements. The cobinamide dose was calculated to achieve a molar equivalent for cyanide neutralization, and the injections were performed intramuscularly in the right gluteus or left pectoralis muscle.
Non-invasive measurements using diffuse optical spectroscopy (DOS)
Diffuse optical spectroscopy (DOS) measurements were obtained through a fiberoptic probe with a light diode emitter and detector at a fixed distance (10 mm) from the source fiber, which was placed on the shaved surface of the right inner thigh of the animal. The broadband DOS system we constructed (11, 19-23) combines multi-frequency domain photon migration with time-independent near infrared spectroscopy to accurately measure bulk tissue absorption and scattering spectra. It employs six laser diodes at discrete wavelengths (661,681,783, 805, 823, and 850 nm), and a fiber coupled avalanche photo diode (APD) detector (Hamamatsu high-speed APD module C5658, Bridgewater, NJ) for the frequency domain measurements. The APD detects the intensity-modulated diffuse reflectance signal at modulation frequencies from 50 to 550 MHz after propagation through the tissue. Absorption and reduced scattering coefficients are measured directly at each of the six laser diode wavelengths using frequency-dependent phase and amplitude data. Reduced scattering coefficients are calculated as a function of wavelength throughout the near infrared region by fitting a power-law to six reduced scattering coefficients. Steady-state acquisition was accomplished using a broadband reflectance measurement from 650 to 1000 nm that follows frequency domain measurements using a tungsten-halogen light source (Ocean Optics HL-2000, Dunedin, FL) and a spectrometer (BWTEK BTC611E, Newark, DE). Intensity of the steady-state reflectance measurements are calibrated to the frequency domain values of absorption and scattering to establish the absolute reflectance intensity (11, 19, 20). Tissue concentrations of oxy- and deoxyhemoglobin are calculated by a linear least squares fit of the wavelength-dependent extinction coefficient spectra of each chromophore. We used oxy- and deoxyhemoglobin absorption spectra reported by Zijlstra et al (24) for subsequent fitting and analysis.
Continuous wave near infrared spectroscopy (CWNIRS)
CWNIRS was used to assess oxy- and deoxyhemoglobin effects of cyanide toxicity and reversal in the brain region. Continuous wave near infrared spectroscopy (CWNIRS) provides rapid, real time measures of tissue oxy- and deoxyhemoglobin concentration changes and penetrates more deeply into tissues than DOS (25); it can , therefore, be used to assess regions such as the CNS, an area particularly sensitive to cyanide toxicity. CWNIRS, however, does not account for scattering effects, and provides only relative information on changes in the concentrations of molecular species (as opposed to absolute concentrations obtained by DOS).
The CWNIRS system consists of a light source (Ocean Optics HL 2000HP, Dunedin, FL), a CCD spectrometer (BWTEK BTC111E, Newark, DE), and customized optical fiber guides (1mm diameter with 10 feet length, RoMack Inc, VA) (25). Continuous wave near infrared light was delivered to the rabbit brain/CNS using a fiber optic probe placed over the forehead, and transmitted light intensities at five wavelengths (732, 758, 805, 840, 880 nm) were measured using the CCD spectrometer every second. We quantified changes in oxy- and deoxyhemoglobin concentrations throughout the experiment using Labview real time display software, and a modified Beer-Lamberts’ law (Labview 7.0, National Instrument, TX). Since it does not account for scattering effect, the unit of oxy- and deoxyhemoglobin concentration is mM/DPF where DPF is a differential pathlength factor (26). Calculated changes in oxyand deoxyhemoglobin were displayed on a computer in real time (25).
Measurement of Red Blood Cell (RBC) Cyanide Concentration
Cyanide in blood is bound almost exclusively to ferric(met)hemoglobin in RBCs; thus, blood cyanide can be measured by separating RBCs from plasma, and acidifying the RBCs to release cyanide as HCN gas (27). Immediately after drawing blood from the rabbits, samples were cooled to 4 °C, centrifuged, and the plasma and RBC fractions separated. All samples were kept at 4 °C and analyzed within 48 hours. For analysis, the RBCs were lysed in ice-cold water. The lysates were placed into glass tubes sealed with stoppers holding plastic center wells (Kontes Glass Co., Vineland, NJ) containing 0.1 M sodium hydroxide (NaOH). A volume of 10% trichloroacetic acid equal to the lysate was injected through the stopper into the tubes, and the tubes were shaken at 37 °C for 75 minutes. After cooling to room temperature, cyanide trapped in the NaOH was measured in a spectrophotometric assay following its reaction with p-nitrobenzaldehyde and o-dinitrobenzene at 560 nm (28). Concentrations were determined from standard curves using freshly prepared KCN dissolved in 0.1 M NaOH. Duplicate samples showed < 15% variation.
Measurement of Plasma Cobinamide Concentration
Plasma cobinamide concentrations were determined by scanning diluted plasma samples between 300 and 600 nm on an Uvikon 960 spectrophotometer (NorthStar Scientific, Leeds, England, NorthStar Scientific Limited - www.nstaruk.com). Cobinamide and dicyanocobinamide have distinctive spectra in this range. To convert all cobinamide in the plasma to one species, i.e., dicyanocobinamide, excess KCN was added to the samples. The cobinamide concentration was calculated based on a least squares regression linear-fit to standard curves generated by adding dicyanocobinamide of known concentrations to baseline plasma samples. Samples were diluted appropriately to assure they were in the linear range of the standard curve, with duplicate measurements performed on each sample.
Cobinamide Solubility and Toxicity
We found that cobinamide sulfite is soluble to at least 350 mM in water, whereas hydroxocobalamin was soluble to about 70 mM. These experiments were performed by dissolving known amounts of the two compounds in the minimal amount of water necessary to achieve a clear homogenous solution. The calculated concentrations were checked spectrophotometrically at 348.5 and 352 nm for cobinamide sulfite and hydroxocobalamin, respectively, using an extinction coefficient of 2.8 × 104.
Cobinamide sulfite could be administered at doses of 300 and 800 mg/kg to rats and mice, respectively, without evidence of significant toxicity. These doses are about 15 and 40 times higher, respectively, than the doses used in the current study. Formal toxicology studies are currently underway.
Statistical Methods
With a two-tailed alpha of 0.05 and 5 and 6 animals in control and cobinamide sulfite group, respectively, we calculated an estimated power of 0.8 to detect a 50% difference in recovery times between groups. Baseline parameters across groups were compared using analysis of variance. Response to treatment across the groups was compared using analysis of variance with repeated measures for cyanide level comparisons. A confidence interval [CI] of 95% was considered significant. Time constants for DOS or CWNIRS changes in tissue hemoglobin oxygenation parameters were compared using analysis of variance. All data were analyzed using a standard statistical package (Systat-12, Systat Software, Inc., Chicago, IL 60606).
