Abstract
Spironolactone and potassium canrenoate are diuretics that are used widely for management of cirrhotic ascites. The administration of spironolactone frequently leads to feminization, which has been noted less frequently with the use of potassium canrenoate, a salt of the active metabolite of spironolactone. The use of these two drugs has been associated with decreases in serum testosterone levels and spironolactone with a reduction in androgen receptor (AR) activity. This decrease in AR has been cited as the cause of the anti androgen effect of these drugs. We therefore assessed the effect of both drugs on levels of androgen and estrogen receptors (ER) in the liver, a tissue that is responsive to sex steroids. Three groups of male rats (n = 12 rats each) were studied. Group 1 (control) received vehicle only; group 2 received spironolactone (5 mg/day); group 3 received potassium canrenoate (5 mg/day). After 21 days of treatment, the animals of all groups were killed and liver tissue was assayed for nuclear and cytosolic AR and ER, and for male specific estrogen binder (MEB), an androgen-responsive protein. Both drugs drastically decreased the nuclear AR content, as compared with the control group, but only spironolactone decreased cytosolic AR. When the total hepatic content of AR is considered, a highly significant decrease is observed only in rats treated with spironolactone. This reduction in hepatic AR content suggested loss of androgen responsiveness of liver. We confirmed this by assessing levels of MEB, and found that livers from group 2 animals had no detectable MEB activity, whereas livers from both group 1 and 3 had normal MEB activity. No changes were observed in nuclear ER and cytosolic ER of group 3 as compared with group 1. Nuclear estrogen receptor decreased and cytosolic ER increased in group 2, but with no change in total ER content. These results indicate that (a) only spironolactone appears to act as an antiandrogen in liver, resulting in a decrease in both AR and male specific estrogen binder content, and (b) neither drug results in elevated hepatic ER content, although spironolactone-treated animals show an altered subcellular localization.
It has been demonstrated by several lines of evidence that liver is an organ responsive to sex hormones. Estrogen receptors (ER) and androgen receptors (AR) have been characterized in the cytosol and nuclei of liver of several animal species and in humans (1–13). The level of these receptors in the liver is dependent to a great extent on the plasma level of the specific hormone, but growth hormone appears to be critical in the regulation of sexual dimorphism of liver function as well. The resultant masculine or feminine pattern of steroid receptors and steroid metabolizing enzymes has an important role in maintaining the sex hormone homeostasis particular to that sex (11,12,14).
Spironolactone and the potassium salt of its active metabolite potassium canrenoate are two drugs used frequently in chronic hepatic disease and to varying extents have been found to lead to feminization, impotence, and decrease of libido in male patients (15–19). These side effects appear to be less frequent with use of potassium canrenoate than with spironolactone (20,21). Different explanations have been offered for the hormonal changes induced by these drugs, and spironolactone in particular. These include reduction in testosterone synthesis (22,23), increase in testosterone clearance (23), reduction of 5α-reductase activity (24), and interference with AR activity (25). To date, no report has defined the effects of these drugs on the sex hormone receptor state of the liver. Considering the critical role of the liver in sex hormone metabolism, a study of this aspect could add new information concerning the mechanisms of feminization by these two drugs. We report here the effect of spironolactone and potassium canrenoate treatment on the sex hormone receptor and androgen responsive protein status of the liver and the effects of these treatments on serum estrogen and testosterone levels.
Materials and Methods
Animals
Three groups of adult male Sprague–Dawley rats (240 g) were studied. Animals in the control group (group 1) were injected intraperitoneally with 0.5 ml of a solution of physiologic saline with Tween 80 (0.1% vol/vol). The rats in the other groups received 5 mg of spironolactone (group 2) or 5 mg of potassium canrenoate (group 3) dissolved in the same vehicle. All animals were injected daily for 21 days, and killed by decapitation after that time.
Materials
Spironolactone and potassium canrenoate were from SPA, Milano, Italy. Radioactive (2, 4, 6, 7. 16, 17)-[3H]estradiol ([3H]E2), 131 Ci/mmol; 17α-methyl-[3H]methyltrienolone ([3H]R1881), 79 Ci/mmol; and non-radioactive R1881 were obtained from New England Nuclear. Boston, Mass. The radiolabeled steroids used in these studies were assayed periodically for purity by thin-layer chromatography on silica gel G in ethyl acetate/hexane/ethanol (85:10:5), and were used only if purity was >95%. The sources of other materials have been described elsewhere (26,27).
