Abstract
Background
Individuals with monoclonal B-cell lymphocytosis (MBL) have been identified in clinic outpatients, in unaffected relatives of patients with chronic lymphocytic leukemia (CLL), and in general populations. MBL and its relationship with CLL have been actively investigated over the last decade. This report systematically reviews the prevalence of MBL in the context of the populations studied and the evolution of laboratory methods used to define MBL.
Methods
To identify published studies that have assessed the prevalence of MBL, we systematically searched the MEDLINE® databases and consulted with members of the International MBL Study Group. We reviewed the 10 articles that were identified by this process. We abstracted information on study populations, laboratory tests, criteria for designating MBL, and the reported frequencies.
Results
Three of the ten studies were published in 2009, three between 2007 and 2008, and four between 2002 and 2004. Reported prevalences varied widely, ranging from 0.12 to 18.2%. This variability was clearly associated with both the laboratory methods and the populations studied. MBL was more common among older individuals and kindred of persons with CLL. The most common MBL subtype was CLL-like MBL.
Conclusions
Large population-based studies of MBL that employ standardized laboratory methods with a consensus case definition are needed to assess prevalence and establish risk factors. These studies should include prospective follow-up of MBL cases to determine the relationship between MBL and CLL. Data from original studies should be reported in sufficient detail to allow future synthesis of information from multiple studies, such as meta-analysis.
Key terms: monoclonal B-cell lymphocytosis, MBL, chronic lymphocytic leukemia, CLL, prevalence, epidemiology
INTRODUCTION
Monoclonal B-cell lymphocytosis (MBL) is an asymptomatic hematologic condition defined by the presence of monoclonal B-lymphocytes detected in peripheral blood of persons who do not have chronic lymphocytic leukemia (CLL), other B-lymphoproliferative disorders (BLPD), or underlying conditions such as infectious and autoimmune diseases (1). MBL and its relationship with CLL and other BLPD have been very active areas of investigation over the last decade. Based on findings from a large population-based cancer screening study, MBL appears to precede CLL by several years (2). Although the majority of individuals with MBL do not develop CLL, some MBL cases are at excess risk of developing CLL (3,4). In fact, the observation that MBL consistently precedes CLL (2) emphasizes the need for future studies designed to delineate the early molecular events leading to CLL. Ultimately, such studies may lead to the identification of novel therapeutic targets that can be used to delay or prevent CLL progression.
CLL is the most common adult leukemia in North America and Europe; it is less frequent in Asia and Africa (5). The reported age-adjusted incidence rate of CLL in the United States between 1975 and 2006 was 4.43 per 100,000 persons (6). However, because of its long asymptomatic period, the incidence of CLL is under-reported in cancer registries (7). Despite this uncertainty, it is clear that the incidence of CLL rises dramatically with age and that it is more common in men than women (7,8). As the proportion of older people has increased with improved life expectancy in the Western world, the burden from CLL has also increased. The American Cancer Society projected 15,490 new cases for 2009, a substantial increase from the 11,168 new cases reported in 2005 (9). The disease burden is also significant in the European Union, with an estimated 46,000 individuals in 2006 living with CLL 5 years post-diagnosis (10).
Currently, detailed information on descriptive epidemiologic characteristics of MBL is not available. The flow cytometry methods used to detect MBL vary from routine clinical settings to detailed research studies. The reported prevalence of MBL also varies widely. This report systematically reviews the reported prevalence of MBL in the context of the populations studied and the evolution of laboratory methods used to define MBL.
METHODS
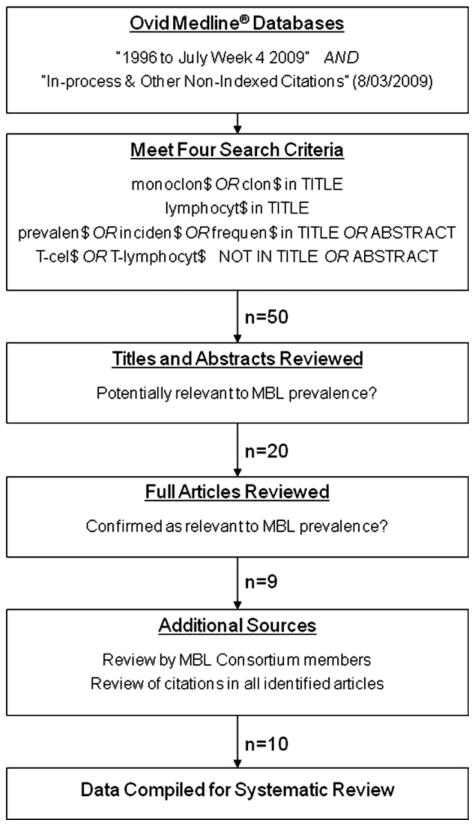
We searched Ovid MEDLINE® database from 1996 to July Week 4, 2009 and the Ovid MEDLINE® In-Process & Other NonIndexed Citations database (dated August 3, 2009) with the multifield search engine using truncation and wild cards. We selected nine articles as outlined in Figure 1.
Fig. 1.
Flow diagram of article identification to be included in this systematic review.
