Abstract
The clinical profile and histopathologic changes in needle biopsies of the liver were studied in 10 cases of acute Epstein-Barr virus infection occurring in liver transplant recipients. The systemic viral syndrome in four cases resembled that seen in infectious mononucleosis, whereas in six others it was characterized by atypical signs and symptoms in the form of jaw pain, arthralgias, joint space effusions, diarrhea, encephalitis, pneumonitis, mediastinal lymphodenopathy, and ascites. Laboratory investigation showed marked elevations in hepatocellular enzymes and circulating atypical lymphocytes in the peripheral blood. Pancytopenia was noted in eight cases. A range of histopathologic changes was noted in the allografts ranging from alterations typically observed in infectious mononucleosis to a distinctive constellation characterized by (a) mixed mononuclear portal and sinusoidal infiltrates containing atypical large noncleaved cells and immunoblasts; (b) associated lobular activity indicative of a hepatitic process, and (c) relatively mild duct damage not in proportion to the severity of the inflammatory infiltrates. The patients responded to reduced immunosuppression, but recurrent viral syndromes occurred in four instances and one patient died of systemic lymphoproliferative disease.
Keywords: Epstein-Barr virus, Transplantation, Liver
Epstein-Barr Virus (EBV) infections are commonly observed in patients after orthotopic liver transplantation and serologic evidence of active disease is demonstrable in 24% of cases (15). Most often this represents asymptomatic infection, but 1–2% of transplant recipients develop persistent or recurrent disease culminating in the development of posttransplant lymphoproliferative disorders, some of which behave like aggressive lymphomas (9). Early recognition of EBV infection and treatment with reduced immunosuppression and acyclovir has the potential of preventing such complications. For this reason we undertook a retrospective analysis to define the clinical presentation, liver function tests, hematological profile, histopathologic changes in liver allograft, and final outcome in 10 cases with EBV syndrome occurring after liver replacement in patients maintained on cyclosporine-prednisone immunosuppression.
It is shown that the observed clinical profile overlaps the syndrome commonly seen in infectious mononucleosis, but, often is different enough that it may go clinically unrecognized. The constellation of changes seen in needle biopsies of the allograft in these cases is sufficiently distinctive for the pathologist to suggest the diagnosis of EBV infection on histopathological grounds alone.
MATERIALS AND METHODS
The cases described in this series were selected by reviewing the clinical and serologic data on adult orthotopic liver transplant recipients who had liver biopsies submitted to the Department of Pathology, Presbyterian University Hospital, Pittsburgh, from 1985 to 1988.
The 10 cases selected for this study fulfilled the following criteria.
There was clinical and serologic evidence of recent EBV infection in the form of fever, sore throat, lymphadenitis, icterus, or splenomegaly accompanied by either a positive anti–viral capsid antigen (VCA) IgM or a recent fourfold rise in titers of anti-VCA IgG antibodies. Cases known to be sero-negative before transplantation were considered primary EBV infection, whereas all other cases were considered to be a reactivation of a latent infection.
Needle biopsies of liver concurrent with the clinical EBV syndrome were available.
Absence of evidence for other known causative agents of mononucleosis-like syndromes such as active cytomegaloviral, hepatitis B, or herpes simplex infections, and drug reactions.
The medical records of these patients were reviewed for clinical presentation, liver function tests, hematologic profile, occurrence of infectious and allograft rejection episodes, and response to reduced immunosuppression and acyclovir administration. Serologic titers of anti-EBV antibodies performed by standard immunofluorescence techniques were recorded as available in the files. Histopathological changes in the liver biopsies were evaluated by examination of 5-μm paraffin-embedded sections stained with hematoxylin eosin, periodic acid-Schiff, and trichrome techniques.
RESULTS
Clinical Presentation
There were five men and five women ranging in age from 18 to 58 years (mean 37, median 35; Table 1). The primary liver diseases for which liver transplantation was performed in these patients were cryptogenic cirrhosis (four cases), primary sclerosing cholangitis, chronic active (clinically non-A non-B) hepatitis with cirrhosis, hepatitis B cirrhosis associated with hepatocellular carcinoma, alcoholic cirrhosis, alpha-1-antitrypsin deficiency, and Wilson’s disease (one case each). Cyclosporine was administered in the dose of 2 mg/kg/day in i.v. on day of surgery followed by 6 mg/kg/day orally. Prednisone was administered in a dose of 200 mg/day i.v. on the day of surgery followed by tapering to an oral maintenance dose of 20 mg/day.
TABLE 1.
Clinical features of Epstein-Barr virus (EBV) Syndrome in orthotopic liver transplant recipients
| Case no. | Age (yr)/sex | Primary disease | Period of onset of EBV syndrome following transplant (days) | Presenting clinical features | Outcome of initial EBV syndrome | Recurrent EBV syndromes | Final outcome of EBV infection |
|---|---|---|---|---|---|---|---|
| 1 | 53/M | CC | 63 | Fever, pharyngitis, cervical and axillary LN | Resolution over 5 wk | PTLD lung and ileum resolved over 8 wk | Resolution |
| 2 | 22/M | PSC | 43 | Fever, pharyngitis generalized LN, Sp, icterus | Resolution over 8 wk | NIL | Resolution |
| 3 | 44/F | CC | 14 | Fever, generalized LN, icterus | Resolution over 8 wk | NIL | Resolution |
| 4 | 28/F | CC | 27 | Arthralgia right knee, petechial rash | Resolution over 2 wk | Systemic PTLD, fatal over 1 mo | Death |
| 5 | 20/M | CC | 603 | Fever, pharyngitis, paratracheal LN, diarrhea, jaw pains, arthralgias, right pleural effusion, Sp | Developed systemic PTLD, death at 8 wk | Death | |
| 6 | 58/M | Alcoholic cirrhosis | 31 | Fever, cervical and axillary LN | Resolution over 2 wk | Meningoencephalitis resolving over 5 days | Resolution |
| 7 | 26/F | α-1ATD | 74 | Icterus, axillary LN | Resolution over 3 wk | Systemic PTLD responsive to immunomodulation | Resolution |
| 8 | 18/F | Wilson’s | 10 | Fever, sinus headaches generalized LN | Resolution over 3 wk | NIL | Resolution |
| 9 | 54/M | CAH-B hepatoma | 36 | Fever, generalized LN | Developed systemic PTLD, fatal over 2 wk | Not applicable | Death |
| 10 | 42/F | CAH cirrhosis | 623 | Fever, pharyngitis, generalized LN, Sp, ascites, dysarthria, dysmetria, pneumonitis | Resolved over 11 wk | NIL | Resolution |
CC, cryptogenic cirrhosis; PSC, primary sclerosing cholangitis; CAH, chronic active hepatitis; α-1ATD, α-1antitrypsin deficiency; LN, lymphadenitis; Sp, splenomegaly; PTLD, posttransplant lymphoproliferative disorders.