Androgen Receptor Studies
Cytosolic and nuclear fractions were prepared as noted in Reference 28. Cytosolic androgen binding was quantitated using the multiple-point [3H]R1881 (0.2–5.0 nM [3H]R1881) binding assay described in detail previously (28,29). Characterization of the [3H]R1881 binding activity as a cytosolic AR has been reported elsewhere (10,12,28). No metabolism of [3H]R1881 could be detected under our incubation conditions. Because of the large number of animals used in these experiments, the one-point assay described previously (26,28,29) was used to quantitate nuclear AR rather than multiple-point analysis. The validity of this assay has been described previously; values obtained from the one-point assay agree well with those from multiple-point assay (29). Evidence that [3H]R1881 binding represents nuclear AR has been described elsewhere (10,28).
Assay of Androgen-Responsive Male Estrogen Binding Protein
Assays for the determination of cytosolic content of a male specific estrogen binder (MEB) has been described previously (27). Briefly, cytosol is preincubated for 1 hat 0°C with 500 nM diethylstilbestrol to block ERs. The cytosol is then incubated with 5 nM [3H]E2, [3H]estradiol in the absence and presence of several concentrations (5 nM–5 μM) of unlabeled estradiol for 2 h at 0°C. Bound and free steroid are separated by centrifuge-assisted BioGel P-6 chromatography (27). This assay is quantitative for MEB and is linear over a broad range of protein concentrations. A typical saturation curve analysis is illustrated in Figure 1 of Reference 27.
Estrogen Binding Studies
The protamine sulfate precipitate method used to assay cytosolic ER and the exchange assay used to measure nuclear ER were identical to those described previously (26,28,29).
Other Methods
Protein concentrations were determined by the method of Bradford (30). Deoxyribonucleic acid concentrations of homogenates and nuclear preparations were determined by the method of Burton (31). Corrections for receptor content expressed on the basis of wet weight of liver, and assessment of purity of subcellular fractions have been described previously (29). Serum testosterone, estradiol, and other hormones were determined by specific radioimmunoassays as described previously (32). Equilibrium dissociation constants and the concentration of cytosolic binding sites for receptor and MEB activity were calculated by the method of Scatchard (33). Unweighted linear regression analysis of Scatchard plots was performed on a TI55-III calculator (Texas-III Instruments, Inc., Dallas, Tex). Statistical analyses were performed using the Student’s t-test program available on the Hewlett-Packard 9815S (Hewlett-Packard, Co., Palo Alto, Calif). All results are expressed as mean ± SD.
Results
Table 1 shows the levels of several gonadal and pituitary hormones in the blood of control rats and those treated with spironolactone and potassium canrenoate. In those animals treated with spironolactone, there is a significant decrease in testosterone levels; all other hormone levels in this group are comparable to those of the control animals. In those treated with potassium canrenoate, no change was observed in either testosterone or estradiol levels, although significant reductions in progesterone and dehydroepiandrosterone sulfate were found.
Table 1.
Plasma Hormone Levels in Control Rats and in Rats Treated With Spironolactone and Potassium Canrenoate
| Control group 1 | Spironolactone group 2 | pa | Potassium canrenoate group 3 | pa | |
|---|---|---|---|---|---|
| Testosterone (ng/ml) | 1.8 ± 0.32 | 0.80 ± 0.24 | <0.05 | 1.5 ± 0.30 | NS |
| Estradiol (pg/ml) | 9.8 ± 1.2 | 7.9 ± 2.1 | NS | 9.6 ± 1.1 | NS |
| Estriol (ng/ml) | 1.26 ± 0.48 | 1.68 ± 0.69 | NS | 1.18 ± 0.39 | NS |
| Progesterone (ng/ml) | 3.93 ± 0.27 | 3.21 ± 0.53 | NS | 2.71 ± 0.38 | <0.05 |
| DHEAS (ng/ml) | 99.60 ± 22.3 | 105.0 ± 22.4 | NS | 38.6 ± 15.3 | <0.05 |
| FSH (mU/ml) | 1.75 ± 0.34 | 2.46 ± 0.37 | NS | 1.19 ± 0.25 | NS |
| PRL (mU/ml) | 7.02 ± 0.77 | 6.8 ± 0.3 | NS | 5.95 ± 0.60 | NS |
| LH (mU/ml) | 6.52 ± 1.02 | 4.76 ± 0.8 | NS | 4.93 ± 0.93 | NS |
DHEAS, dehydroepiandrosterone sulfate; FSH, follicle-stimulating hormone; LH, luteinizing hormone; NS, not significant; PRL, prolactin. Group 1 was treated with vehicle. The other groups received spironolactone (group 2) and potassium canrenoate (group 3) at the dosage of 5 mg/day for 21 days. Values are expressed as mean ± SD.
