Abstract
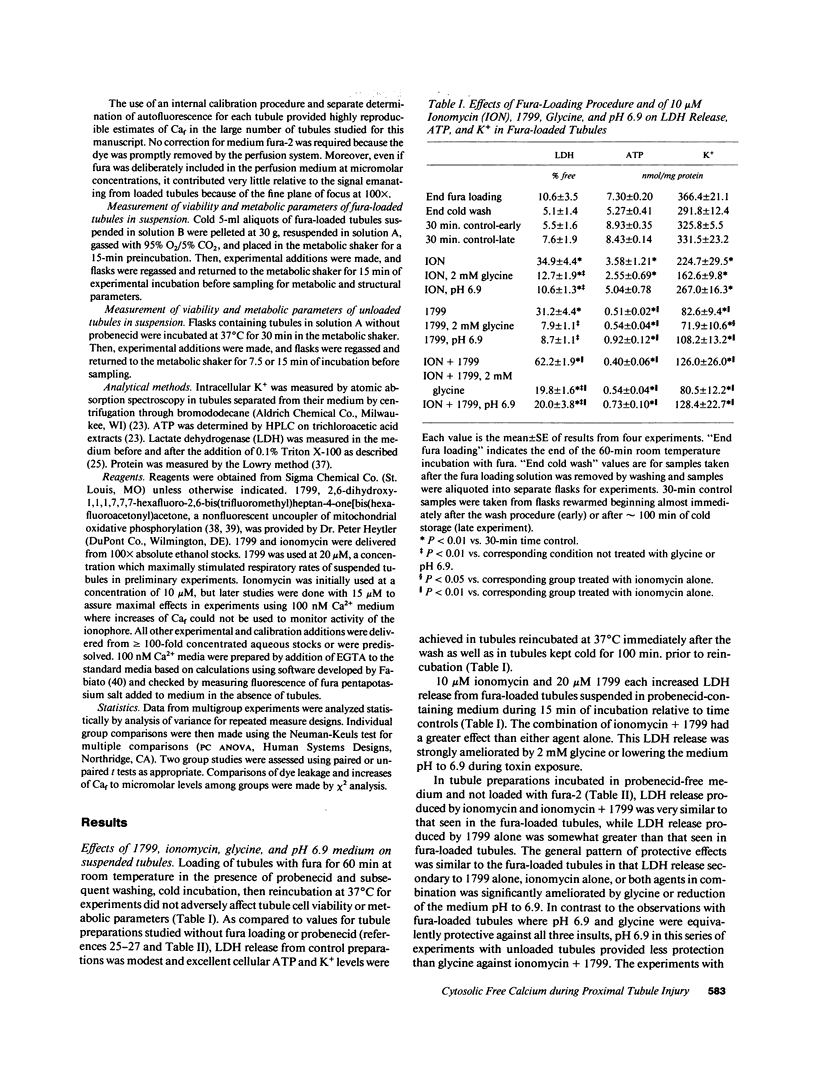