These articles were provided to participants in the International MBL Study Group (11), who identified one additional article. The citations contained in this final set of 10 articles were reviewed for any salient articles that had been missed; none were identified.
The ten articles were reviewed and information on study population, laboratory tests, criteria for designating MBL, and the frequency of MBL were abstracted. Where possible, we recalculated prevalence rates for subgroups of MBL from the data reported in each study. We did not calculate an aggregated prevalence estimate across all studies because the laboratory methods utilized and the populations studied varied widely.
RESULTS
Characteristics of the Included Studies
Of the ten articles identified, nine were cross-sectional surveys (3,12–19) and one was a longitudinal cohort study incorporating both baseline and follow-up data (20). All were published between 2002 and 2009: three studies in 2009, three studies between 2007 and 2008, and four between 2002 and 2004.
Six studies originated from Europe (Italy n = 2; United Kingdom n = 3; Spain n = 1) and four from North and South America (United States n = 3; Brazil n = 1). No studies from other geographic regions met the inclusion criteria. The individuals studied came from diverse settings. Three studies targeted general populations (12,13,20), one investigated a blood donor population (14), three included outpatients in general clinics (3,15,16), and three targeted clinically unaffected relatives of CLL patients (17–19). The number of individuals included in these studies varied greatly, ranging from 33 in a study of CLL kindred (18) to 5141 in a study of blood donors (14). Most studies targeted older adults, with minimum age in the 40s. However, three studies included individuals less than 20-years-old (12,14,17). Of the ten studies, only one study selected individuals based on the presence of lymphocytosis (3).
Laboratory Methods and Criteria for MBL
Although all studies used multicolor flow cytometry to detect MBL, there were significant differences in the methodologies employed. Some studies utilized two color or three color antibody-fluorochrome combinations and analyzed relatively few cellular events. These methods produced lower prevalence estimates (14,20).
Other recent studies made use of instrument and technical advances that allowed multiplexed analysis with more antibody-fluorochrome combinations. These studies also collected larger numbers of cells, which facilitated detection of very small populations of MBL cells among normal lymphocytes. Four color to eight-color antibody-fluorochrome combinations have been used to analyze as many as 5 × 105–5 × 106 total cellular events. These protocols produced higher prevalence estimates (12,13).
Another common finding among most studies is the presence of three different types of MBL cases, defined on the basis of CD19 positivity, CD5 presence or absence, and CD20 intensity. The most common type is CLL-like MBL. These MBL cells coexpress CD19 and CD5 with dim expression of CD20, and are immunopheno-typically indistinguishable from CLL cells. The second type is termed atypical CLL-like MBL, which differs from the typical form in that CD20 expression is bright. The third type of MBL includes cases that do not express CD5; these are classified as CD5− MBL or non-CLL-like MBL.
Although most studies attempted to detect immunoglobulin heavy-chain variable (IGHV) gene rearrangement in MBL samples, negative results did not preclude MBL that had been detected phenotypically. However, positive results did provide information on IGHV gene family usage in MBL (12).
Prevalence of MBL: General Populations and Blood Donors
Table 1 summarizes the prevalence estimates obtained from three studies that investigated general populations and one study that investigated blood donors. The most recently published studies included volunteers in geographically well-defined populations in Spain (13) and Italy (12). The study in Spain examined 608 individuals who were over 40 years of age; this study acquired the highest number of leukocyte events noted in this systematic review (eight-color staining panel, acquisition of 5 × 106 events). The overall prevalence of MBL in this Spanish study population was 14.3%. The study in Italy found an overall prevalence of 7.4% among 1,725 individuals aged 18 years or older (five-color staining panel, acquisition of 5 × 105 events). In contrast, a much lower prevalence of MBL was reported from two studies that were conducted in the United States using less sensitive flow cytometry methods. One was a blood donor study, which reported a prevalence of 0.12% among 5,141 individuals 17 years or older (14). The other was an environmental health study, which reported a prevalence of 0.6% among 1,926 individuals aged 40 years or older (20).
Table 1.