Values that differ from control values.
Figure 1 reports the activity and subcellular distribution of AR in the livers of these animals. Cytosolic AR levels (Figure 1C) are significantly higher in those rats treated with potassium canrenoate than in either the control animals or the animals treated with spironolactone. There were no variations in the affinity of the cytosolic AR for its ligand in any groups studied. Specifically, the equilibrium dissociation constant values for cytosolic AR were 0.26 ± 0.06, 0.25 ± 0.08, and 0.31 ± 0.04 nM for groups 1, 2, and 3, respectively. The activity of nuclear AR, however, presents a markedly different picture, and is significantly decreased in the animals treated with either spironolactone or potassium canrenoate (Figure 1B). As a result of these variations, the total AR content of the liver (Figure 1A) changed significantly only in those animals treated with spironolactone (50% decrease from normal levels). Interestingly, the activity of total AR in the spironolactone-treated animals shows a decrease proportional to that of plasma testosterone (Figure 1D).
Figure 1.
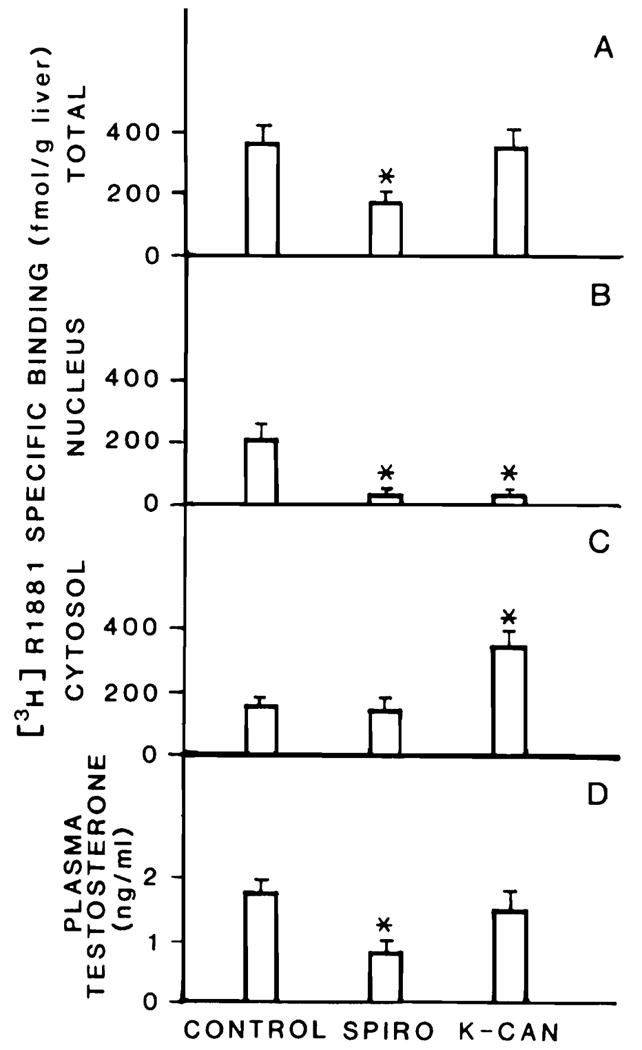
Variation in specific [3H]R1881 in rat liver of male rats treated with spironolactone (SPIRO) or potassium canrenoate (K-CAN). Specific [3H]R1881 binding was quantitated as described in Methods and is expressed as femtomoles per gram liver. Total hepatic receptor content is shown in A, nuclear receptor in B, and cytosolic receptor in C. Serum testosterone levels are shown in D. All groups consisted of at least 5 animals; the values are expressed as mean ± SD. *Different from control, p < 0.05.
Because total hepatic AR activity and plasma testosterone values were reduced in the spironolactone-treated animals, the activity of a hepatic androgen responsive protein, a MEB, was also assessed. Figure 2 shows that the activity of this protein is undetectable in the livers of those animals treated with spironolactone; this loss of MEB activity is comparable to that seen in rats castrated at least 15 days before death (10,12). However, the level of MEB in the rats treated with potassium canrenoate is actually higher than that observed in the control rats.