Viral syndromes were clinically recognized 10–623 days following transplantation (mean 219, median 39). Fever (n = 8), pharyngitis (n = 4), and lymphadenopathy (n = 9) were the most common manifestations. Lymph node enlargement was typically most noticeable in the cervical and axillary regions (n = 4) but was also frequently generalized (n = 5). The spleen was palpable in four cases. Hepatomegaly was not described but icterus was noted in three cases. Atypical symptoms in the form of jaw pain, arthralgias, joint space effusions, diarrhea, skin rash, encephalitis, pneumonitis, mediastinal lymph node enlargement, ascites, and ileal perforation were observed in six instances either at initial presentation or on subsequent follow-up. Multiple other infections and rejection episodes were documented in these patients before and after the diagnosis of EBV infection (Table 2).
TABLE 2.
Occurrence of infectious and allograft rejection episodes in relation to onset of EBV syndrome
| Case no. | Timing of episodes of allograft rejectiona |
Infectious episodes |
||
|---|---|---|---|---|
| Before diagnosis of EBV (days) | After diagnosis of EBV (days) | Before diagnosis of EBV | After diagnosis of EBV | |
| 1 | None | +17 | Blood cultured staphylococci and CMV | Candida pharyngitis, CMV pneumonitis, staphylococcal septicemia |
| 2 | −35, −13 | +18 | None | Escherichia coli (abdominal wound) |
| 3 | None | +5, +38, +111 | Herpes simplex (lip) | None |
| 4 | −10 | +133, +169, +307 | CMV viremia | Urine-cultured CMV, E coli, and S fecalis |
| 5 | None | None | CMV hepatitis, pneumocytis infection | S viridans pharyngitis |
| 6 | −20 | + 11, +80 | Herpes simplex, Streptococcus viridans pharyngitis | Cholangitis, bile-cultured coagulase negative staphylococci, S fecalis |
| 7 | −33 | +8 | CMV uveitis | Bile-cultured citrobacter, S fecium, candida |
| 8 | −2 | +13 | Urine-cultured Candida stellatoidea | Groin wound infection by S fecalis, coagulase-negative staphylococci |
| 9 | −3, −37 | None | None | None |
| 10 | None | +25 | None | Urine cultured E coli, enterococci, candida. Bronchoalveolar lavage–cultured candida |
−, number of days rejection preceded the onset of a clinical syndrome; +, number of days that rejection occurred after the diagnosis of viral syndrome.
Liver Function Tests and Hematologic Profile
Liver function tests (Table 3) showed elevations of bilirubin up to 11.7 mg%, the conjugated form constituting 17–86% of the total. Serum glutamic-oxaloacetic transaminase (SGOT) and serum glutamic-pyruvic transaminase (SGPT) levels were raised moderately and usually less than 1,000 IU/L, except in case 9, where massive hepatic necrosis was documented at autopsy. The alkaline phosphatase and gamma glutamyl transferase tended to be somewhat higher and peak values of 1,198 IU/L and 1,438 IU/L respectively, were recorded. Hematological parameters (Table 4) typically showed a low hemoglobin, thrombocytopenia, and mildly deranged prothrombin time and partial thromboplastin time. Total leukocyte counts showed a varied picture with leukopenia seen in some patients but leukocytosis in others. Differential counts showed lymphocytic preponderance (38–84%), and circulating atypical lymphocytes (6–55%) were always demonstrable.
TABLE 3.
Peak values of abnormalities in liver function tests concurrently with the clinical viral syndrome
| Case no. | Bilirubin (mg/dl) |
SGOT (IU/L) | SGPT (IU/L) | Alkaline phosphatase | γ Glutamyl transferase | ||
|---|---|---|---|---|---|---|---|
| Total | Direct | % Direct | |||||
| 1 | 2.0 | 0.4 | 16.6 | 128 | 273 | 789 | 243 |
| 2 | 5.6 | 4.7 | 85.9 | 66 | 80 | 1,056 | 572 |
| 3 | 7.6 | 5.3 | 69.7 | 862 | 518 | 1,198 | 1,032 |
| 4 | 5.0 | 3.2 | 64.0 | 45 | 57 | 255 | 356 |
| 5 | 0.7 | NA | NA | 73 | 17 | 109 | 47 |
| 6 | 1.7 | 1.2 | 70.6 | 102 | 99 | 741 | 341 |
| 7 | 10.2 | 8.8 | 86.3 | 107 | 160 | 988 | 1,438 |
| 8 | 2.6 | 1.7 | 65.3 | 124 | 335 | 475 | 446 |
| 9 | 11.7 | 8.8 | 75.2 | 3,233 | 1,043 | 701 | 201 |
| 10 | 0.7 | NA | NA | 306 | 281 | 441 | 278 |
NA, not available.
TABLE 4.
Hematological parameters in patients with EBV syndromes on cyclosporin-prednisone immunosuppression following liver transplantation
| Case no. | Total leukocytic count/1,000 mm3 |
Lymphocytes (%) (% atypical forms) | Hemoglobin trougha (g/dl) | Hemoglobin peaka (g/dl) | Lowest platelet count/1,000 mm3 | Peak PTa (control 11.8 seconds) | Peak PTTa (Control 25.0 seconds) | |
|---|---|---|---|---|---|---|---|---|
| Trougha | Peaka | |||||||
| 1 | 3.1 | 6.9 | 84 (20) | 9.7 | 10.4 | 45 | 13.5 | 31.4 |
| 2 | 2.4 | 5.0 | 57 (7) | 8.1 | 11.9 | 113 | 14.3 | 39.5 |
| 3 | 7.5 | 16.5 | 39 (19) | 8.9 | 12.0 | 110 | 14.5 | 31.9 |
| 4 | 3.1 | 6.7 | 43 (29) | 7.8 | 10.2 | 73 | 16.1 | 33.8 |
| 5 | 2.3 | 6.4 | 84 (42) | 5.7 | 11.1 | 11 | 18.3 | 33.3 |
| 6 | 5.8 | 17.4 | 68 (55) | 9.3 | 10.9 | 99 | 13.5 | 34.1 |
| 7 | 3.6 | 30.1 | 62 (12) | 6.7 | 12.7 | 378 | 13.7 | 34.4 |
| 8 | 3.0 | 9.3 | 38 (11) | 7.5 | 10.2 | 43 | 14.1 | 31.8 |
| 9 | 3.9 | 11.0 | 44 (16) | 9.3 | 12.5 | 41 | 22.1 | 71.4 |
| 10 | 1.2 | 3.8 | 65 (6) | 8.8 | 10.8 | 92 | 13.3 | 32.7 |
PT, prothrombin time; PTT, partial thromboplastin time.