Overview of Studies that Reported the Frequency of MBL Among Healthy Residential Populations and Blood Donors
| Authors, year (reference) | Country, study period, and study population | Laboratory tests and clinical evaluation | MBL criteria | Frequency of MBL N (%) |
|---|---|---|---|---|
| Dagklis et al., 2009 (12) | Italy 2005–2007 Healthy volunteers in a geographically isolated rural area Total n: 1725 Age: 18–102 years Male 758; female 967 |
Flow cytometry Acquisition: 5 × 105 events/sample Five color: CD20/CD5/CD19/k/λ PCR amplification of IGHV-D-J rearrangements and sequence analysis Clinical records |
Mean B-cell count <5 × 109/L CLL-like: CD5bright CD20dim expression on CD19+ B-cells with a normal or an unbalanceda k:λ ratio Non-CLL like: CD5− MBL or atypical CLL-MBL with an unbalanced k:λ ratioa within CD5− or CD5+ B-cells |
All MBL: 128/1725 (7.4%) Male/female: (9.9%)/(5.5%) CLL-like: 89/1725 (5.2%) Male/female: (6.7%)/(3.9%) Age: <50 (0.8%), 50–69 (6.8%), >69 (9.2%) Unbalanced k/λ ratio: 83/89 Monoclonal B-cells/total B-cells: (mean = 6.9%) Non-CLL-like: 39/1725 (2.3%) Age: <50 (1.3%), 50–69 (1.5%), >69 (5.1%) CD5− MBL: 20/1725 (1.2%) |
| Nieto et al., 2009 (13) | Spain 12 month period (not otherwise specified) Healthy volunteers from the primary health care system region of Salamanca Total n: 608 Age: >40 years Male 284; female 324 |
Flow cytometry Acquisition: 5 × 106 leukocytes/sample Eight color: CD20/CD45/CD8 +λ/CD56+k/CD4/CD19/CD3/CD38; CD20/CD45/Bcl2/CD23/CD19/CD10/CD5/CD38; CD20/k/λ/CD19/CD10/CD5 PCR amplification of IGH gene rearrangements |
A clonal B-cell cluster of minimum 50 cellular events CLL-like: “Circulating monoclonal B-cells with a CLL-phenotype” Non-CLL like (not otherwise specified) Normal lymphocyte counts |
All MBL: 87/608 (14.3%) Age: 40–49 (5.1%), 50–59 (5.3%), 60–69 (17.5%), 70–79 (21.7%), 80–89 (27.3%), >89 (75.0%) CLL-like MBL: 73/608 (12.0%) Male/female (13.8%)/(11.0%) k Restricted: 48/73 λ Restricted: 11/73 Biclonal: 14/73 Monoclonal B-cells/total B-cells: (median = 0.38%) Non-CLL like: 14/608 (2.3%) |
| Shim et al., 2007 (20) | United States 1991–1994: baseline Volunteers living near or far from waste sites Total n: 1,926 Age: 40–76 years Male 934; female 992 1997, 2003: Follow-ups B-cell lymphocytosis cases at baseline only Total n: 74 |
Flow cytometry: Acquisition: not reported Two color (baseline), three color (1997), four color (2003) Extended panel for differential diagnosis (2003) Medical records review in the 2003 follow-up |
MBL: CLL-like phenotype determined by “CD20, CD19, and CD5 staining patterns” or the presence of a monoclonal population determined by an unbalanceda k:λ ratio No known history of a lymphoproliferative disorder |
Overall MBL: 11/1,926 (0.6%) Baseline: 9 and 1997 follow-up: 2 Male/female (0.6%)/(0.5%) Residents near waste sites (0.8%); far from waste sites (0.2%) |
| Rachel et al., 2007 (14) | United States 2000–2002 Blood donors in a blood center Total n: 5141 Age: 17 years or older |
Flow cytometry Acquisition: not reported Two-color screening (CD19/CD5) Extended panel for cases with ≥40% CD19+ or >15% CD19/5 coexpression: CD45, CD14, CD8, CD3, HLA-DR, CD2, CD19, CD10, CD20, CD23, CD7, k, λ PCR analysis for IGH gene clonality |
B-cell count <5 × 109/L MBL: monoclonal B-cells expressing either “classical CLL” or “non-CLL subtypes” with a k:λ ratio >3:1 or <1:3 |
6/5141 (0.12%) k Restricted: 1/5141 (0.02%) λ Restricted: 5/5141 (0.1%) |
unbalanced k:λ ratio: a k:λ ratio of >3:1 or <1:3
Two of these four studies reported the MBL prevalence by subtype. The overall prevalence of CLL-like MBL was 12.0% in the Spanish study and 5.2% in the Italian study. The overall prevalence of non-CLL like subtype was 2.3% for both studies. Although the CLL-like subtype represented the majority of the MBL cases detected in the two studies, the proportion of CLL-like MBL among all MBL cases was somewhat higher in the Spanish study (83.9%) than in the Italian study (69.5%). Both studies reported increasing prevalence rates with increasing age. For overall MBL, the age-specific prevalences in the Spanish study were 5.1% for the 40–49 years group and 5.3% for the 50–59 years group. The prevalence sharply increased in the older age groups: 17.5% (60–69 years), 21.7% (70–79 years), 27.3% (80–89 years) and 75% (>89 years) (13). In the Italian study population, the age-specific prevalences of CLL-like MBL were 0.8, 6.8, and 9.2% for participants aged <50 years, 50–69 years, and >69 years, respectively. In contrast, the age-specific prevalence of non-CLL like MBL did not rise until the >69 age group (12). Neither study provided the breakdown of individuals by age group; therefore the stability of the age-specific estimates could not be ascertained. Three studies reported male: female ratios, which ranged from 1.2 (20) to 1.8 (12).
Prevalence of MBL: Outpatient Populations
Three clinic-based studies (3,15,16) reported the prevalence of MBL among outpatients with normal blood counts (Table 2). The flow cytometry methods used in these studies were similar, except that the more recent study by Rawstron et al. (3) acquired 2.5 times more events per sample. Of these three clinic-based studies, the two studies that included only individuals in their 60s or older reported similar prevalence rates of overall MBL: 6.4 (15) and 6.9% (3). The similarity in prevalence estimates is remarkable, considering that the two studies are from two different countries. The other study, which included younger outpatients, reported a lower prevalence (4.5%) (16).