Figure 2.
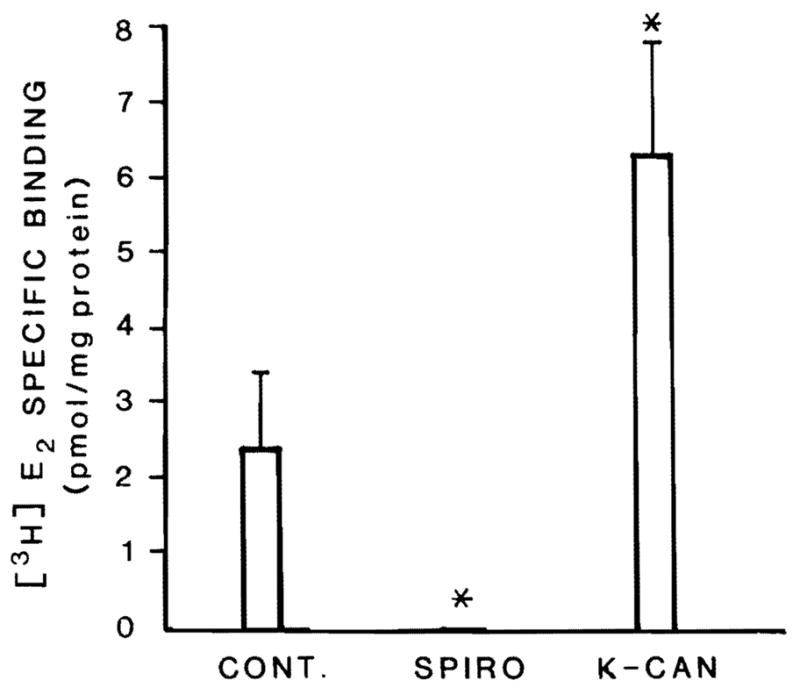
Activity of an androgen responsive protein in liver of male rats treated with spironolactone (SPIRO) or potassium canrenoate (K-CAN). Cytosolic MEB was measured by its [3H]estradiol ([3H]E2) binding activity as described in Methods. All groups consisted of at least 5 animals; the values are expressed as mean ± SD. *Different from control, p < 0.05.
The loss of expression of MEB after spironolactone treatment suggests that this drug may be interfering with androgen action. To determine whether either of these drugs might interfere by competing for androgen binding to its receptor, we tested whether they were capable of competing for AR binding in an in vitro assay. As shown in Figure 3, spironolactone, at a 100-fold and 1000-fold excess, is partially effective as a competitor for [3H]R1881 binding in rat liver cytosol. The strong androgens R1881 and dihydrotestosterone, however, are much more effective competitors than is spironolactone. In contrast, potassium canrenoate is incapable of competing for [3H]R1881 binding at any concentration tested. When these substances were tested at 100-fold excess using nuclear preparations, the competition observed was virtually identical to that for the cytosolic AR except that, in nuclei, dihydrotestosterone was equivalent to R1881 in effectiveness.
Figure 3.
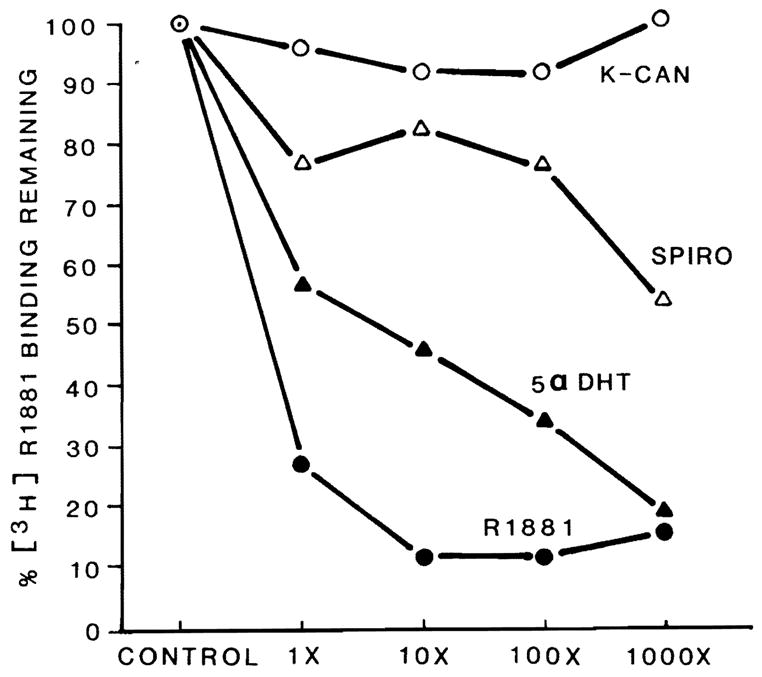
Specificity of binding to cytosolic proteins in male rat liver cytosol. Two hundred microliters of male rat liver cytosol were incubated with 1.5 nM [3H]R1881 in the absence (control, 100%) and presence of 1-, 10-, 100-, and 1000-fold excess of potentially competing drugs or hormones.  , control = 100%; ●-●, [3H]R1881; ▲-▲, 5 α-dihydrotestosterone; △-△, spironolactone (SPIRO); ○-○, potassium canrenoate (K-CAN).