Peak and trough refers to the high and low values obtained during the course of the viral syndrome.
Serology
Serologic studies demonstrated recent EBV infection in all patients (Table 5). Comparison of pre- and posttransplantation anti-VCA titers available in nine cases allowed five cases to be characterized as primary and four as reactivation infection. Development of anti–early antigen (EA) antibody was noted in six cases, and anti–Epstein-Barr nuclear antigen (EBNA), antibody in two cases.
TABLE 5.
Serological profile of EBV infections occurring in immunosuppressed liver transplant recipients
| Case no. | Pretransplant serology | Posttransplant serology |
Type of infection | ||
|---|---|---|---|---|---|
| Anti-VCA | Anti-EA | Anti-EBNA | |||
| 1 | −VE | 1:10 | 1:10 | −VE | Primary |
| 2 | −VE | 1:20 | −VE | −VE | Primary |
| 3 | Anti-VCA IgG 1:40, anti-EA 1:10 | 1:640 | 1:20 | 1:20 | Reactivation |
| 4 | −VE | 1:80 | 1:20 | NA | Primary |
| 5 | NA | 1:640 | −VE | −VE | Indeterminate |
| 6 | ANTI VCA 1:320, ANTI-EA 1:5 | 1:1280 | 1:20 | NA | Reactivation |
| 7 | −VE | 1:640 | 1:80 | −VE | Primary |
| 8 | Anti-VCA IgG 1:50, anti-VCA IgM-VE, anti-EBNA 1:8 | 1:50 IgG | 1:20 | 1:2 | Reactivation |
| 1:10 IgM | NA | ||||
| 9 | Anti-VCA 1:20, anti-EA 1:10 | 1:80 | 1:20 | NA | Reactivation |
| 10 | −VE | 1:320 | 1:80 | NA | Primary |
VCA, viral capsid antigen; EA, early antigen; EBNA, Epstein-Barr nuclear antigen; −VE, negative antibody profile; NA, not available.
Histopathology
Histopathologic examination showed prominent portal and periportal mononuclear infiltrates. In six cases small and large atypical lymphocytes with differentiation to immunoblasts and plasma cells was present (Fig. 1). Five cases demonstrated spillover of the infiltrate into the lobule (Fig. 2). The lobule typically showed features of a “reactive” or lowgrade hepatitis in the form of hepatocellular swelling, focal acidophilic necrosis, and mild lobular disarray. Regenerative activity was seen in the form of double-layered liver cords, pseudorosette formation, cytoplasmic basophilia, nuclear pleomorphism, and mitotic figures. The sinusoids contained linearly aligned (Fig. 3) and focally aggregated (Fig. 4) mononuclear cells, similar to those seen in the portal tracts. In one case the infiltrate was granulomatoid (Fig. 5). Bile duct damage affecting 10–70% of the total number of ducts present was seen in seven cases, but the severity and prevalence of damage appeared not to be in proportion to that expected for rejection. Subendothelial localization of lymphocytes was seen in the portal or central veins in six cases and mild focal canalicular cholestasis occurred in four cases. The changes observed were not always florid or uniformly distributed throughout the specimens. Three cases (cases 1, 4, and 5) merely showed a nonspecific reactive type hepatitis. The liver sinusoids showed surprisingly mild lymphocytosis despite a concurrent systemic posttransplant lymphoproliferative disease. It was also noticed that the infiltration in zone 1 varied in severity in the same specimen, and portal triads with only sparse mononuclear cells (Fig. 6) could be recognized in biopsies with dense portal lymphocytic and immunoblastic infiltrates elsewhere. Sequential biopsies available in cases 2, 3, 6, 7, and 10 showed histological resolution of liver cell injury concomitant with clinical improvement in the viral syndrome after a reduction in immunosuppression. Complete resolution of hepatitis could be documented in cases 3, 6, and 10 at 69–106 days after the initial biopsy. In cases 1 and 4, an initial clinical improvement was followed by the development of a systemic posttransplant lymphoproliferative disorder. A sufficient number of biopsies were not available in cases 5, 8, and 9 for any meaningful temporal clinicopathologic correlation. Another general observation made on serial biopsies was an increase in bile duct damage, venous “endothelialitis” and a mixed inflammatory infiltrate in the portal tracts (Fig. 7). This presumably reflected enhanced cellular rejection secondary to the reduced immunosuppression instituted in these patients after the diagnosis of EBV infection. Attempts to detect the EBV genome within the liver tissue using a commercially available in situ hybridization kit was unsuccessful (EBV Pathogene kit, ENZO, New York).
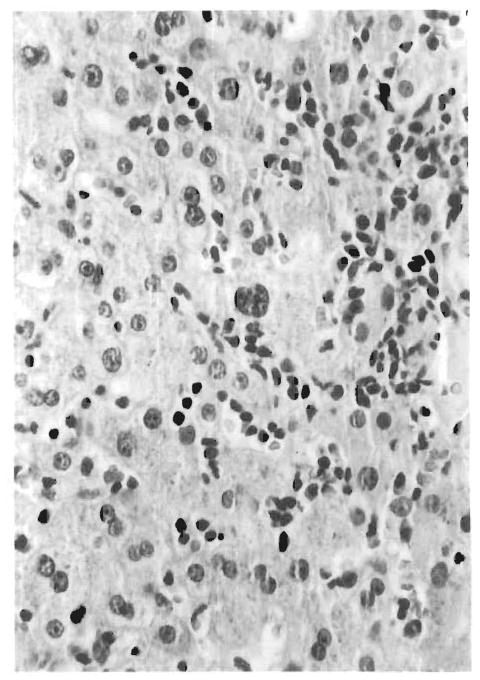
FIG. 1.
Portal tract infiltrate comprising small and large atypical lymphocytes with differentiation to immunoblasts and mature plasma cells. The normal portal tract landmarks are obscured.