Results
DOS monitoring of peripheral muscle region cyanide toxicity and recovery
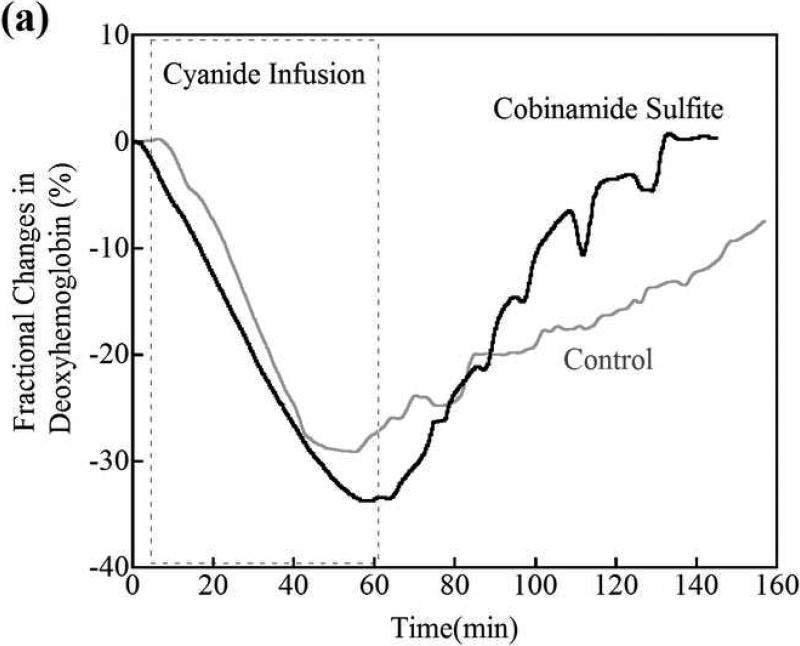
The deoxygenated hemoglobin concentration in peripheral muscle fell progressively during the 60 minutes of cyanide infusion [Fig. 2a; fractional changes in deoxyhemoglobin (deoxyhemoglobin) concentration from the baseline (pre cyanide infusion) concentration are shown for a representative animal]. A fall in deoxygenated hemoglobin would be expected, because of cyanide inhibiting cellular respiration and decreasing tissue oxygen consumption. When the cyanide infusion was stopped at 60 minutes, the deoxyhemoglobin concentration slowly returned over >60 minutes towards baseline values in control non-cobinamide sulfite-treated animals (Fig. 2a, grey line). The rate of return of deoxyhemoglobin levels in the cobinamide sulfite-treated animals was much faster than in control animals, particularly in the first 10 minutes after the intramuscular injection, which would be a critical time period for recovery (Fig. 2a, black line).
Figure 2.
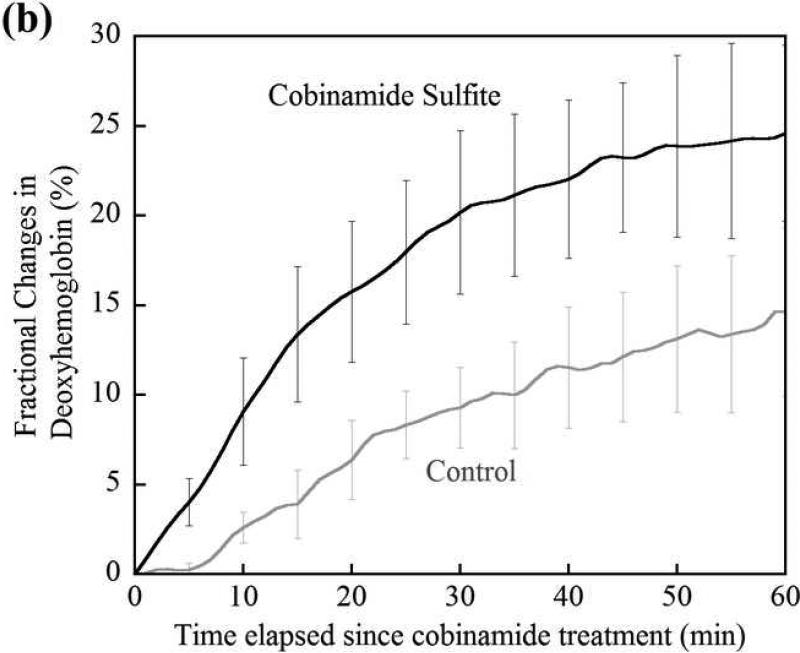
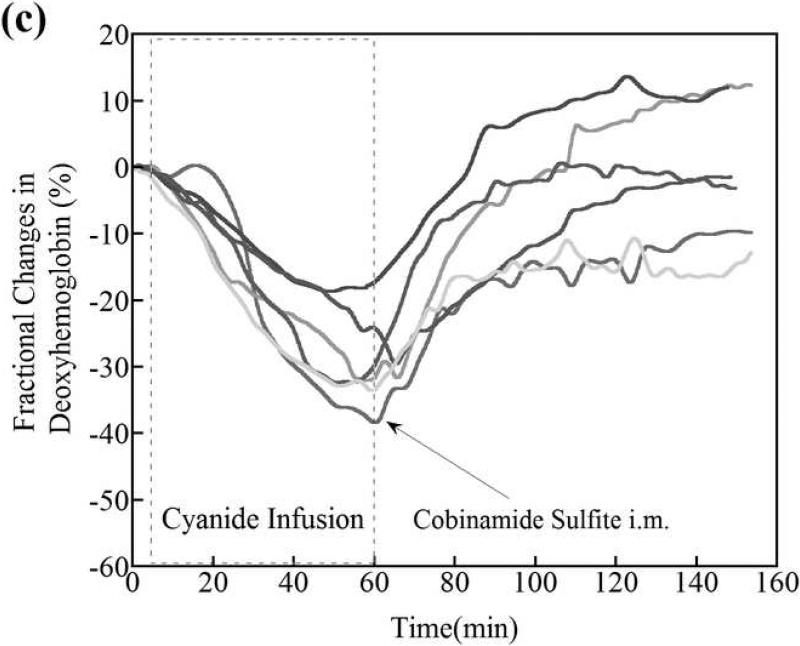
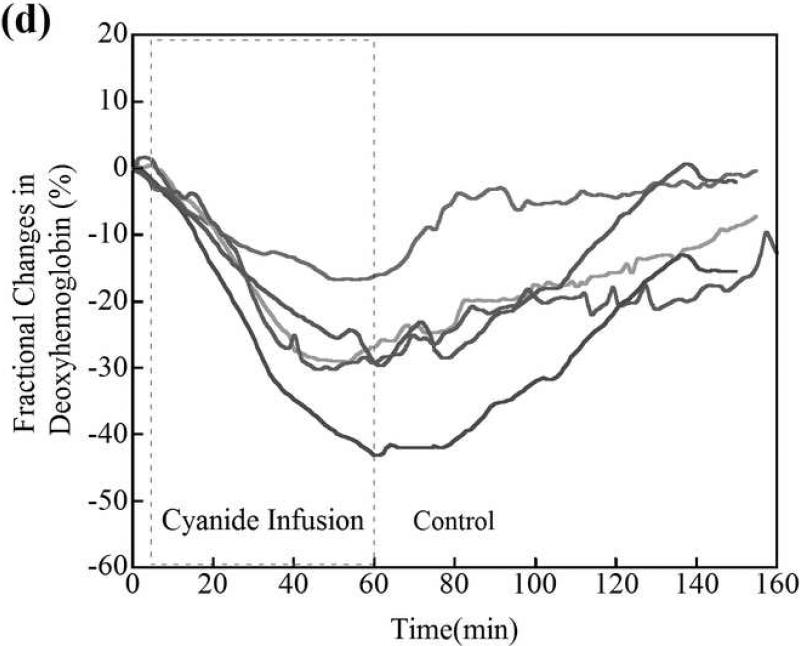
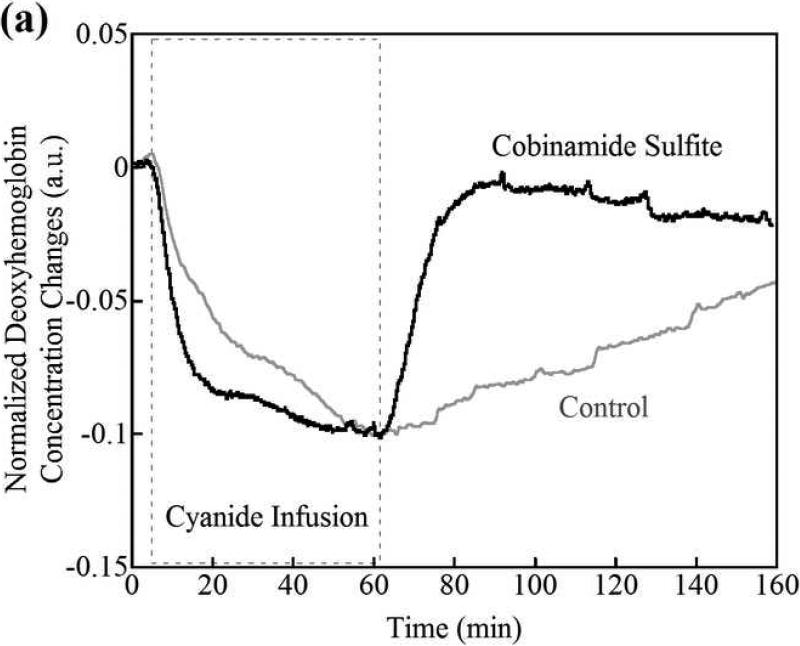
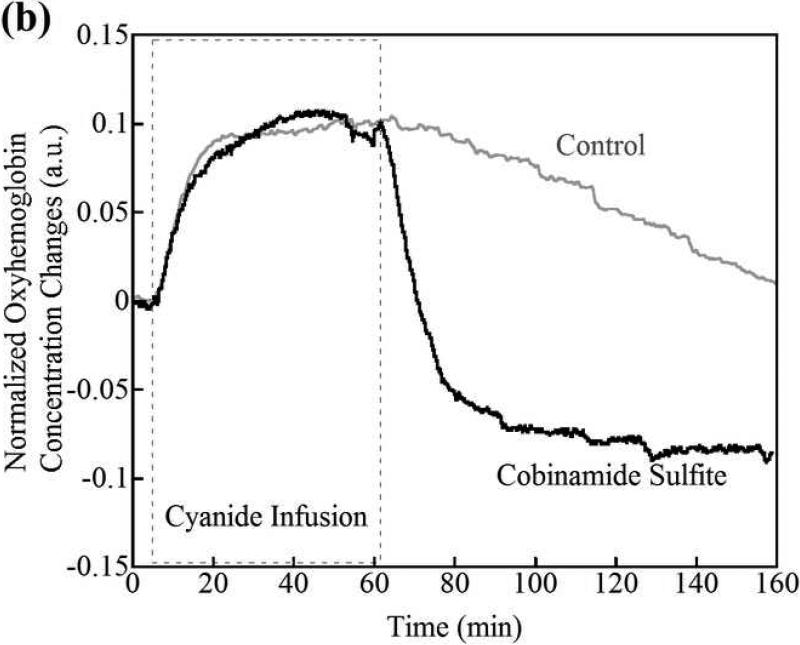
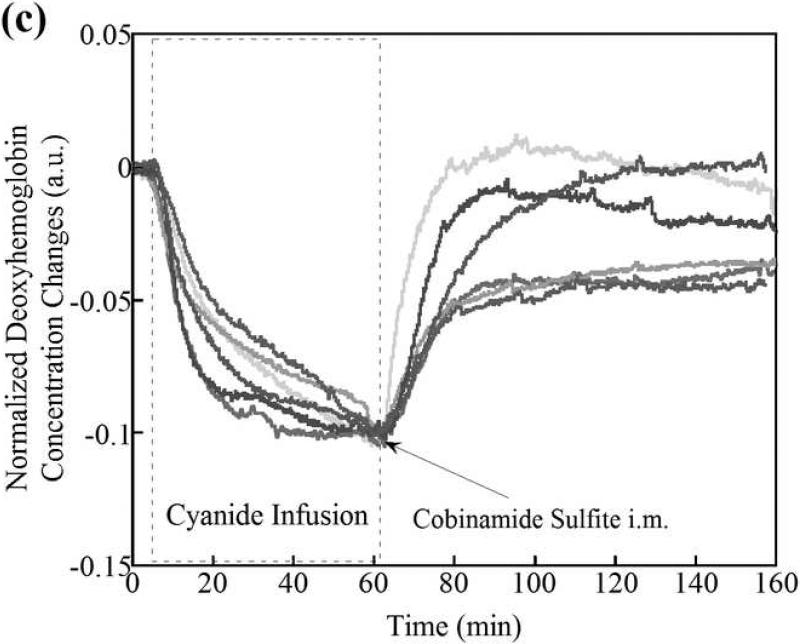
(a) Changes in tissue deoxyhemoglobin concentrations as measured by DOS during and immediately after cyanide infusion in rabbits. The decrease in deoxyhemoglobin concentration during the 60 minutes of cyanide infusion is due to the inability of tissues to remove oxygen from circulating blood, leaving more hemoglobin in the oxygenated state. At 60 minutes when the cyanide infusion was stopped, the animals received either phosphate-buffered saline (control, gray line) or cobinamide sulfite by intramuscular injection (black line). In the control animal, deoxyhemoglobin levels gradually increased toward baseline after the cyanide infusion was discontinued; however, deoxyhemoglobin levels still remained below baseline levels at more than 140 minutes (> 80 minutes following completion of the cyanide infusion). In the cobinamide sulfite-treated animal, the deoxyhemoglobin concentration showed a rapid reversal following cobinamide sulfite administration, returning to baseline values on average within 24 minutes after injection. The data are expressed as the percent fractional change in deoxyhemoglobin from baseline. Individual animal data from both control and cobinamide sulfite treated group are also available to view in the Annals of Emergency Medicine online repository. (Fig. 2c and 2d)