, control = 100%; ●-●, [3H]R1881; ▲-▲, 5 α-dihydrotestosterone; △-△, spironolactone (SPIRO); ○-○, potassium canrenoate (K-CAN).
In other studies, the activity and subcellular distribution of hepatic estrogen receptors in the three groups of animals were examined. The activity of cytosolic ER was virtually unchanged among the three groups (group 1, 445 ± 61; group 2, 553 ± 150; group 3, 441 ± 94 fmol/g liver), nor did the affinity of cytosolic ER for estradiol vary among the groups of animals (equilibrium dissociation constant values of 0.26 ± 0.07, 0.45 ± 0.12, and 0.36 ± 0.08 nM for groups 1, 2, and 3, respectively). The activity of nuclear ER was decreased significantly (p < 0.05) only in that group of animals treated with spironolactone (178 ± 44, 67.2 ± 16, and 209 ± 44 fmol/g liver for groups 1, 2, and 3, respectively). However, the total hepatic ER content does not vary in the three groups of animals studied (group 1, 673 ± 113; group 2, 600 ± 88; and group 3, 650 ± 90 fmol/g liver). This latter finding is consistent with the similar plasma estradiol values in these three groups of animals (Table 1).
Discussion
Liver is a sex steroid-responsive organ in both male and female animals. Many biochemical events, particularly those regarding drug and sex hormone metabolism, are influenced by the sex of the animal. This sexual dimorphism of liver function has been observed in both animals and humans (reviewed in Reference 11). In male rats, androgen is the major determinant of the masculine pattern of liver function. Androgen stimulates the expression of both androgen receptor and certain enzymes and proteins such as MEB (10,12). This masculine pattern can be feminized by certain changes in serum hormone levels occurring after castration (10,12), chronic alcohol ingestion (5), estrogen treatment (34), and portal vein ligation (35), and during liver regeneration (26,29).
It is known from the clinical literature that spironolactone has feminizing action. There have been many reports addressing the mechanism of this action. Menard et al. (22) demonstrated, in several species, that spironolactone treatment decreases the microsomal cytochrome P450 content of the testes and reduces the activity of 17-hydroxylase, an enzyme necessary for testosterone synthesis. In women treated with spironolactone to reduce hirsutism, this drug has been shown to increase testosterone clearance (23) and decrease genital skin fibroblast steroid 5α reductase (24). Loriaux et al. (36) showed, using an in vitro assay, that spironolactone inhibits the binding of dihydrotestosterone to the AR. Chopra et al. (37) reported that in general such feminization is a result of an alteration of testosterone-estrogen balance.
Our results present further observations on the action of spironolactone. Administration of this drug resulted in a significant decrease in plasma of testosterone levels and hepatic AR and the complete loss of activity of the androgen responsive hepatic protein MEB. As we have reported previously (4,12,27), MEB is a cytosolic protein that has a moderate affinity for estradiol, high binding capacity, and specificity for steroidal estrogen. Its function in the cytosol of male rats is unknown, but may be that of an intracellular estrogen scavenger (4,27). The complete disappearance of MEB in rats treated with spironolactone is significant because, first, its loss represents interruption of hepatic androgen response, and second, its absence might lead to an increased intracellular estrogen concentration.