Table 2.
Overview of Studies that Reported the Frequency of MBL Among Outpatient Clinics, Excluding Familial-CLL Clinics
| Authors, year (reference) | Country, study period, and study population | Laboratory tests and clinical evaluation | MBL Criteria | Frequency of MBL N (%) |
|---|---|---|---|---|
| Rawstron et al., 2008 (3) | United Kingdom Cohort 1: Outpatients with entirely normal blood counts Total n: 1520 Age: 62–80 years Male 630; female 890 Cohort 2: 1995–2000 Patients referred for current or previous lymphocytosis Total n: 2228 Age: 39–99 years |
Flow cytometry Acquisition: 5 × 105 events/sample Four color screening: CD5/CD19/k/λ Extended panela for cases with a k:λ ratio of >2.1:1 or <1:1 FISH PCR analysis for IGHV gene |
B-cell count <5 × 109/L CLL-phenotype MBL: CD19+, CD5+, CD23+, CD20weak, CD79bweak, and either k or λ Ig light chains Non–CLL-phenotype MBL: light-chain–restricted CD19+ B-cells with CD5− and strong CD20+ No history of cancer |
Cohort 1 All MBL:105/1520 (6.9%) CLL-phenotype: 78/1520 (5.1%) Non-CLL-phenotype: 27/1520 (1.8%) Cohort 2 CLL-phenotype: 309/2228 (13.9%) |
| Ghia et al., 2004 (15) | Italy Study period: “20 months” Outpatients from three clinics outside Turin; referred for routine blood tests; normal blood cell counts; and had no history or suspicion of malignancy Total n: 500 Age: > 65 years Male 231; female 269 |
Flow cytometry Acquisition: ≥2 × 105 events/sample Four color: CD5/CD19/k/λ for all 500 cases and CD5/CD20/CD79b/CD19 for 350 cases Extended panel for cases with CLL-like phenotype or unbalanced k:λ ratiob: CD10, CD20, CD23, CD79b, IgM, IgD, IgG, FMC7 PCR analysis for IGHV, bcl-1, bcl-2 genes |
Unbalanced k:λ ratiob CLL-like MBL: CD20low, CD23+, CD5bright, FMC7−, CD10−, IgMlow, CD79blow Atypical CLL-like MBL: CD20high, CD23+, CD5bright FMC7−, CD10−, IgMlow, CD79blow Non–CLL-like MBL: CD20high, CD23−, CD5−, FMC7+, CD10−, IgM1+/low, CD79b+ |
All MBL: 32/500 (6.4%) Male/female: (7.4%)/(5.6%) k restricted (includes two biclonal MBLs): 25/500 (5.0%) λ restricted: 7/500 (1.4%) CLL-like: 22/500 (4.4%) Atypical CLL-like: 3/500 (0.6%) Non–CLL-like (includes 2 biclonal MBLs): 7/500 (1.4%) |
| Rawstron et al., 2002 (16) | United Kingdom Hospital outpatients with normal hematologic parameters; samples not sent to assess any malignancy; had not been seen at hematology, oncology, transplantation clinic; <24 h sample available Total n: 910 Age: > 40 years Male 425; female 485 |
Flow cytometry Acquisition: ≥2 × 105events/sample Four color screening: CD20/CD76b/CD19/CD5; k/λ/CD19/CD5 Extended panel for cases with CLL-phenotype: CD19, CD5 or CD20; CD3/CD3; k/λ; CD5 or CD79b, CD20/CD79b; CD11a/CD23; IgM/CD38; CD10/CD22; IgG/CD27 PCR analysis for IGHV gene |
Clonal B-cell cluster of minimum 50 cellular events CLL-phenotype MBL: CD20low, CD5bright, CD79b+, and abnormal Ig light-chain expressionc Non-CLL phenotype MBL: normal expression of CD5/20/79b and light chain restriction |
All MBL: 41/910 (4.5%) CLL-phenotype: 32/910 (3.5%) Male/female: (4.9%)/(2.3%) Age: 40–59 (2.1%), ≥60 (5.0%); Non-CLL phenotype: 9/910 (1.0%) |
Extended panel for lymphocytosis cases:CD19, CD3/CD3 (control), CD20/CD5, CD10/CD38, k/λ, FMC7/CD22, CD11a/CD23, IgM/IgD, IgG/CD76b and for cases with normal blood count: CD19, CD5, CD20/CD79b, FMC7/CD23.
unbalanced k:λ ratio: a k:λ ratio of >3:1 or <1:3.
abnormal Ig light-chain expression: a k/λ ratio of >4.0 or <0.5, or >25% lacking sIg.
One of these three studies also investigated CLL-like MBL among 2,228 outpatients aged 39–99 years who were referred for investigation of previous or current lymphocytosis (3). The prevalence was 13.9%, which was much higher than that observed among outpatients with normal blood counts using similar laboratory methods (3.5–5.1%) (3,16). Among all MBL cases with normal blood counts, the proportion of CLL-like MBL ranged from 68.6 (15) to 78.0% (16). As in the population-based studies, the prevalence of CLL-like MBL was higher in the older age group (16). Two studies reported a higher MBL prevalence among men (male: female ratios of 1.3 and 2.1) (15,16).