(b) Fractional change in deoxyhemoglobin concentrations in all control and cobinamide sulfite-treated animals. The deoxyhemoglobin concentration began to rise gradually with cessation of the cyanide infusion in control animals. In contrast, deoxyhemoglobin concentration rose much more rapidly in cobinamide sulfite-treated animals. The data are the mean values for five control animals and six cobinamide sulfite-treated animals, with error bars representing the standard deviation.
Changes in deoxyhemoglobin concentrations for all animals during the recovery/treatment phase are shown in Fig. 2b, which illustrates the progression of fractional change in deoxyhemoglobin during the 60 minutes following injection of phosphate buffered saline (control) or cobinamide sulfite. Post cyanide infusion fractional deoxyhemoglobin values of the control was in a mean of -23.7%, compared to -28.7% in treatment animals; a difference of 5% (95% confidence interval [CI] -15.7, 4.6%), where the deoxyhemoglobin concentration at the completion of the cyanide infusion and start of the treatment period is designated as zero. From the data in Fig. 2b, the time constant for recovery of deoxygenated hemoglobin to baseline, pre-cyanide poisoning levels is calculated as: (~ (1-exp(-t/τ), where τ is a time constant). τ from fittings are listed below in Table 1 to determine the effective “time constant” to recovery where W is the classic time to recovery of an exponential decay fit curve to 63.2% of baseline (25). Using this formula, the time constant for recovery of deoxygenated hemoglobin was in a mean of 76 minutes in control animals, compared to 24 minutes for cobinamide sulfite-treated animals; a difference of 52 minutes (95% CI -5, 110 minutes). In the crucial first 10 minutes following cyanide poisoning, the slope of recovery of deoxygenated hemoglobin for the cobinamide sulfite-treated group is almost three times greater than for controls (Table 1).
Table 1.