The loss of MEB only in the spironolactone-treated animals is interesting, as both drug groups demonstrated reduced nuclear AR levels. However, only the spironolactone group demonstrated reduced serum testosterone. It is possible that the nuclear AR has either a higher affinity in vivo for spironolactone than for testosterone, or the spironolactone is available at much higher concentrations than testosterone to interact with the nuclear AR. Because we did not measure serum levels of either drug, it is not possible to differentiate between these possibilities. It is clear, however, that the presence of spironolactone inhibits the expression of MEB; this reduced expression is likely to result from an unproductive interaction of the drug with AR.
In spite of the loss of MEB activity, our results display no action of spironolactone either on plasma estradiol or estriol content, or on the total ER activity in liver. It is possible that our treatment period was too short to produce a feminization of (i.e., an increase in) these activities. It is more likely, however, that in liver disease patients, feminization occurs because spironolactone treatment interferes in maintaining the androgenic influence necessary to sustain a masculine pattern of liver function. Such interference, coupled with an already compromised ability to excrete estrogens, may permit full feminization of liver and other peripheral tissues, such as the breast. However, our experimental animals display no liver disease; thus, treatment of normal male animals with spironolactone may not result in the pronounced feminization that has been noted in some patients with liver disease.
Our results in animals treated with potassium canrenoate, the major active metabolite of spironolactone, confirmed the clinical data indicating that this drug has less antiandrogenic effect than spironolactone. Use of potassium canrenoate has been shown to reverse the gynecomastia produced during treatment with spironolactone (20,38), and shows a much lower incidence of the feminizing side effects of cirrhotic patients (38). It has been demonstrated, in castrated animals, that potassium canrenoate does not inhibit cytochrome P450, as does spironolactone, and produces significantly less androgenic antagonism (39). Thus, our results in animals chronically treated with potassium canrenoate confirm the clinical findings. In fact, no decrease of plasma testosterone, total hepatic AR, or MEB, and no inhibition of androgen binding to its receptor were found to occur in the liver of these animals. Although no decrease was observed in these androgen-related functions, it was noted that potassium canrenoate also resulted in a decrease in nuclear AR. Perhaps the normal serum testosterone in these animals was sufficient to maintain an adequate androgenic response in spite of the reduced nuclear AR levels. Another possibility is that this drug somehow promotes release of the nuclear AR into the cytosolic fraction. The latter possibility is consistent with our findings of increased cytosolic AR in these animals.
In conclusion, the data reported in this paper demonstrate that spironolactone treatment of male rats decreased plasma testosterone and exhibited a powerful anti androgenic effect, as documented by the low levels of hepatic AR and disappearance of an androgen responsive hepatic protein, MEB. We also demonstrated that potassium canrenoate did not show any of the antiandrogenic actions of spironolactone in the liver, suggesting that the clinical improvement reported in patients treated with potassium canrenoate as compared with spironolactone is the result of this biochemical difference. Also, our results indicate that in animals with no underlying liver disease, there is no increase in plasma estrogen levels or total hepatic ER content.
Acknowledgments
This study was supported by the Veterans Administration, grants AM29961, AM30001, and AM31577 from the National Institutes of Health, and by grant 885/02 16544 from Consiglio Nazionale delle Ricerche, Italy.