Prevalence of MBL: Clinically Unaffected Relatives of CLL Patients
Table 3 summarizes MBL prevalence among clinically unaffected relatives of CLL patients. In a study of clinically unaffected first-degree relatives of familial-CLL patients (18), the overall prevalence of MBL was 18% (6/33). In another study of clinically unaffected first-degree relatives of familial-CLL patients, the prevalence of CLL-like MBL was 13.5% (8/59) (19).
Table 3.
Overview of Studies that Reported Frequency of MBL Among Clinically Unaffected Relatives of CLL Patients
| Authors, year (reference) | Country, study period, and study population | Laboratory tests and clinical evaluation | MBL criteria | Frequency of MBL N (%) |
|---|---|---|---|---|
| Matos et al., 2009 (17) | Brazil Study period not specified First-degree relatives of 42 randomly selected families with sporadic (non-familial) CLL Total n: 167 Age: 18–84 years Male 73; female 94 |
Flow cytometry Acquisition: 3 × 105 events/sample Four color screening: CD20/CD79b/CD19/CD5;k/λ/CD19/CD5; polyclonal k/polyclonal λ/CD19/CD5 Extended panel for cases with CLL-phe notype: CD11a, CD23, CD38, CD49c, CD49d, CD54, FMC7 PCR analysis for IGHV and T-cell receptor genes FISH |
A clonal B-cell cluster of minimum 100 cellular events MBL: CLL-specific immunophenotype or an overall k/λ ratio >3:1 or <0.3:1; stable monoclonal population over a 3-month period; no lymphadeno-pathy, organomegaly, autoimmune or infectious diseases; BALC <5 × 109/L CLL-like MBL: CD5+, CD23+, CD20dim Atypical CLL-like MBL: CD5+, CD23+/−, CD20bright |
All MBL: 7/167 (4.2%) Male/female: (6.8%)/(2.1%) Age: <40 (0%), 40–60 (2.5%), >60 (15.6%) CLL-like MBL: 6/167 (3.6%) Atypical CLL: 1/167 (0.6%) |
| Marti et al., 2003 (18) | United States Study period not specified First-degree relatives of CLL cases among 9 kindreds enrolled in a familial-CLL registry Total n: 33 |
Flow cytometry Two and three color: CD45/CD14; CD3/CD19; CD3/CD16+CD56; CD4/CD8; CD19/CD5;CD20/CD5; CD19/CD23; k/λ/CD19; CD10/CD34/CD19; k/CD22/CD20; λ/CD22/CD20; PCR analysis for IGHV gene Clinical evaluation |
Lymphocyte count <5 × 109/L MBL: A flow cytometric detection of CLL-phenotype or a detectable population of CD5− monoclonal B-cells Absence of a history of B-cell leukemia or other related lymphoproliferative diseases |
All MBL: 6/33 (18%) |
| Rawstron et al., 2002 (19) | United Kingdom Study period not specified Healthy, first-degree relatives of CLL cases in 21 families with 2 or more members who had CLL Total n: 59 Age: 23–86 years Healthy spouses Total n: 23 Age: 23–79 years |
Flow cytometry Four color: CD20/CD79b/CD19/CD5; k/λ/CD19/CD5 Extended panel for cases representing more than 50 events in all 3 CLL regions: CD19, CD20, CD5, and either CD11a, CD22, CD23, CD27, CD38, k, λ, or FMC7 |
CLL-phenotype MBL: CD20weak or negative, CD5positive, CD79bweak or negative, CD22weak, FMC7weak | First-degree relatives: CLL-phenotype MBL: 8/59 (13.6%) Spouse: CLL-phenotype MBL 1/23 (4.3%) |
With sporadic CLL, the prevalence of MBL was lower: 4.1% (3.6% for CLL-like and 0.6% for atypical) among the 167 first-degree relatives of 42 families with only one CLL patient per family (17). In this study, the prevalence was higher among men (male: female ratio of 3.2) and higher in the older age group (2.5% for the 40–60 years-old participants and 15.6% for those older than 60 years).
DISCUSSION
As immunophenotyping by flow cytometry came into common use, it was evident that monoclonal B-cells with the CLL-phenotype could be detected in the peripheral blood of some individuals who did not have CLL or any other B-cell malignancy. A unified nomenclature for this condition was lacking (21) until the term MBL was introduced in 2005 by the International Familial-CLL Consortium (1). The 10 articles included in this systematic review collectively reveal several important considerations in assessing the prevalence of MBL. Both the laboratory methods used and the populations studied have a major impact on the reported prevalence rates. Although it is difficult to quantify their respective contributions, some general tendencies are apparent.