Deoxyhemoglobin recovery times in peripheral tissues following cyanide infusion. Time constant values for deoxyhemoglobin recovery fitted by using an exponential model shown as mean SEM standard deviation. The time constant is the standard exponential decay constant representing the time to recover to 63.2% of baseline value. Analysis was performed using a two sample t-test assuming unequal variances, for the intramuscular cobinamide sulfite versus controls; p<0.05 for the difference in deoxyhemoglobin time constant between control and cobinamide sulfite-treated animals. Oxy- and deoxyhemoglobin recovery times in CNS region following cyanide infusion. CWNIRS Time constant values for cyanide recovery of oxyhemoglobin and deoxyhemoglobin fitted by using an exponential model shown as mean SEM standard deviation, with 95% confidence interval in parentheses. Analysis was performed using two sample t-test assuming unequal variances; time constant p values for intramuscular cobinamide sulfite versus controls were <0.05 for both oxy- and deoxyhemoglobin. Slope of recovery versus time for deoxy- and oxyhemoglobin in the critical first 10 minutes after treatment. The above results are from fitting the initial 10 minutes of hemodynamic recovery in CNS after cobinamide sulfite administration shown as mean SEM standard deviation, with 95% confidence interval in parentheses. DPF is a differential pathlength factor (see the materials and methods section)
| Control (n=5) | Cobinamide sulfite (n=6) | 95% CI for the mean differences | |
|---|---|---|---|
| Recovery time to 63% of baseline values (minutes) | |||
| Peripheral Muscle | |||
| Deoxyhemoglobin, mean (SD) | 76 (42.7) | 24 (14.7) | 7 to 98 |
| CNS | |||
| Deoxyhemoglobin, mean (SD) | 1032 (610) | 9 (2.5) | 116 to 1,874 |
| Oxyhemoglobin, mean (SD) | 261 (225) | 10 (1.6) | 28 to 358 |
| Slope of the first 10 minutes recovery times (□M/DPF/minutes) | |||
| Deoxyhemoglobin, mean (SD) | 0.6 (0.4) | 2.3 (0.8) | 0.9 to 2.5 |
| Oxyhemoglobin, mean (SD) | −1.1 (0.3) | −4.4 (1.2) | 2.1 to 4.5 |
CWNIRS monitoring of CNS region cyanide toxicity and recovery
CWNIRS monitoring over the CNS region yielded analogous results to DOS monitoring over muscle, but with a more pronounced effect of cyanide and response to treatment. Specifically, cobinamide sulfite markedly increased the rate of recovery of CNS region deoxyhemoglobin toward baseline values (Fig. 3a). The time constant for recovery of deoxyhemoglobin concentrations was a mean > 1000 minutes in control animals and 9 minutes in cobinamide sulfite-treated animals with a difference of > 900 minutes (95% CI 116, 1874 minutes) (Table 1). In the critical first 10 minutes after the end of the cyanide infusion when cobinamide sulfite was injected, the slope of recovery of the deoxyhemoglobin concentration was almost four times greater in the cobinamide sulfite-treated animals than in controls (Table 1). CNS region oxyhemoglobin returned to baseline much faster in the cobinamide sulfite-injected animals than in control animals (Fig. 3b). The time constant for returning oxyhemoglobin concentrations was in a mean > 250 minutes in control animals and 10 minutes for cobinamide sulfite-treated animals with a difference of 250 minutes (95% CI 28, 358 minutes) (Table 1). Again, the rate of return of the oxyhemoglobin concentration during the first 10 minutes after stopping the cyanide infusion in the CNS region was about four times greater in the cobinamide sulfite-treated animals than in control animals (Table 1).
Figure 3.
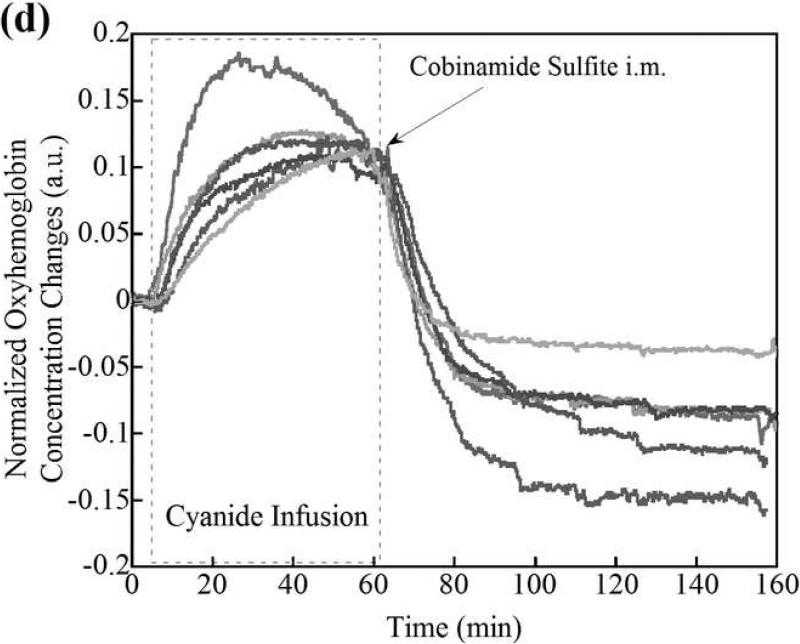
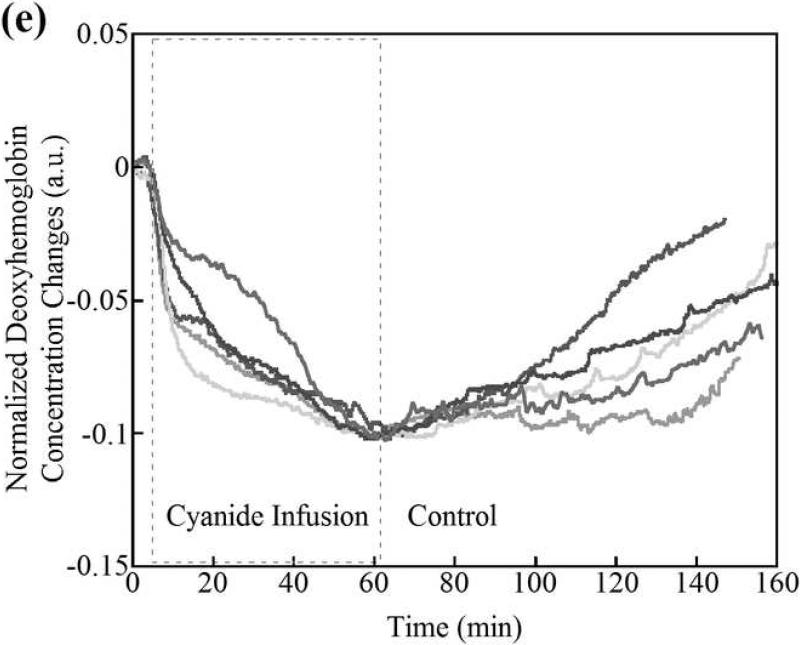
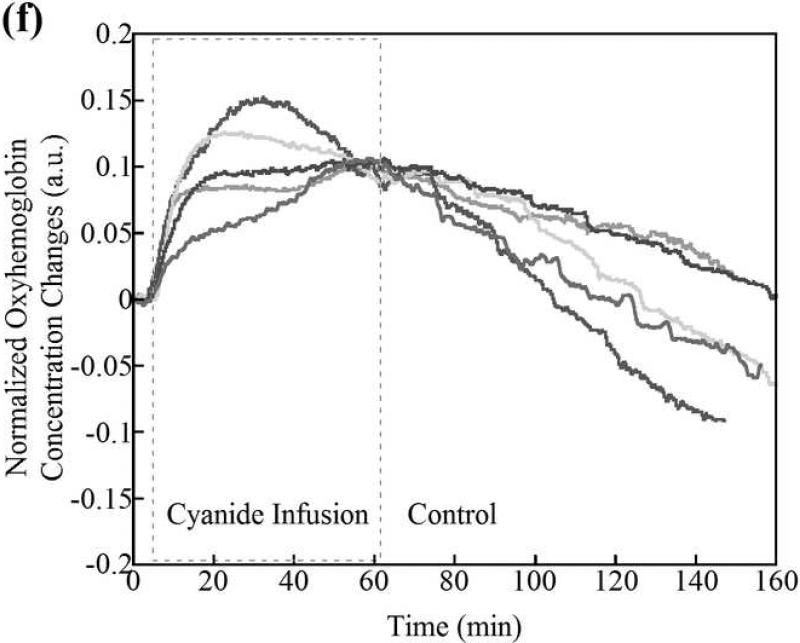
Deoxyhemoglobin (a) and oxyhemoglobin (b) levels in the CNS region as measured by CWNIRS. Representative animals are shown. The change in oxyhemoglobin and deoxyhemoglobin are normalized to the 0.1 and -0.1 with the values at the time point of CN infusion cessation, respectively, to be easy to compare the recovery rate between control and cobinamide sulfite-treated animals. As shown, the return of oxy- and deoxyhemoglobin levels to baseline is dramatically improved after cobinamide sulfite injection, which is similar to findings using DOS in Fig. 2. Individual animal data from both control and cobinamide sulfite treated group are also available to view at the online web site. (Fig. 3c, 3d, 3e, and 3f)
Total Tissue Hemoglobin Levels
Total tissue hemoglobin levels measured by DOS did not change during the post cobinamide sulfite injection recovery time or in control animals during the recovery period. The changes in total hemoglobin concentration during the post cobinamide sulf ite injection recovery time or in control animals are -0.6 μM (95% CI -2.2, 1.1μM) and -1.1 μM (95% CI -2.4, 0.2 μM), respectively.