Abbreviations used in this paper
- AR
androgen receptor
- ER
estrogen receptor
- [3H]R1881
17 α-methyl-[3H]methyltrienolone
- MEB
male specific estrogen binder
References
- 1.Duffy MJ, Duffy GJ. Estradiol receptors in human liver. J Steroid Biochem. 1978;9:233–5. doi: 10.1016/0022-4731(78)90154-1. [DOI] [PubMed] [Google Scholar]
- 2.Aten RF, Dickson RB, Eisenfeld AJ. Estrogen receptor in adult male rat liver. Endocrinology. 1978;103:1629–35. doi: 10.1210/endo-103-5-1629. [DOI] [PubMed] [Google Scholar]
- 3.Powell-Jones W, Thompson C, Nayfeh SN, Lucier GW. Sex differences in estrogen binding by cytosolic and nuclear components of rat liver. J Steroid Biochem. 1980;13:219–29. doi: 10.1016/0022-4731(80)90195-8. [DOI] [PubMed] [Google Scholar]
- 4.Eagon PK, Fisher SE, Imhoff AF, et al. Estrogen binding proteins of male rat livers: influences of hormonal changes. Arch Biochem Biophys. 1980;201:486–99. doi: 10.1016/0003-9861(80)90537-8. [DOI] [PubMed] [Google Scholar]
- 5.Eagon PK, Zdunek JR, Van Thiel DH, et al. Alcohol-induced changes in hepatic estrogen binding proteins: a mechanism explaining feminization in alcoholics. Arch Biochem Biophys. 1981;211:48–54. doi: 10.1016/0003-9861(81)90428-8. [DOI] [PubMed] [Google Scholar]
- 6.Kneifel R, Katzenellenbogen BS. Comparative effects of estrogen and antiestrogen on plasma renin substrate levels and hepatic estrogen receptors in rats. Endocrinology. 1981;108:545–52. doi: 10.1210/endo-108-2-545. [DOI] [PubMed] [Google Scholar]
- 7.Norstedt G, Wrange O, Gustafsson JA. Multihormonal regulation of the estrogen receptor in rat liver. Endocrinology. 1981;108:1190–6. doi: 10.1210/endo-108-4-1190. [DOI] [PubMed] [Google Scholar]
- 8.Porter LE, Elm MS, Van Thiel DH, Dugas MC, Eagon PK. Characterization and quantitation of human hepatic estrogen receptor. Gastroenterology. 1983;84:704–12. [PubMed] [Google Scholar]
- 9.Bannister P, Sheridan P, Losowsky MS. Identification and characterization of the human hepatic androgen receptor. Clin Endocrinol. 1985;23:495–502. doi: 10.1111/j.1365-2265.1985.tb01108.x. [DOI] [PubMed] [Google Scholar]
- 10.Eagon PK, Seguiti SM, Willett JE, Rogerson BJ. Hepatic androgen receptor: effects of hormonal alteration (abstr) Hepatology. 1985;5:1046. (abstract 397) [Google Scholar]
- 11.Eagon PK, Porter LE, Francavilla A, DiLeo A, Van Thiel DH. Estrogen and androgen receptors in liver; their role in liver disease and regeneration. Semin Liver Dis. 1985;5:59–69. doi: 10.1055/s-2008-1041758. [DOI] [PubMed] [Google Scholar]
- 12.Turocy JF, Chiang AN, Seeley DH, Eagon PK. Effect of H2 antagonists on androgen imprinting of male hepatic functions. Endocrinology. 1985;117:1953–61. doi: 10.1210/endo-117-5-1953. [DOI] [PubMed] [Google Scholar]
- 13.Porter LE, Elm MS, Van Thiel DH, Eagon PK. Hepatic estrogen receptor in human liver disease. Gastroenterology. 1987;92:735–45. doi: 10.1016/0016-5085(87)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Gustafsson JA, Mode A, Norstedt G, et al. The hypothalamopituitary-liver axis: a new hormonal system in control of hepatic steroid and drug metabolism. Biochem Action Horm. 1980;7:47–89. [Google Scholar]
- 15.Clark E. Spironolactone therapy and gynecomastia. JAMA. 1965;193:163–4. doi: 10.1001/jama.1965.03090020077026. [DOI] [PubMed] [Google Scholar]
- 16.Fromatin M. Surveillance clinique et hormonale des traitements prolonges de l’hypertension arterielle par les anti aldosterones. Mises J Cardiol. 1980;19:95–8. [Google Scholar]
- 17.Greenblatt DJ, Koch-Weser J. Adverse reactions to spironolactone. JAMA. 1973;225:40–3. doi: 10.1001/jama.225.1.40. [DOI] [PubMed] [Google Scholar]
- 18.Huffman DH, Kampmann JP, Hignite CE, Azarnoff DL. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther. 1978;24:465–73. doi: 10.1002/cpt1978244465. [DOI] [PubMed] [Google Scholar]
- 19.Niewoehner CB, Nuttal FQ. Gynecomastia in a hospitalized male population. Am J Med. 1984;37:633–8. doi: 10.1016/0002-9343(84)90353-x. [DOI] [PubMed] [Google Scholar]