Regarding laboratory methodology, the major factor that influences MBL detection is the number of B-cells acquired for analysis (22). This impact is most clearly demonstrated by Nieto et al. (13), who acquired 5 × 106 leukocytes and reported the highest prevalence of MBL among all general population studies. Their reported prevalence approached that in familial-CLL kindreds reported by Marti et al. (18). In the Nieto study, 50 monoclonal B-cell events were required to define MBL, and the clonal B-cells in many of the MBL cases represented a very low proportion (median: 0.38%, interquartile range: 0.14–4.2%) of the total B-cell population (13). It is not clear whether the study of familial-CLL kindreds was also capable of detecting such low levels of monoclonal B-cells. It would be interesting to know what the MBL prevalence would have been in the kindred study if the laboratory methods employed by Nieto et al. had been used. In any case, the most clinically relevant issue is the likelihood that MBL progresses to CLL or some other significant endpoint. The size of the clone in absolute and relative terms, as well as the total B-cell count and the total lymphocyte count, should be explored to identify the most predictive combination of prognostic factors.
Another laboratory source of variability that could influence MBL prevalence estimates is the ability to identify unique clonotypes by combinatorial patterns of staining. The studies that employed two different fluorescent labels could identify only four phenotype combinations. Five-color methods can distinguish 32 combinations, and eight-color methods can distinguish 256. Although it is not possible to visualize so many combinations at once, distinct populations can be identified by sequential gating or graphical representation of multivariate analysis (e.g. principal component analysis of flow cytometry data) (23). In addition, the intensity of staining for each receptor reflects differential expression, providing further capability to identify unique clonotypes (24).
Despite the variability in prevalence estimates resulting from the differences in laboratory methodologies, it is clear from this systematic review that MBL is more common among older adults and is most common among first-degree relatives of familial-CLL patients. The age-related trend was consistent across the studies regardless of the sources of the study population. These features parallel those of CLL, which is one of the most age-related of all malignancies and has a strong familial risk.
Other demographic risk factors are less certain or remain unexplored. Although male excess has been well documented in CLL, our systematic review shows that the gender difference appears to be less pronounced in MBL. This difference may be real or may be an artifact.
The male: female ratio ranged from 1.2–1.8 in general population-based studies and from 1.3–2.1 in outpatient-based studies. However, none of the studies reported the age-sex specific prevalence, and it is possible that women included in the studies were older than men. In fact, we speculate that future refinements in defining subtypes of MBL may delineate a condition with a higher probability of progression, and in this group the male: female ratio may coincide with that of CLL more closely.
No prevalence study of MBL from Asia or Africa was available for the review, and the studies included in this review did not report MBL prevalence by ethnic group. Given the fact that CLL is reportedly less common among Asians (5), one might speculate that this could be due to a lower prevalence of MBL, or a lower risk of progression from MBL to CLL, or a combination. To our knowledge, at this time, no study has been conducted to address these questions.
The reported subgroupings of MBL contain intriguing but inconsistent characterizations that do not allow a formal classification scheme. This inconsistency is due partly to differences in laboratory immunophenotyping methods and partly to a lack of consensus case definitions for MBL subgroups. Of particular importance is the distinction between “low-count” and “high-count” MBL. The total lymphocyte count, total B-cell count, and the proportion of monoclonal B-cells are all important parameters to explore in optimizing the definition of low-count and high-count subtypes. An optimized classification system is essential in assessing etiology, pathogenesis, and the likelihood of progression (25).
Studies of MBL will provide a greater understanding of the interaction between genetic and environmental factors in the natural history of CLL and other BLPD. Although CLL has strong familial associations, the overall genetic basis for the disease remains unclear (26). Both biological agents (27) and chemical exposures have been linked to CLL (28), including the herbicide Agent Orange (29). However, studies to date have not been able to establish a statistically significant association between any environmental agent and CLL, and an extrinsic environmental cause for CLL remains elusive. As MBL occurs earlier and has a much higher prevalence than CLL, it provides a more sensitive marker of biological effect for epidemiologic studies (30).
In summary, large population-based studies of MBL that employ standardized laboratory methods with consensus case definition are needed to assess prevalence and establish risk factors. These studies should include follow-up of MBL cases to determine the relationship between MBL and B-cell malignancies (particularly CLL), including risk factors associated with progression. They also should explore correlations with molecular markers and potential gene-environment interactions. Finally, our review underscores the importance of reporting data from original studies in sufficient detail to allow future synthesis of information from multiple studies, including meta-analysis.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Agency for Toxic Substances and Disease Registry and Centers for Disease Control and Prevention.