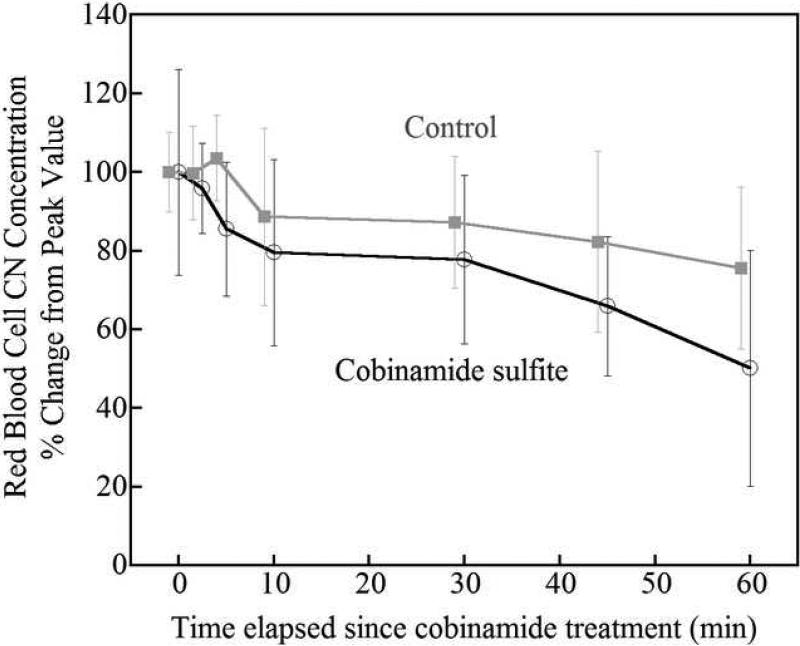
Red blood cell cyanide concentrations
The concentration of cyanide in red blood cells decreased significantly more rapidly following intramuscular cobinamide sulfite injection than in control animals (Fig. 4). At 30 minutes, cyanide concentrations had fallen to in a mean 68 % of peak values in cobinamide sulfite-treated animals compared to 82 % in control animals with a difference of 14 % (95% CI -35, 6 %). Analysis of variance with repeated measures revealed a significant difference between the cobinamide sulfite-treated group and controls over time (F=5.7, p<0.008).
Figure 4.
The change of red blood cell cyanide concentrations during 60 minutes of recovery after the cessation of CN infusion. Values are normalized as percent of peak cyanide level at the completion of cyanide infusion and are shown as mean and standard deviation. Cobinamide sulfite-treated animals had significantly faster reduction in blood cyanide levels compared to control animals. The values of cobinamide sulfite treated group are slightly shifted to prevent the overlap of error bars between two groups.
Cobinamide concentrations
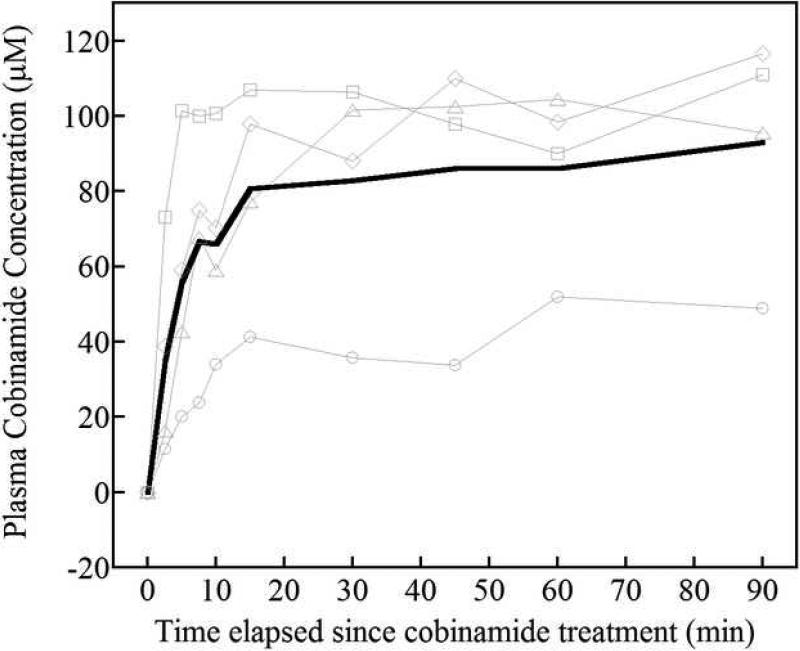
Plasma cobinamide concentrations rose rapidly after intramuscular administration. Cobinamide was undetectable in the plasma at baseline and at the completion of the cyanide infusion. By 2.5 minutes following the intramuscular injection, cobinamide concentrations had risen to a mean of 35 μmol/liter (95% CI 11, 59 μmol/liter), and by 5 minutes were at 56 μmol/liter (95% CI 27, 85 μmol/liter); the cobinamide concentrations rose to 81 μmol/liter by 15 minutes (95% CI 56, 106 μmol/liter), after which time they remained relatively constant until 90 minutes (Fig. 5). Thus, cobinamide sulfite injected intramuscularly is absorbed quickly and, at least over the relatively short time frame of these experiments, is eliminated slowly. The half-life □ for the rise in cobinamide concentration was 6.4 minutes (95% CI 3.2, 9.6 minutes), which is close to the τ for the CNS region return of oxy- and deoxy hemoglobin concentrations.
Figure 5.
Plasma cobinamide concentrations after intramuscular administration of cobinamide sulfite. Whole blood was obtained from rabbits at the indicated times after intramuscular injection of 1 ml of .082 mmoles cobinamide sulfite. The blood was centrifuged, and KCN was added to the plasma fractions to convert all of the cobinamide to dicyanocobinamide. The latter was measured by scanning the samples between 300 and 600 nm, and performing a least squares regression comparison to spectra obtained from standards as described in Methods. Four thin gray lines indicate the data from individual animals and a thick black line represent the mean values from four rabbits.