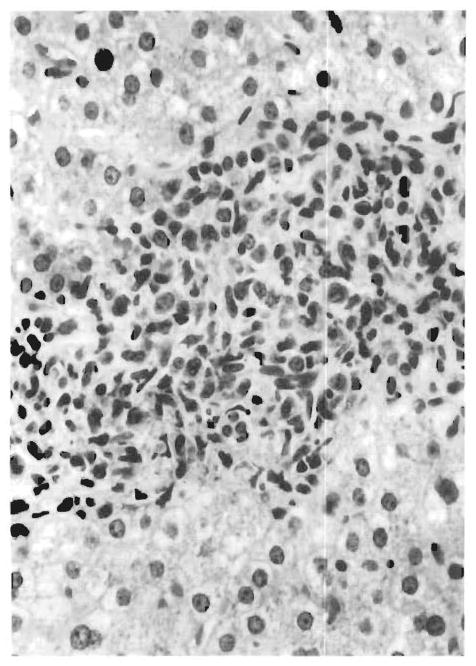
FIG. 2.
Atypical mononuclear cells in the portal tract (see also inset) are seen to spill over into the hepatic lobule associated with hepatocyte necrosis.
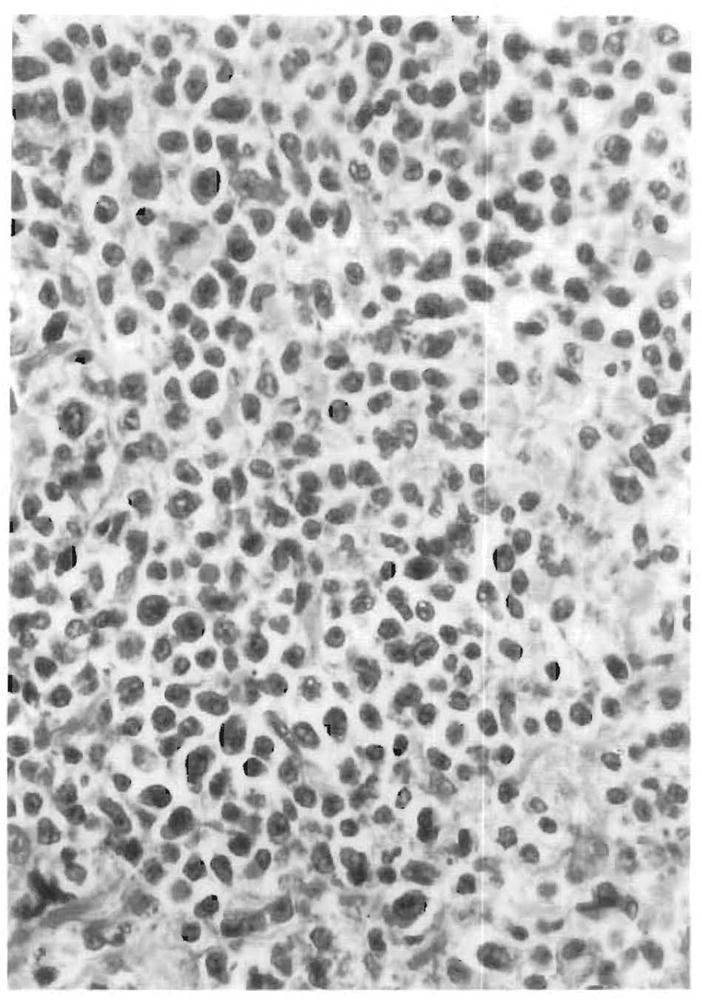
FIG. 3.
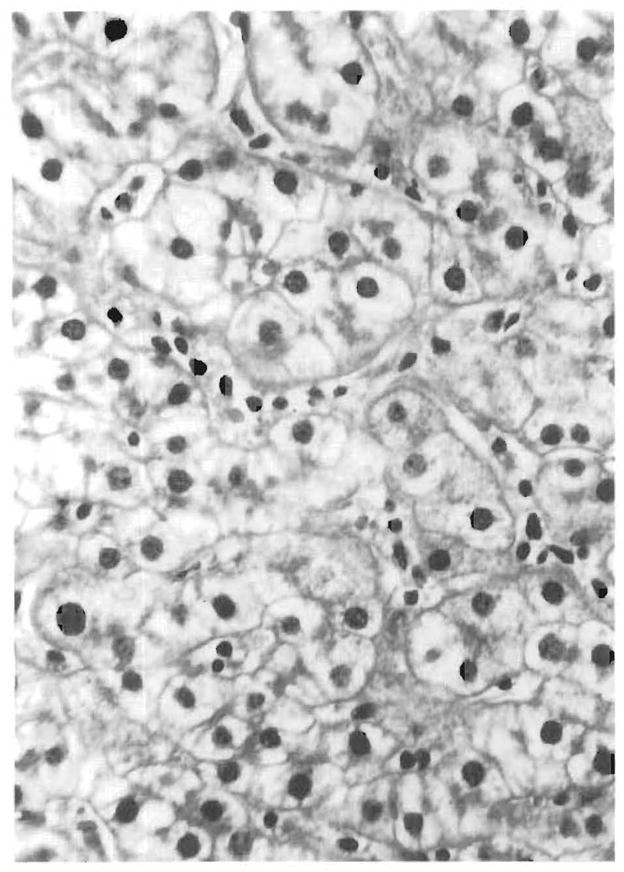
The sinusoids show mononuclear cells arranged in a linear beadlike fashion. The surrounding hepatocytes show mild lobular disarray of swollen liver cells forming pseudorosettes.
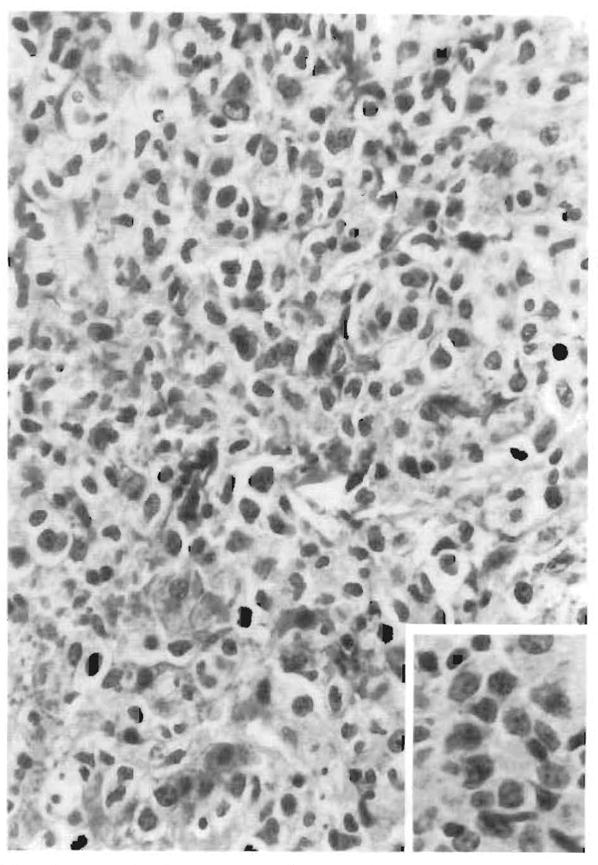
FIG. 4.