LITERATURE CITED
- 1.Marti GE, Rawstron AC, Ghia P, Hillmen P, Houlston RS, Kay N, Aurran-Schleinitz T. Caporaso N on behalf of The International Familial CLL Consortium. Diagnostic criteria for monoclonal B-cell lymphocytosis (MBL) Br J Haematol. 2005;130:325–332. doi: 10.1111/j.1365-2141.2005.05550.x. [DOI] [PubMed] [Google Scholar]
- 2.Landgren O, Albitar M, Ma W, Abbasi F, Hayes RB, Ghia P, Marti GE, Caporaso NE. B-cell clones as early markers for chronic lymphocytic leukemia. N Engl J Med. 2009;360:659–667. doi: 10.1056/NEJMoa0806122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawstron AC, Bennett FL, O’Connor SJ, Kwok M, Fenton JA, Plummer M, de Tute R, Owen RG, Richards SJ, Jack AS, Hillmen P. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med. 2008;359:575–583. doi: 10.1056/NEJMoa075290. [DOI] [PubMed] [Google Scholar]
- 4.Shanafelt TD, Kay NE, Rabe KG, Call TG, Zent CS, Maddocks K, Jenkins G, Jelinek DF, Morice WG, Boysen J, Schwager S, Bowen D, Slager SL, Hanson CA. Brief Report: Natural history of individuals with clinically recognized monoclonal B-cell lymphocytosis compared with patients with Rai 0 chronic lymphocytic leukemia. J Clin Orthod. 2009;27:3959–3963. doi: 10.1200/JCO.2008.21.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linet MS, Devesa SS, Morgan GJ. The leukemias. In: Schottenfeld D, Fraumeni JF, editors. Cancer Epidemiology and Prevention. 3. New York: Oxford University Press; 2006. pp. 841–871. [Google Scholar]
- 6.Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK, editors. SEER Cancer Statistics Review 1975–2006. Bethesda, MD: National Cancer Institute; 2009. Available at: http://seer.cancer.gov/csr/1975_2006/ (based on November 2008 SEER data submission, posted to the SEER web site, 2009) [Google Scholar]
- 7.Dores GM, Anderson WF, Curtis RE, Landgren O, Ostroumova E, Bluhm EC, Rabkin CS, Devesa SS, Linet MS. Chronic lymphocytic leukaemia and small lymphocytic lymphoma: Overview of the descriptive epidemiology. Br J Haematol. 2007;139:809–819. doi: 10.1111/j.1365-2141.2007.06856.x. [DOI] [PubMed] [Google Scholar]
- 8.Redaelli A, Laskin BL, Stephens JM, Botteman MF. Pashos CL (2004) The clinical and epidemiological burden of chronic lymphocytic leukaemia. Eur J Cancer Care (Engl) 2004;13:279–287. doi: 10.1111/j.1365-2354.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Cancer Statistics Working group. Statistics Working Group. United States Cancer Statistics: 1999–2005 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2009. Available at: www.cdc.gov/uscs. [Google Scholar]
- 10.Watson L, Wyld P, Catovsky D. Disease burden of chronic lymphocytic leukaemia within the European Union. Eur J Haematol. 2008;81:253–258. doi: 10.1111/j.1600-0609.2008.01114.x. [DOI] [PubMed] [Google Scholar]
- 11.Goolsby C, Peterson L, Ma S, Rosen S. Cytometry Part B Clin Cytom. Suppl 1. 78B. 2010. A special supplement on MBL. Why? (editorial) pp. S2–S3. this issue. [DOI] [PubMed] [Google Scholar]
- 12.Dagklis A, Fazi C, Sala C, Cantarelli V, Scielzo C, Massacane R, Toniolo D, Caligaris-Cappio F, Stamatopoulos K, Ghia P. The immunoglobulin gene repertoire of low-count CLL-like MBL is different from CLL: Diagnostic implications for clinical monitoring. Blood. 2009;114:26–32. doi: 10.1182/blood-2008-09-176933. [DOI] [PubMed] [Google Scholar]
- 13.Nieto WG, Almeida J, Romero A, Teodosio C, López A, Henriques AF, Sánchez ML, Jara-Acevedo M, Rasillo A, González M, Fernández-Navarro P, Vega T, Orfao A the Primary Health Care Group of Salamanca. Increased frequency (12%) of circulating CLL-like B-cell clones in healthy individuals using a high-sensitive multicolor flow cytometry approach. Blood. 2009;114:33–37. doi: 10.1182/blood-2009-01-197368. [DOI] [PubMed] [Google Scholar]
- 14.Rachel JM, Zucker ML, Fox CM, Plapp FV, Menitove JE, Abbasi F, Marti GE. Monoclonal B-cell lymphocytosis in blood donors. Br J Haematol. 2007;139:832–836. doi: 10.1111/j.1365-2141.2007.06870.x. [DOI] [PubMed] [Google Scholar]
- 15.Ghia P, Prato G, Scielzo C, Stella S, Geuna M, Guida G, Caligaris-Cappio F. Monoclonal CD5+ and CD5-B-lymphocyte expansions are frequent in the peripheral blood of the elderly. Blood. 2004;103:2337–2342. doi: 10.1182/blood-2003-09-3277. [DOI] [PubMed] [Google Scholar]