- 20.Dupont A. Disappearance of spironolactone-induced gynaecomastia during treatment with potassium canrenoate. Lancet. 1985;ii:731. doi: 10.1016/s0140-6736(85)92975-7. [DOI] [PubMed] [Google Scholar]
- 21.Marco J, Constans R, Alibelli MJ, Baradat G, Dardenne P. Effets sexuels secondaires de la spironolactone: interet d’une therapeutique substitutive par la canrenone. Nouv Presse Med. 1978;7:3668–74. [Google Scholar]
- 22.Menard RH, Stripp B, Gillette JR. Spironolactone and testicular cytochrome P-450: decreased testosterone formation in several species and changes in hepatic drug metabolism. Endocrinology. 1974;94:1628–36. doi: 10.1210/endo-94-6-1628. [DOI] [PubMed] [Google Scholar]
- 23.Boisselle A, Tremblay RR. New therapeutic approach to the hirsute patient. Fertil Steril. 1979;32:276–9. doi: 10.1016/s0015-0282(16)44232-9. [DOI] [PubMed] [Google Scholar]
- 24.Serafini PC, Catalino J, Lobo RA. The effect of spironolactone on genital skin 5α reductase. J Steroid Biochem. 1985;23:191–4. doi: 10.1016/0022-4731(85)90236-5. [DOI] [PubMed] [Google Scholar]
- 25.Rifka SM, Pita JC, Vigersky RA, Wilson YA, Loriaux DL. Interaction of digitalis and spironolactone with human sex steroid receptors. J Clin Endocrinol Metab. 1978;46:338–44. doi: 10.1210/jcem-46-2-338. [DOI] [PubMed] [Google Scholar]
- 26.Francavilla A, DiLeo A, Eagon PK, et al. Regenerating rat liver: correlations between estrogen receptor localization and DNA synthesis. Gastroenterology. 1984;86:552–7. [PMC free article] [PubMed] [Google Scholar]
- 27.Rogerson BJ, Eagon PK. A male specific hepatic estrogen binding protein; properties and binding characteristics. Arch Biochem Biophys. 1986;250:70–85. doi: 10.1016/0003-9861(86)90703-4. [DOI] [PubMed] [Google Scholar]
- 28.Francavilla A, Eagon PK, DiLeo A, et al. Circadian rhythm of hepatic cytosolic and nuclear estrogen and androgen receptors. Gastroenterology. 1986;91:182–8. doi: 10.1016/0016-5085(86)90456-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francavilla A, Eagon PK, DiLeo A, et al. Sex hormone related functions in regenerating male rat liver. Gastroenterology. 1986;91:1263–70. doi: 10.1016/s0016-5085(86)80026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 31.Burton K. Determination of DNA concentration with diphenylamine. Methods Enzymol. 1968;12:163–6. [Google Scholar]
- 32.Van Thiel DH, Gavaler JS, Cobb CF, Sherins RJ, Lester R. Alcohol induced testicular atrophy in the adult male rat. Endocrinology. 1979;105:888–94. doi: 10.1210/endo-105-4-888. [DOI] [PubMed] [Google Scholar]
- 33.Scatchard G. The attractions of proteins for small molecules and ions. Ann NY Acad Sci. 1949;51:660–72. [Google Scholar]
- 34.Gustafsson JA, Mode A, Norstedt G, Skett P. Sex steroid induced changes in hepatic enzymes. Ann Rev Physiol. 1983;45:51–60. doi: 10.1146/annurev.ph.45.030183.000411. [DOI] [PubMed] [Google Scholar]
- 35.Farrell GC, Koltai A, Zaluzny L, Murray M. Effects of portal vein ligation on sex hormone metabolism in male rats: relationships to lowered hepatic cytochrome P-450 levels. Gastroenterology. 1986;90:299–305. doi: 10.1016/0016-5085(86)90924-8. [DOI] [PubMed] [Google Scholar]
- 36.Loriaux DL, Menard R, Taylor A, Pita J, Sarton R. Spironolactone and endocrine dysfunction. Ann Intern Med. 1976;85:630–6. doi: 10.7326/0003-4819-85-5-630. [DOI] [PubMed] [Google Scholar]
- 37.Chopra IJ, Tulchinsky D, Greenway FL. Estrogen-androgen imbalance in hepatic cirrhosis. Ann Intern Med. 1973;79:198–203. doi: 10.7326/0003-4819-79-2-198. [DOI] [PubMed] [Google Scholar]
- 38.Bellati G, Ideo G. Gynaecomastia after spironolactone and potassium canrenoate. Lancet. 1986;i:626. doi: 10.1016/s0140-6736(86)92856-4. [DOI] [PubMed] [Google Scholar]
- 39.Armanini D, Karbowiak I, Goi A, Mantero F. Spironolactone and potassium canrenoate: biological effect and plasma androgen-receptor activity in castrated mouse. J Endocrinol Invest. 1983;6(Suppl 1):115. [Google Scholar]