The sinusoidal infiltrates in this case are arranged in focal aggregates. Hepatocyte regenerative activity is seen in the form of binucleate cells and nuclear pleomorphism.
FIG. 5.
Ill-defined granulomas located sinusoidally were noted in this biopsy. Gram, Grocott, and acid fast bacilli (AFB) stains were negative.
FIG. 6.
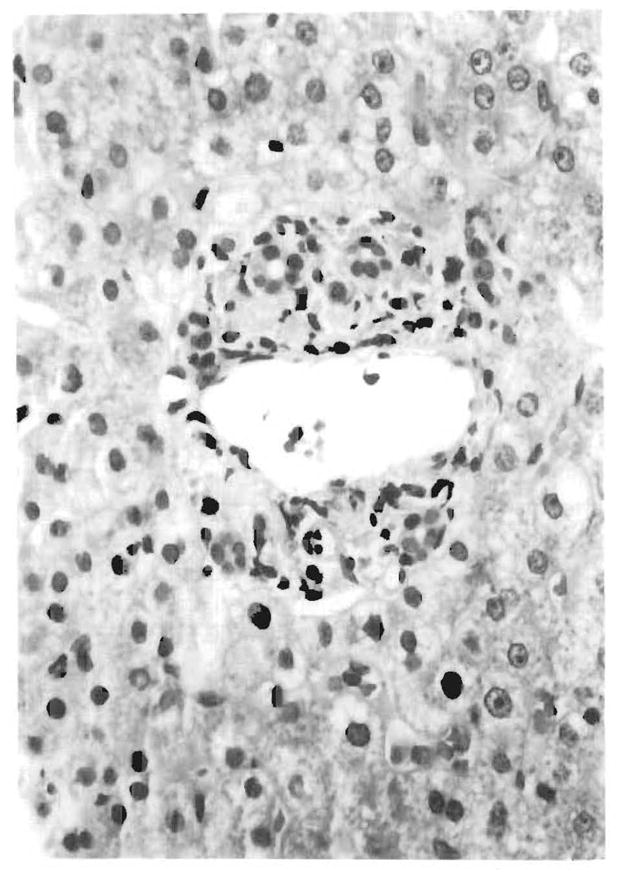
Illustration of a case with portal tracts containing a very sparse inflammatory infiltrate. One portal tract in the same biopsy, however, contained dense mononuclear infiltrates similar to those shown in Fig. 1. Such focal lesions are liable to be missed in small specimens.
FIG. 7.
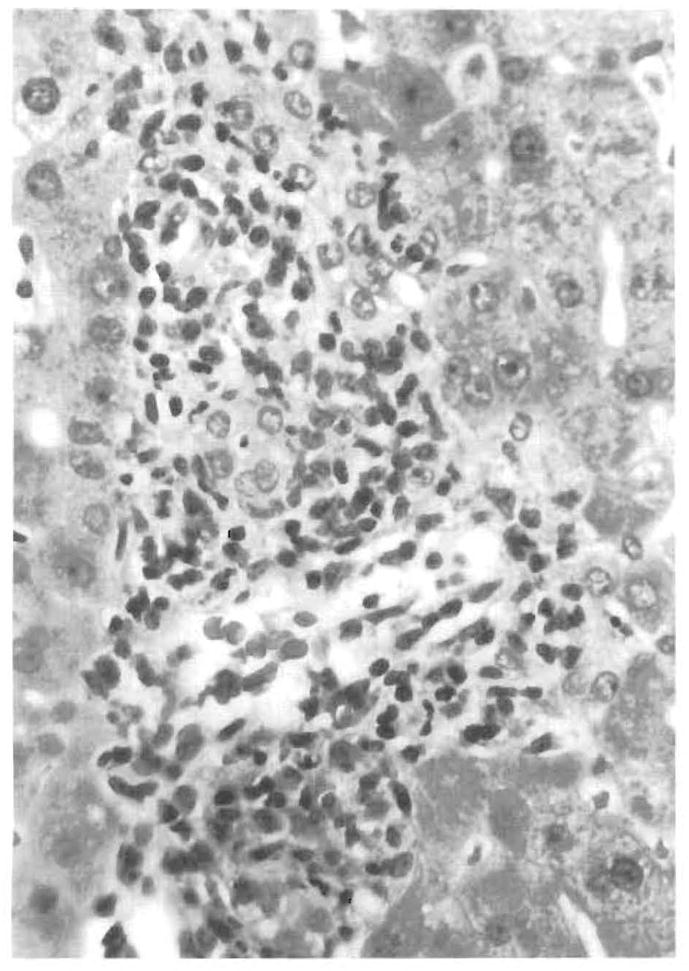
Bile duct damage and subendothelial inflammation of a portal vein developed in this case after the suggested diagnosis of EBV hepatitis on a preceding biopsy. This led the clinical team to reduce the level of immunosuppression in this patient.
Treatment and Clinical Course
Patient 4 received no specific treatment. The diagnosis was missed because of the atypical presentation and a serologic clue to EBV infection was obtained only after the patient had been discharged. The patient presented 9 months later with recurrent EBV infection complicated by diffuse intraabdominal PTLD and died (Figs. 8–11). The remaining cases were treated by a reduction of immunosuppression and a course of acyclovir therapy. Five cases had a benign self-limited course with a return of clinical and hematological parameters to normal over a period of 2–8 weeks. Recurrent viral syndromes developed in 4 cases (cases 1, 4, 6, and 7). Interestingly three of the recurrences (cases 1, 6, and 7) also responded to reduced immunosuppression.
FIG. 8.