- 16.Rawstron AC, Green MJ, Kuzmicki A, Kennedy B, Fenton JA, Evans PAS, O’connor SJM, Richards SJ, Morgan GJ, Jack AS, Hillmen P. Monoclonal B lymphocytes with the characteristics of “indolent” chronic lymphocytic leukemia are present in 3.5% of adults with normal blood counts. Blood. 2002;100:635–639. doi: 10.1182/blood.v100.2.635. [DOI] [PubMed] [Google Scholar]
- 17.Matos DM, Ismael SJ, Scrideli CA, de Oliveira FM, Rego EM, Falcao RP. Monoclonal B-cell lymphocytosis in first-degree relatives of patients with sporadic (non-familial) chronic lymphocytic leukemia. Br J Haematol. 2009;147:339–346. doi: 10.1111/j.1365-2141.2009.07861.x. [DOI] [PubMed] [Google Scholar]
- 18.Marti GE, Carter P, Abbasi F, Washington GC, Jain N, Zenger VE, Ishibe N, Goldin L, Fontain L, Weissman N, Sgambati M, Fauget G, Bertin P, Voght RF, Slade B, Noguchi PD, Stetlet-Stevenson MA, Caporaso N. B-cell monoclonal lymphocytosis and B-cell abnormalities in the setting of familial B-cell chronic lymphocytic leukemia. Cytometry Part B Clin Cytom. 2003;52B:1–12. doi: 10.1002/cyto.b.10013. [DOI] [PubMed] [Google Scholar]
- 19.Rawstron AC, Yuille MR, Fuller J, Cullen M, Kennedy B, Richards SJ, Jack AS, Matutes E, Catovsky D, Hillmen P, Houlston RS. Inherited predisposition to CLL is detectable as subclinical monoclonal B-lymphocyte expansion. Blood. 2002;100:2289–2290. doi: 10.1182/blood-2002-03-0892. [DOI] [PubMed] [Google Scholar]
- 20.Shim YK, Vogt RF, Middleton D, Abbasi F, Slade B, Lee KY, Marti GE. Prevalence and natural history of monoclonal and polyclonal B-cell lymphocytosis in a residential adult population. Cytometry Part B Clin Cytom. 2007;72B:344–353. doi: 10.1002/cyto.b.20174. [DOI] [PubMed] [Google Scholar]
- 21.Caporaso NE, Marti GE, Vogt RF, Shim YK, Middleton D, Landgren O on behalf of the International MBL Study Group. Evolution of a precursor. Cytometry Part B Clin Cytom. 2010;78B:1–2. doi: 10.1002/cyto.b.20508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogt RF, Champion PD, Shim YK. A computer simulation for exploring the detection of monoclonal B-cell lymphocytosis (MBL) by flow cytometry. Cytometry Part B Clin Cytom. 2010;78B(Suppl 1):S110–S114. doi: 10.1002/cyto.b.20557. this issue. [DOI] [PubMed] [Google Scholar]
- 23.Pedreira CE, Costa ES, Almeida J, Fernandez C, Quijano S, Flores J, Barrena S, Lecrevisse Q, Van Dongen JJM, Orfao A on behalf of the EuroFlow Consortium. A probabilistic approach for the evaluation of minimal residual disease by multiparameter flow cytometry in leukemic B-cell chronic lymphoproliferative disorders. Cytometry A. 2008;73A:1141–1150. doi: 10.1002/cyto.a.20638. [DOI] [PubMed] [Google Scholar]
- 24.Vogt RF, Schwartz A, Marti GE, Whitfield WE, Henderson LO. Quantitative fluorescence cytometry. In: Faguet GB, editor. Hematologic malignancies: Methods and techniques. Totowa NJ: Humana Press; 2001. pp. 255–277. [Google Scholar]
- 25.Rawstron AC, Shanafelt T, Lanasa MC, Landgren O, Hanson C, Orfao A, Hillmen P, Ghia P. Different biology and clinical outcome according to the absolute numbers of clonal B-cells in monoclonal B-cell lymphocytosis (MBL) Cytometry Part B Clin Cytom. 2010;78B(Suppl 1):S19–S23. doi: 10.1002/cyto.b.20533. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caporaso N, Goldin L, Plass C, Calin G, Marti G, Bauer S, Raveche E, McMaster ML, Ng D, Landgren O. Slager Chronic lymphocytic leukaemia genetics overview. Br J Haematol. 2007;139:630–634. doi: 10.1111/j.1365-2141.2007.06846.x. [DOI] [PubMed] [Google Scholar]
- 27.Landgren O, Gridley G, Check D, Caporaso NE, Morris Brown L. Acquired immune-related and inflammatory conditions and subsequent chronic lymphocytic leukaemia. Br J Haematol. 2007;139:791–798. doi: 10.1111/j.1365-2141.2007.06859.x. [DOI] [PubMed] [Google Scholar]
- 28.Blair A, Purdue MP, Weisenburger DD, Baris D. Chemical exposures and risk of chronic lymphocytic leukaemia. Br J Haematol. 2007;139:753–61. doi: 10.1111/j.1365-2141.2007.06874.x. [DOI] [PubMed] [Google Scholar]
- 29.Committeeto Review the Health Effects in Vietnam Veterans of Exposure to Herbicides. Veterans and Agent Orange: Update 2004. Washington, DC: The National Academies Press; 2004. [Google Scholar]
- 30.Vogt RF, Shim YK, Middleton DC, Buffler PA, Campolucci SS, Lybarger JA, Marti GE. Monoclonal B-cell lymphocytosis as a biomarker in environmental health studies. Br J Haematol. 2007;139:690–700. doi: 10.1111/j.1365-2141.2007.06861.x. [DOI] [PubMed] [Google Scholar]