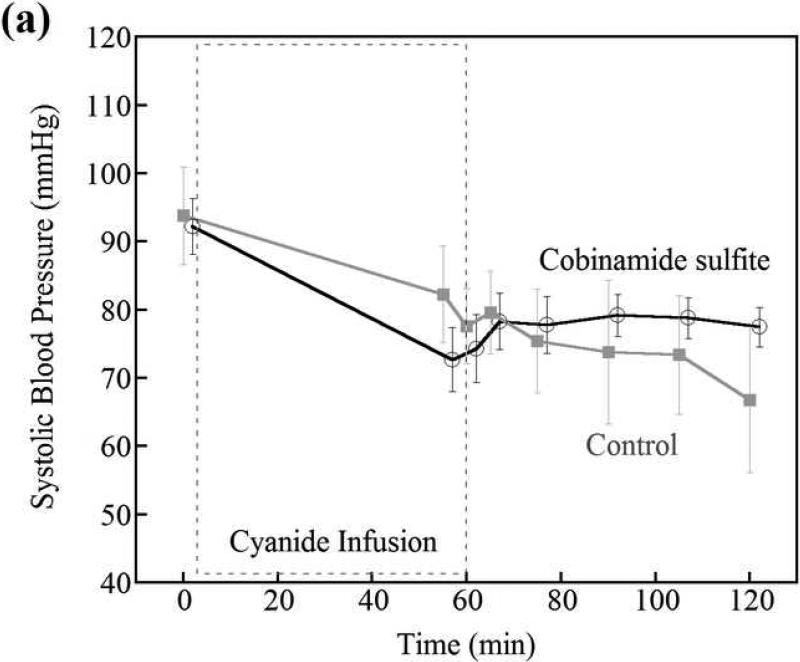
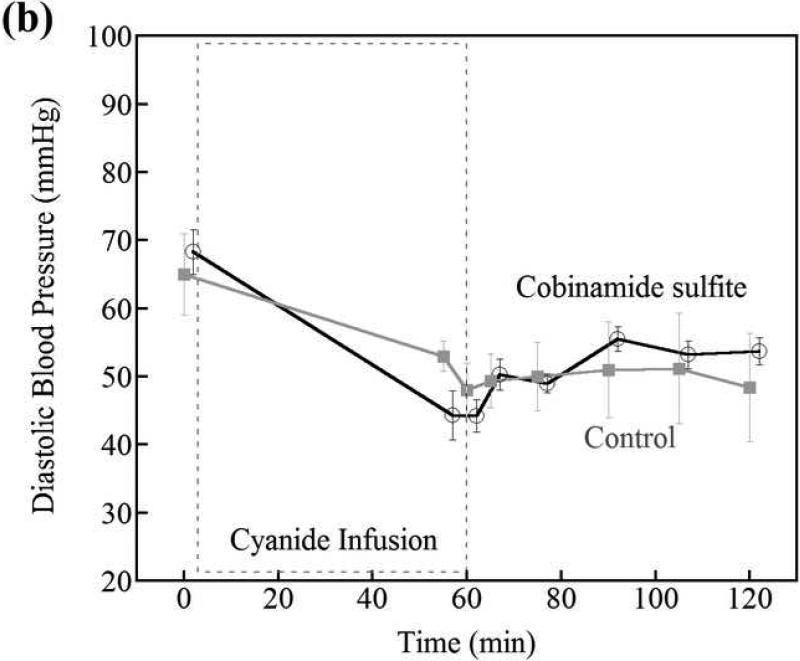
Blood pressure
There was no difference in systolic, diastolic, or mean blood pressure in the cobinamide sulfite treated animals compared to control animals at any time during the study (Fig. 6). Prior to initiation of cyanide infusion, systolic blood pressures were in a mean of 92 mmHg (95% CI 82, 103 mm Hg) and 94 mmHg (95% CI 74, 114 mm Hg) in the cobinamide sulfite-treated and control groups, respectively. At 5 and 45 minutes following intramuscular injection, systolic blood pressure was 73 mmHg (95% CI 61, 85 mm Hg) and 79 mm Hg (95% CI 71, 87 mm Hg) versus 82 mmHg (95% CI 60, 105 mm Hg) and 73 mmHg (95% CI 49, 98 mm Hg) in cobinamide sulfite-treated verus control animals, respectively.
Figure 6.
Systolic and diastolic blood pressures in control versus cobinamide sulfite-treated animals; cobinamide sulfite was administered at 60min. Blood pressure was not different between control and cobinamide sulfite-treated animals. The data are the mean and standard deviation for each group of animals. The values of cobinamide sulfite treated group are slightly shifted to prevent the overlap of error bars between two groups.
Discussion
We investigated the feasibility of an intramuscular cobinamide sulfite injection for reversing the physiologic effects of cyanide poisoning in an animal model (11). Cobinamide has many characteristics that suggest it may be effective in this role, including high binding affinity for cyanide, rapid binding kinetics, and high solubility in aqueous media (8, 29).
Cyanide can be lethal within minutes, therefore, mobilization of an intramuscular drug must be very rapid and capable of neutralizing cyanide's effects before irreversible damage occurs. In preliminary experiments performed prior to initiating the present study, we injected cobinamide (without addition of sodium sulfite) dissolved in phosphate-buffered saline intramuscularly into cyanide toxic rabbits (at and above doses that were known to be effective when administered intravenously). However, DOS and CWNIRS data, as well as red blood cell cyanide concentrations, and blood-gas evaluations, all showed cobinamide was not effective by this intramuscular route, even at high doses. A relatively slow rate of transfer of cobinamide into the blood was seen, and dissection of intramuscular injection sites revealed high amounts of cobinamide remaining in the muscle following sacrifice. While cobinamide has an extraordinarily high binding affinity for cyanide, it also reacts with and binds to nitric oxide (30). We hypothesized that the injected cobinamide induced localized vasoconstriction around the site of injection from binding nitric oxide, resulting in sequestration in the muscle bed. To overcome this problem, we generated cobinamide sulfite by adding sodium sulfite in slight molar excess (1.25 fold higher than the cobinamide dose) to the cobinamide solution prior to injection. Sulfite forms a co-ordinate bond with cobalamin (31), and, we, therefore, presume that sulfite forms a similar co-ordinate bond with cobinamide. Sulfite binds to cobalamin with moderate affinity (KA, 107 M-1) (31), and is likely to bind to cobinamide with at least as great an affinity. We further hypothesized that sulfite bound to cobinamide would prevent cobinamide from scavenging nitric oxide, thereby allowing cobinamide to be mobilized from the muscle bed. Because cobinamide binds cyanide with a much higher affinity than it binds sulfite, sulfite does not interfere with cyanide binding to cobinamide, as we showed in in vitro studies. Sulfite exhibits relatively low levels of toxicity to animals, and is used as a food preservative (32-34). Injecting cobinamide sulfite at a dose calculated to neutralize the administered cyanide rapidly reversed optical evidence of cyanide toxicity within minutes, with cobinamide appearing in the plasma 2-3 minutes after injection. Dissection of the muscle at the injection site demonstrated good mobilization of the injected solution. The calculated cobinamide sulfite dose required to neutralize a lethal human dose of cyanide could be administered as a 3-4 mL injection.
Rapid diagnosis and continual assessment of treatment response is essential to optimize cyanide countermeasures, particularly in the mass casualty setting. Having an antidote such as cobinamide that is stable, and can be administered intramuscularly in small volumes would allow for stockpiling and distribution of antidote kits to areas of potential risk including industrial sites, military installations, and to rapid response disaster and antiterrorist teams. However, sodium sulfite in liquid form is rapidly oxidized, and in the oxidized state does not prevent nitric oxide (NO) binding to cobinamide. Therefore, the sodium sulfite must be dissolved and mixed with cobinamide shortly before IM injection. In practice, this can be accomplished using a dual-chambered syringe with solid sodium sulfite in one chamber and a cobinamide solution in the other chamber; the two mix immediately before injection.
As a vitamin B12 analog, cobinamide appears to be relatively non-toxic (35-38), and we have administered cobinamide sulfite to mice and rabbits at doses 15-40 times higher than we used in this study without evidence of toxicity. A high safety index would allow cobinamide sulfite to be used for potential victims in whom cyanide poisoning could not be confirmed with certainty prior to antidote administration. Furthermore, because cobinamide is a direct binding agent, and does not induce methemoglobin formation, it has the potential to be used in patients with combined carbon monoxide and cyanide poisoning, commonly seen in smoke inhalation injury, in contrast to the concerns of nitrite treatment in such cases (39).
Cyanide is not easily detected by rapid assay, either in blood samples, or through noninvasive monitoring. Furthermore, blood cyanide levels do not correlate closely with the degree of toxicity for a number of well described reasons (13, 40-43). While diffuse optical spectroscopy does not measure cyanide levels directly, it can assess tissue oxy- and deoxyhemoglobin concentrations, as an indirect, but quantitative, noninvasive measure of the impact of cyanide on physiologic functions (11). It is of interest that blood cyanide levels did not decrease as quickly as would be expected by the rapid recovery seen in optical oxy- and deoxyhemoglobin measurements. This is consistent with reversing cytochrome-c oxidase inhibition after only partial unblocking of cyanide binding as demonstrated by Leavesley et al. (44).