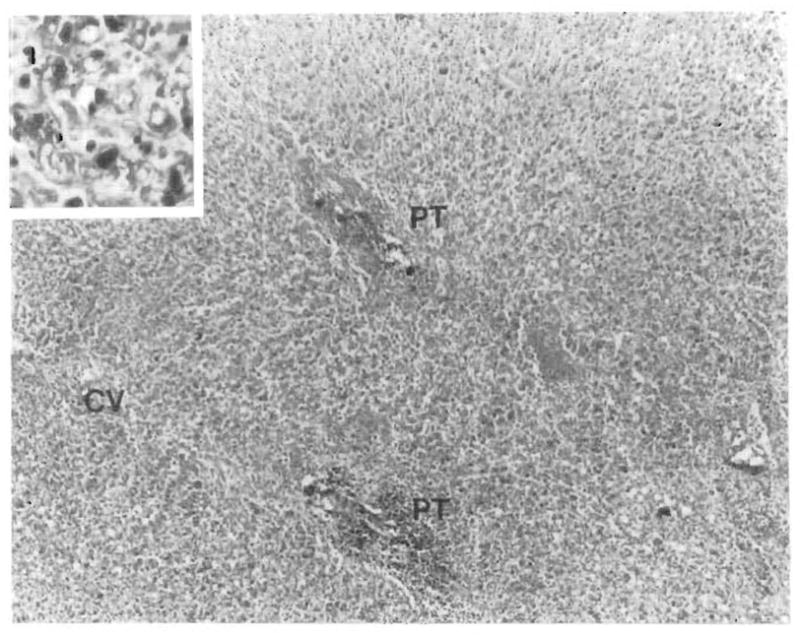
Panlobular necrosis observed in a fatal case with EBV-associated hepatitis. Systemic PTLD involving the lungs, gastro-intestinal tract, lymph nodes, spleen, bone marrow, adrenals, pancreas, and testes was documented at autopsy. Inset shows necrotic liver tissue at a higher magnification (PT, portal tract; CV, central vein).
FIG. 11.
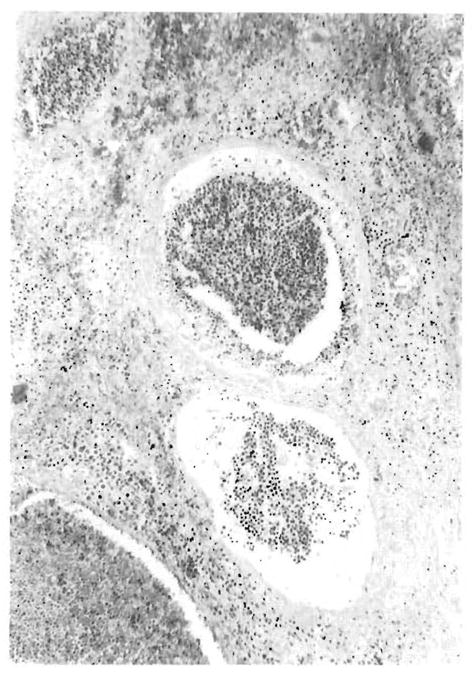
Portal veins from the deep hilum of case 4 studied at necropsy. The cells seen intraluminally had the same atypical and blastic features as those seen in the portal tracts in the antemortem needle biopsy. The systemic dissemination of these infiltrates by the vascular route is perhaps one of the pathways leading to the development of PTLD lesions in multiple organ systems.
DISCUSSION
The predominant clinical manifestations of EBV infection seen in these transplant recipient patients, namely fever, lymphadenitis, pharyngitis, splenomegaly, and icterus, do not differ from those of classic infectious mononucleosis (IM). However, atypical signs and symptoms, prolonged viral syndromes, and recurrent attacks were noted in several patients. This presumably represents modification of the natural course of EBV infection by an underlying state of immunosuppression as has been observed for salmonellosis (13), cytomegalovirus (18), herpes simplex, and varicella zoster (15) infections.
Liver function tests in uncomplicated IM have been studied by several authors. Serum bilirubin elevations have been noted in up to 21% of cases, but values exceeding 6 mg% are unusual. Serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), or gamma glutamyl transferase (GGT) in excess of 500–600 IU/L are uncommon (4), and alkaline phosphatase activity generally does not exceed 400 IU/L (5, 14). Our data indicate that EBV hepatitis in immunosuppressed subjects may show biochemical hepatocellular injury of greater magnitude than is usually observed in immunocompetent hosts.
The hematological profile commonly showed atypical lymphocytosis, thrombocytopenia, granulocytopenia, and anemia. Thrombocytopenia and granulocytopenia are common in IM (1, 7). Significant bleeding and fatal agranulocytosis have been described occasionally (10, 17). The underlying mechanism may be a combination of hypersplenism antiplatelet antibodies and bone marrow suppression. Anemia, however, is not a characteristic feature of classic IM in contrast to the universal occurrence of significant anemia in the present series. Concurrent thrombocytopenia and neutropenia suggest bone marrow depression as the principal underlying factor. Hemophagocytosis as a cause of progressive pancytopenia in EBV infections has also been documented (8, 12, 19).
The available serologic titers to EBV antigens conclusively established that five of 10 symptomatic cases (50%) represent individuals with no prior exposure to EBV. In general, because 90% of patients referred to our institution for transplantation are EBV seropositive (and the majority of these have an uneventful postoperative course), it implies that exposure to EBV for the first time under the setting of posttransplant immunosuppression carries a high risk of developing atypical systemic EBV syndromes.
Our experience with evaluation of needle biopsies of liver performed on these patients indicates that histopathologic changes associated with EBV syndrome are sufficiently distinctive in many cases to at least suggest this diagnosis to the treating physicians, who could then obtain serologic confirmation and proceed to reduce the level of immunosuppression. Infectious mononucleosis can be histopathologically confused with non-A, non-B hepatitis, and serologic correlations are advisable. The most distinctive cases of EBV involvement of the allograft are characterized by a mononuclear portal infiltrate comprising small lymphocytes, plasma cells, large cleaved/noncleaved cells, and immunoblasts. Granulomas may also be found (6). Although a mononuclear portal infiltrate occurring in a posttransplant liver biopsy normally points to the onset of allograft rejection (16), a monomorphous portal and sinusoidal infiltrate lacking polymorphs or eosinophils, containing a significant number of atypical large noncleaved cells, and associated with hepatitic changes should arouse the suspicion of a viral syndrome. These changes overlap with, but are distinct from, those of cellular rejection (3). EBV-induced bile duct damage has been demonstrated in fatal cases of X-linked lymphoproliferative syndrome (2, 8). Arteritis and venulitis have also been documented in EBV pneumonitis in immunosuppressed heart-lung transplant recipients (11). However, when a mixed polymorphonuclear eosinophilic and mononuclear inflammatory infiltrate occurs, duct damage and vascular lesions are readily apparent, and allograft rejection should be considered the primary process. In difficult cases access to information such as the occurrence of febrile syndromes, serology, and blood cyclosporine levels can aid in resolving the differential diagnosis between acute cellular rejection and EBV hepatitis. Of course these two processes may coexist or occur sequentially. In fact, a reduction in immunosuppression following a diagnosis of EBV hepatitis frequently triggers allograft rejection to a variable degree.