In control animals, deoxyhemoglobin recovery time constants were very different between muscle and CNS (Tables 1), with the CNS recovering much more slowly. In contrast, in cobinamide sulfite-treated animals, the CNS deoxyhemoglobin recovery rate was much faster than in muscle. These results suggest that cyanide detoxification by cobinamide is very effective in recovering CNS oxy- and deoxyhemoglobin extraction function compared to peripheral muscle. We speculate that the high blood flow rate in the CNS may account for this effect.
In addition to binding cyanide, cobinamide can bind nitric oxide (9, 30), but the binding constant is several orders of magnitude lower for NO than for cyanide (9, 30). Thus, cobinamide preferentially binds cyanide, but cobinamide can bind NO when given in excess of available cyanide (9, 30). NO binding by cobinamide could result in vasoconstriction and increased blood pressure. In preliminary dose ranging studies, we observed oxy- and deoxyhemoglobin overshoot, accompanied by significant hypertension, when cobinamide (without sulfite) was administered intravenously in substantial excess of cyanide.
There are a number of limitations to this study. Utilizing DOS and CWNIRS based hemoglobin oxygenation as the major outcome indicator for cyanide toxicity reversal must be interpreted with caution, since this is not a lethal model, and we have not demonstrated the ability to increase survival in cyanide poisoning with IM Cobinamide sulfite injection. In addition, we do not demonstrate any evidence of CNS function recovery or improvements. Therefore, we cannot draw conclusions regarding effectiveness of cobinamide in treating lethal cyanide poisoning from this study.
With regard to specific limitations, animals were anesthetized for comfort and safety in compliance with animal welfare regulations, but this caused no adverse hemodynamic or other detectable events. Second, the number of animals studied was limited, as was the duration of follow-up, preventing subtle toxicities from cyanide poisoning or cobinamide from being detected. As this is a sub-lethal model, determining whether similar beneficial effects and complete toxicity reversal will be seen in a higher dose lethal model will require separate investigations.
While an intramuscular injection may be ideal for mass casualty settings, intravenous administration of a cyanide antidote may be preferable for individual exposures because of potentially more rapid systemic distribution. However, the time required to establish intravenous access could offset the advantage of faster distribution of intravenous versus intramuscular drug administration, and hydroxocobalamin and other cyanide antidotes must be infused over 15 minutes (45). Further studies will be needed to address these issues as well. Specific DOS and CWNIRS limitations for real time assessment of cyanide toxicity and reversal are that DOS and CWNIRS measure average tissue constituents to a depth of 4-8 mm (at the source detector separations used in these studies). Deeper tissue effect and organ specific toxicities cannot be assessed using the current study design. Furthermore, as with all near-infrared optical absorption technologies, other potential optically interfering agents, or medical conditions that might alter oxyhemoglobin levels could affect the ability to diagnose and monitor cyanide toxicity, including commonly encountered combined carbon monoxide and cyanide toxicity states. DOS capabilities in these scenarios will need to be investigated as well.
We used DOS to monitor oxy- and deoxyhemoglobin in peripheral muscles and CWNIRS to monitor oxy- and deoxyhemoglobin in the CNS because DOS is limited in maximal source detector separations and signal intensity. Therefore the maximal DOS depth of penetration is shallower, with more limited capabilities for CNS measurements. The two methods yield similar results when simultaneously monitoring over two separate muscle beds, validating their use.
In conclusion, this study demonstrates the feasibility of an intramuscular injection of cobinamide sulfite for rapidly reversing the physiologic effects of cyanide toxicity on oxy- and deoxyhemoglobin as an indicator of cytochrome oxidase blockage and tissue oxygen extraction capabilities. In addition, the study provides further evidence of the value of DOS and CWNIRS as tools for real-time, quantitative assessment of cyanide toxicity effects and comparisons between various potential cyanide treatment regimens. If additional studies confirm the efficacy and safety of intramuscular cobinamide sulfite in lethal cyanide exposures, this approach may be paradigm shifting in treating mass exposures to cyanide.
Acknowledgements
The investigators would like to thank Tanya Burney, David Jett, and Tim Bigby for their input, advice, and guidance in the development of these studies and this manuscript. We would like to thank Kathy Osann, PhD for her statistical analysis of the cyanide data. Please note that the views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of the agencies providing support for this work.
Funding:
This work was supported by the AF [9550-04-1-0101, 9550-08-1-0384]; the Laser Microbeam and Medical Program from the National Center for Research Resources [P41RR001192]; and the National Institutes of Health [1U54NS063718, U01-NS058030].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Martin CO, Adams HP., Jr. Neurological aspects of biological and chemical terrorism: a review for neurologists. Arch Neurol. 2003;60(1):21–25. doi: 10.1001/archneur.60.1.21. [DOI] [PubMed] [Google Scholar]
- 2.Gracia R, Shepherd G. Cyanide poisoning and its treatment. Pharmacotherapy. 2004;24(10):1358–1365. doi: 10.1592/phco.24.14.1358.43149. [DOI] [PubMed] [Google Scholar]
- 3.Eckstein M. Cyanide as a chemical terrorism weapon. JEMS. 2004;(8)(suppl):29, 22–31. [PubMed] [Google Scholar]
- 4.Baskin SI, Brewer TG. Medical aspects of chemical and biological warfare. Chapter 10, Cyanide Poisoning. In: Sidell FR, Takafuji ET, Franz DR, Borden Institute (U.S.), editors. Textbook of military medicine Part I, Warfare, weaponry, and the casualty. Washington, D.C.: 1997. pp. 272–286. [Google Scholar]
- 5.Dzombak DA, Ghosh RS, Wong-Chong GM. Cyanide in Water and Soil: Chemistry, Risk, and Management. CRC Press; 2006. [Google Scholar]
- 6.Suskind R, editor. The one percent doctrine: Deep inside america's pursuit of its enemies since 9/11. Simon & Schuster; New York, NY: 2006. [Google Scholar]
- 7.Cummings TF. The treatment of cyanide poisoning. Occup Med (Lond) 2004;54(2):82–85. doi: 10.1093/occmed/kqh020. [DOI] [PubMed] [Google Scholar]
- 8.Broderick KE, Potluri P, Zhuang S, et al. Cyanide detoxification by the cobalamin precursor cobinamide. Exp Biol Med (Maywood) 2006;231(5):641–649. doi: 10.1177/153537020623100519. [DOI] [PubMed] [Google Scholar]
- 9.Sharma VS, Pilz RB, Boss GR, et al. Reactions of nitric oxide with vitamin B12 and its precursor, cobinamide. Biochemistry. 2003;42(29):8900–8908. doi: 10.1021/bi034469t. [DOI] [PubMed] [Google Scholar]
- 10.Hayward GC, Hill HAO, Pratt JM, et al. The chemistry of vitamin B(12). Part IV.1 The thermodynamic trans-effect. J Chem Soc. 1965:6485–6493. [PubMed] [Google Scholar]
- 11.Lee J, Armstrong J, Kreuter K, et al. Non-invasive in vivo diffuse optical spectroscopy monitoring of cyanide poisoning in a rabbit model. Physiol Meas. 2007;28(9):1057–1066. doi: 10.1088/0967-3334/28/9/007. [DOI] [PubMed] [Google Scholar]
- 12.Borron SW. Recognition and treatment of acute cyanide poisoning. J Emerg Nurs. 2006;32(4 Suppl):S12–18. doi: 10.1016/j.jen.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Borron SW, Baud FJ. Acute cyanide poisoning: clinical spectrum, diagnosis, and treatment. Arh Hig Rada Toksikol. 1996;47(3):307–322. [PubMed] [Google Scholar]
- 14.Alexander K, Baskin SI. The inhibition of cytochrome oxidase by diaminomaleonitrile. Biochim Biophys Acta. 1987;912(1):41–47. doi: 10.1016/0167-4838(87)90245-7. [DOI] [PubMed] [Google Scholar]
- 15.Egekeze JO, Oehme FW. Cyanides and their toxicity: a literature review. Tijdschr Diergeneeskd. 1980;105(8)(suppl 2):104–114. [PubMed] [Google Scholar]
- 16.Panda M, Robinson NC. Kinetics and mechanism for the binding of HCN to cytochrome c oxidase. Biochemistry. 1995;34(31):10009–10018. doi: 10.1021/bi00031a024. [DOI] [PubMed] [Google Scholar]
- 17.Lee PA, Sylvia AL, Piantadosi CA. Cyanide-related changes in cerebral O2 delivery and metabolism in fluorocarbon-circulated rats. Toxicol Appl Pharmacol. 1988;94(1):34–44. doi: 10.1016/0041-008x(88)90334-1. [DOI] [PubMed] [Google Scholar]
- 18.Koschel MJ. Management of the cyanide-poisoned patient. J Emerg Nurs. 2006;32(4 Suppl):S19–26. doi: 10.1016/j.jen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Bevilacqua F, Berger AJ, Cerussi AE, et al. Broadband absorption spectroscopy in turbid media by combined frequency-domain and steady-state methods. Appl Opt (USA) 2000;39(34):6498–6507. doi: 10.1364/ao.39.006498. [DOI] [PubMed] [Google Scholar]
- 20.Pham TH, Coquoz O, Fishikin JB, et al. Broad bandwidth frequency domain instrument for quantitative tissue optical spectroscopy. Rev Sci Instrum. 2000;71:2500–2513. [Google Scholar]
- 21.Merritt S, Gulsen G, Chiou G, et al. Comparison of water and lipid content measurements using diffuse optical spectroscopy and MRI in emulsion phantoms. Technol Cancer Res Treat. 2003;2(6):563–569. doi: 10.1177/153303460300200608. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, El-Abaddi N, Duke A, et al. Noninvasive in vivo monitoring of methemoglobin formation and reduction with broadband diffuse optical spectroscopy. Journal of Applied Physiology. 2006;100(2):615–622. doi: 10.1152/japplphysiol.00424.2004. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, Saltzman DJ, Cerussi AE, et al. Broadband diffuse optical spectroscopy measurement of hemoglobin concentration during hypovolemia in rabbits. Physiological Measurement. 2006;27(8):757–767. doi: 10.1088/0967-3334/27/8/009. [DOI] [PubMed] [Google Scholar]
- 24.Zijlistra WG, Buursma A, Assendelft OWV. Visible and Near Infrared Absorption Spectra of Human and Animal Haemoglobin: Determination and Application: VSP. 2000.
- 25.Kim JG, Liu H. Investigation of biphasic tumor oxygen dynamics induced by hyperoxic gas intervention: the dynamic phantom approach. Appl Opt. 2008;47(2):242–252. doi: 10.1364/ao.47.000242. [DOI] [PubMed] [Google Scholar]
- 26.Kim JG, Lee J, Roe J, et al. Hemodynamic changes in rat leg muscles during tourniquet-induced ischemia-reperfusion injury observed by near-infrared spectroscopy. Physiol Meas. 2009;30(7):529–540. doi: 10.1088/0967-3334/30/7/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundquist P, Rosling H, Sorbo B. Determination of cyanide in whole blood, erythrocytes, and plasma. Clin Chem. 1985;31(4):591–595. [PubMed] [Google Scholar]
- 28.Guilbault G, Kramer D. Ultrasensitive, specific method for cyanide using p-notrobenzaldehyde and o-dintrobenzene. Anal Chem. 1966;28:834–836. [Google Scholar]
- 29.Broderick KE, Balasubramanian M, Chan A, et al. The cobalamin precursor cobinamide detoxifies nitroprusside-generated cyanide. Exp Biol Med (Maywood) 2007;232(6):789–798. [PubMed] [Google Scholar]
- 30.Broderick KE, Singh V, Zhuang S, et al. Nitric oxide scavenging by the cobalamin precursor cobinamide. J Biol Chem. 2005;280(10):8678–8685. doi: 10.1074/jbc.M410498200. [DOI] [PubMed] [Google Scholar]
- 31.Dolphin D, editor. Vitamin B12. Wiley Interscience; New York, NY: 1981. [Google Scholar]
- 32.Nair B, Elmore AR. Final report on the safety assessment of sodium sulfite, potassium sulfite, ammonium sulfite, sodium bisulfite, ammonium bisulfite, sodium metabisulfite and potassium metabisulfite. Int J Toxicol. 2003;22(Suppl 2):63–88. doi: 10.1080/10915810305077x. [DOI] [PubMed] [Google Scholar]
- 33.Evaluation of certain food additives Fifty-first report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organ Tech Rep Ser. 2000;891:i–viii. 1–168. [PubMed] [Google Scholar]
- 34.Til HP, Feron VJ. Toxicology of sulphiting agents. I: Animal studies. Food Addit Contam. 1992;9(5):587–595. doi: 10.1080/02652039209374112. [DOI] [PubMed] [Google Scholar]
- 35.Stabler SP, Brass EP, Marcell PD, et al. Inhibition of cobalamin-dependent enzymes by cobalamin analogues in rats. J Clin Invest. 1991;87(4):1422–1430. doi: 10.1172/JCI115148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinberg JB, Shugars DC, Sherman PA, et al. Cobalamin inhibition of HIV-1 integrase and integration of HIV-1 DNA into cellular DNA. Biochem Biophys Res Commun. 1998;246(2):393–397. doi: 10.1006/bbrc.1998.8629. [DOI] [PubMed] [Google Scholar]
- 37.Kondo H, Iseki T, Goto S, et al. Effects of cobalamin, cobalamin analogues and cobalamin binding proteins on P388D1 mouse leukemic cells in culture. Int J Hematol. 1992;56(2):167–177. [PubMed] [Google Scholar]
- 38.Coates ME, Davies MK, Dawson R, et al. The activity for chicks of some vitamin B12-like compounds. Biochem J. 1956;64(4):682–686. doi: 10.1042/bj0640682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shepherd G, Velez LI. Role of hydroxocobalamin in acute cyanide poisoning. Ann Pharmacother. 2008;42(5):661–669. doi: 10.1345/aph.1K559. [DOI] [PubMed] [Google Scholar]
- 40.Brierley JB, Brown AW, Calverley J. Cyanide intoxication in the rat: physiological and neuropathological aspects. J Neurol Neurosurg Psychiatry. 1976;39(2):129–140. doi: 10.1136/jnnp.39.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brierley JB, Prior PF, Calverley J, et al. Cyanide intoxication in Macaca mulatta. Physiological and neuropathological aspects. J Neurol Sci. 1977;31(1):133–157. doi: 10.1016/0022-510x(77)90011-9. [DOI] [PubMed] [Google Scholar]
- 42.Groff WA SF, Sr, Kaminskis A, Froehlich HL, Johnson RP. Plasma free cyanide and blood total cyanide: A rapid completely automated microdistillation assay. Clinical Toxicology. 1985;23:133–163. doi: 10.3109/15563658508990623. [DOI] [PubMed] [Google Scholar]
- 43.Baud FJ, Barriot P, Toffis V, et al. Elevated blood cyanide concentrations in victims of smoke inhalation. N Engl J Med. 1991;325(25):1761–1766. doi: 10.1056/NEJM199112193252502. [DOI] [PubMed] [Google Scholar]
- 44.Leavesley HB, Li L, Prabhakaran K, et al. Interaction of Cyanide and Nitric Oxide with Cytochrome c Oxidase in: Implications for Acute Cyanide Toxicity. Toxicol Sci. 2007 doi: 10.1093/toxsci/kfm254. [DOI] [PubMed] [Google Scholar]
- 45.Cyanokit afpiN, CA: Dey, LP. 2006. 7-29-08 hwccA.