In summary, EBV-associated hepatitis in immunosuppressed patients encompasses a very wide clinicopathological spectrum. Examination of some biopsies reveals only a mild nonspecific hepatitis, whereas other specimens show dense portal and sinusoidal infiltrates with large atypical mononuclear cells. Massive panlobular necrosis can also occur, as has been reported in sporadic fatal infectious mononucleosis (2). The ultimate clinical outcome (Fig. 12) in most cases is a resolution in response to reduced immunosuppression, but there is a tendency to the occurrence of recurrent viral syndromes and occasional progression to posttransplant lymphoproiiferative disorders.
FIG. 12.
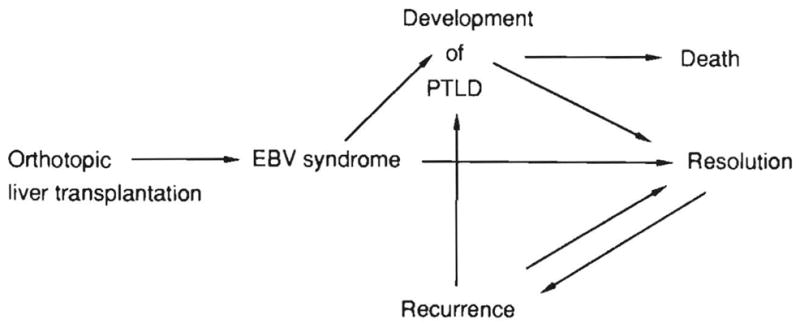
Illustration of the possible outcomes of EBV infection in immunosuppressed liver transplant recipients.
FIG. 9.
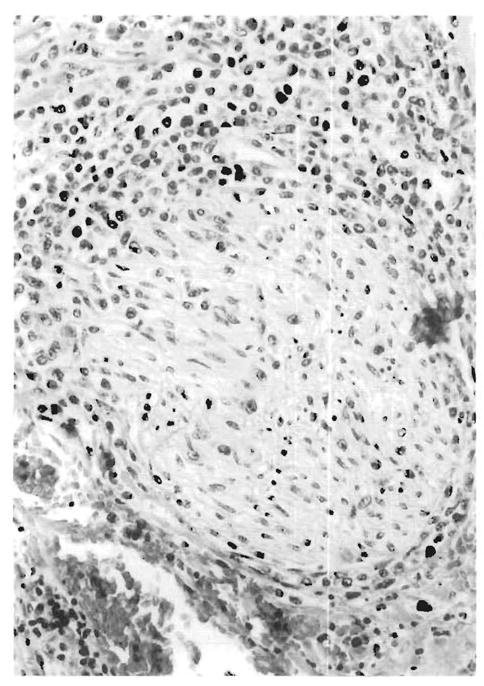
Deep hilar section from the case illustrated in Fig. 8. The mononuclear infiltrate is seen involving a nerve bundle (autopsy material, case 4).
FIG. 10.
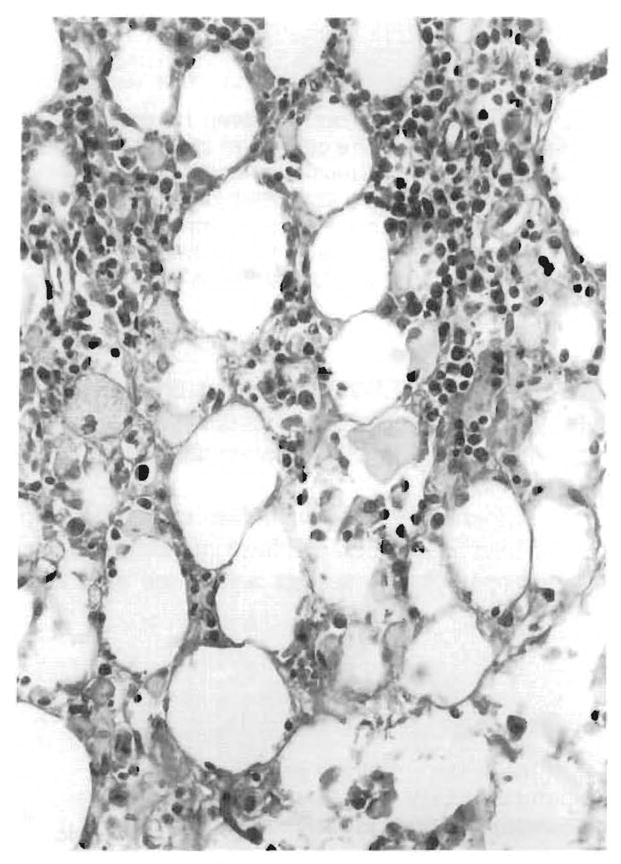
The “malignant potential” of the mononuclear infiltrate in fatal EBV-associated hepatitis is illustrated by its extension beyond the confines of the liver into the hilar fat (autopsy material, case 4).
FIG. 13.
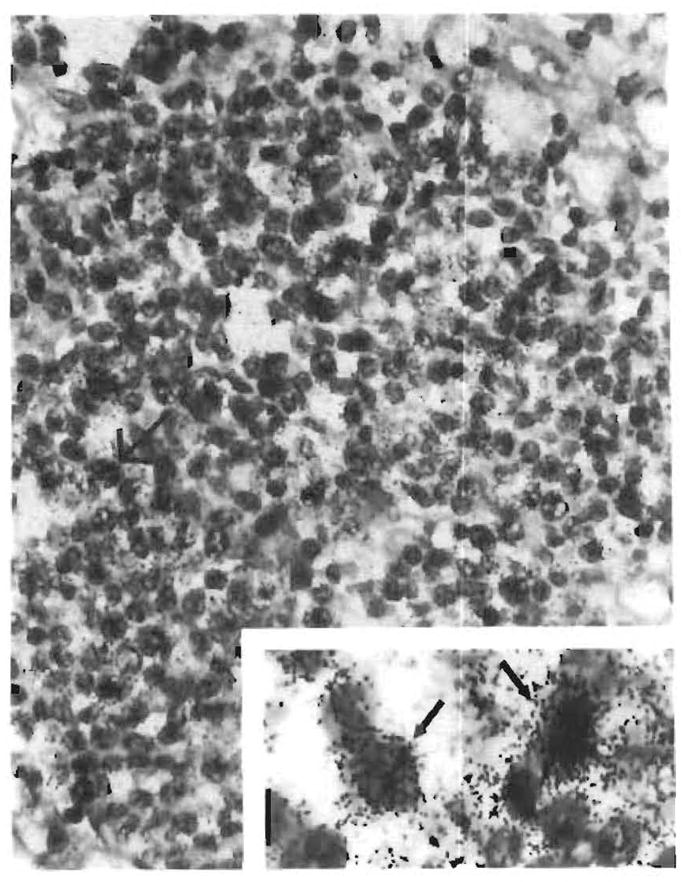
Autopsy liver tissue from a case with posttransplant lymphoproliferative disease: portal tract infiltrates hybridized strongly (arrows, inset) with a 35S-dCTP labelled probe consisting of the Bam H1-W fragment of EBV DNA.
Acknowledgments
We thank Dr. Ron Jaffe for critically reviewing the manuscript and Mary Ann Mient for typing the manuscript.
Footnotes
Note added in proof. Attempts to demonstrate EBV genomes within the lesions using the EBV pathogene kit were unsuccessful, and exhausted the entire tissue embedded in the paraffin blocks of these cases. In situ hybridization studies performed on an additional case by Dr. Lawrence Weiss, City of Hope National Medical Center, Duarte, CA, demonstrated EBV nuclei acids within the lymphoid infiltrates in portal and sinusoidal locations (Fig. 13).
References
- 1.Cantow EF, Kostinas JE. Studies on infectious mononucleosis IV. Changes in granulocyte series. Am J Clin Pathol. 1966;46:43–7. doi: 10.1093/ajcp/46.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Chang MY, Campbell WG. Fatal infectious mononucleosis associated with liver necrosis and herpes like particles. Arch Pathol. 1975;99:185–91. [PubMed] [Google Scholar]
- 3.Demetris AJ, Jaffe R, Starzl TE. A review of adult and pediatric post-transplant liver pathology. Pathology Annu. 1987;2:347–86. [PubMed] [Google Scholar]
- 4.Gelb D, West M, Zimmerman HJ. Serum enzymes in disease IX. Analysis of factors responsible for elevated values in infectious mononucleosis. Am J Med. 1962;33:249–61. doi: 10.1016/0002-9343(62)90023-2. [DOI] [PubMed] [Google Scholar]
- 5.Horwitz CA, Burke MD, Grimes P, Tombers J. Hepatic function studies in infectious mononucleosis. Clin Chem. 1980;26:243–61. [PubMed] [Google Scholar]
- 6.Ishak KG. Granulomas of liver. In: Joachim HL, editor. Pathology of granulomas. New York: Raven; 1983. pp. 308–9. [Google Scholar]
- 7.Jandl JH. Blood: textbook of haematology. Boston: Little Brown; 1987. Infectious mononucleosis; pp. 537–44. [Google Scholar]
- 8.Markin RS, Linder J, Zuerlein K, Mroczek E, Grierson HL, Brichacek B, Purtilo DT. Hepatitis in fatal infectious mononucleosis. Gastroenterology. 1987;93:1210–7. doi: 10.1016/0016-5085(87)90246-0. [DOI] [PubMed] [Google Scholar]
- 9.Nalesnik MA, Jaffe R, Starzl TE, et al. The pathology of posttransplant lymphoproliferative disorders occurring in the setting of cyclosporine A-prednisone immunosuppression. Am J Pathol. 1988;133:173–92. [PMC free article] [PubMed] [Google Scholar]
- 10.Neel EU. Infectious mononucleosis: death due to agranulo-cytosis and pneumonia. JAMA. 1976;236:1493–4. doi: 10.1001/jama.236.13.1493. [DOI] [PubMed] [Google Scholar]
- 11.Randhawa PS, Yousem SA, Paradis IL, Dauber JA, Griffith BP, Locker J. The clinical spectrum, pathology and clonal analysis of Epstein-Barr virus associated lymphoproliferative disorders in heart-lung transplant recipients. Am J Clin Pathol. 1989;92:47–55. doi: 10.1093/ajcp/92.2.177. [DOI] [PubMed] [Google Scholar]
- 12.Risdall RK, McKenna RW, Nesbit ME, Krivit W, Balfour HH, Simmons RL, Brunning RD. Virus associated hemophagocytic syndrome. A benign histiocytic proliferation distinct from malignant histiocytosis. Cancer. 1979;44:993–1002. doi: 10.1002/1097-0142(197909)44:3<993::aid-cncr2820440329>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Samra Y, Shaked Y, Maier MK. Non typhoid salmonellosis in renal transplant recipients: report of five cases and review of the literature. Rev Infect Dis. 1986;8:431–5. doi: 10.1093/clinids/8.3.431. [DOI] [PubMed] [Google Scholar]
- 14.Shuster FS, Ognibene AJ. Disassociation of serum bilirubin and alkaline phosphatase in infectious mononucleosis. JAMA. 1969;209:267–8. [PubMed] [Google Scholar]
- 15.Singh N, Dummer JS, Kusne S, Breinig MK, Armstrong JA, Makowka L, Starzl TE, Ho M. Infections with cytomegalo-virus and other herpes viruses in 121 liver transplant recipients: transmission by donated organ and the effect of OKT3 antibodies. J Infect Dis. 1988;158:124–31. doi: 10.1093/infdis/158.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snover DC, Freese DK, Sharp HL, Blomer JR, Najarian JS, Ascher NL. Liver allograft rejection. An analysis of the use of biopsy in determining outcome of rejection. Am J Surg Pathol. 1987;11:1–10. doi: 10.1097/00000478-198701000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Thangarajan D. Hemorrhagic complication: severe thrombocytopenia with subarachnoid hemorrhage in infectious mononucleosis. Am J Med Sci. 1976;272:221–2. [Google Scholar]
- 18.Whelchel JP, Pass RF, Diethelm AG, Whitley RJ, Alford CA. Effect of primary and recurrent CMV infection upon graft and patient survival after renal transplantation. Transplantation. 1979;28:443–6. [PubMed] [Google Scholar]
- 19.Wilson ER, Malluh A, Stagno S. Fatal Epstein-Barr virus–associated hemophagocytic syndrome. J Pediatrics. 1981;98:260–2. doi: 10.1016/s0022-3476(81)80654-3. [DOI] [PubMed] [Google Scholar]