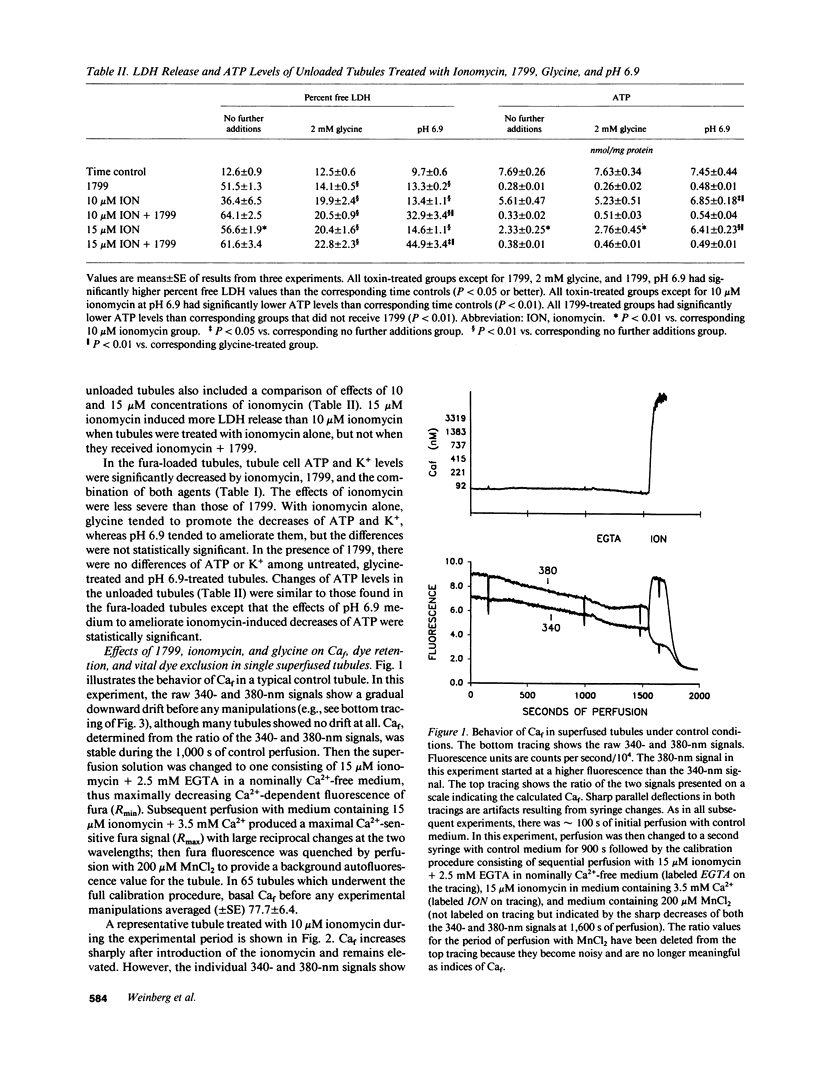
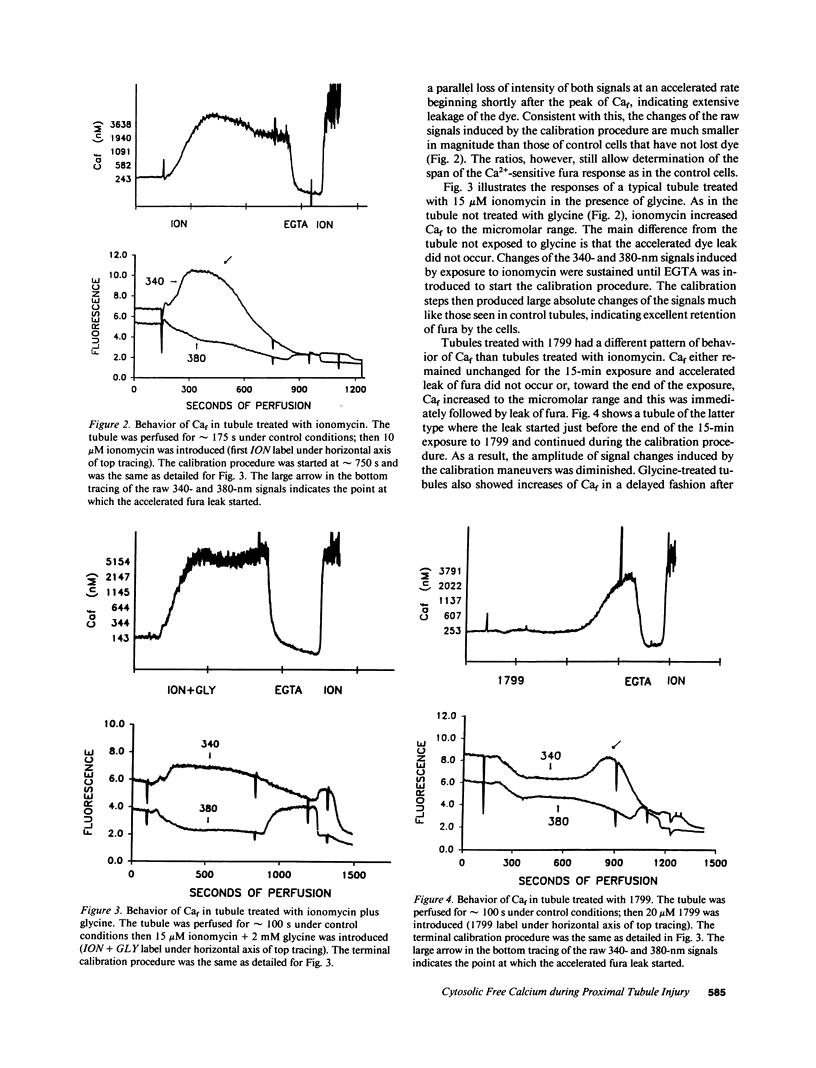
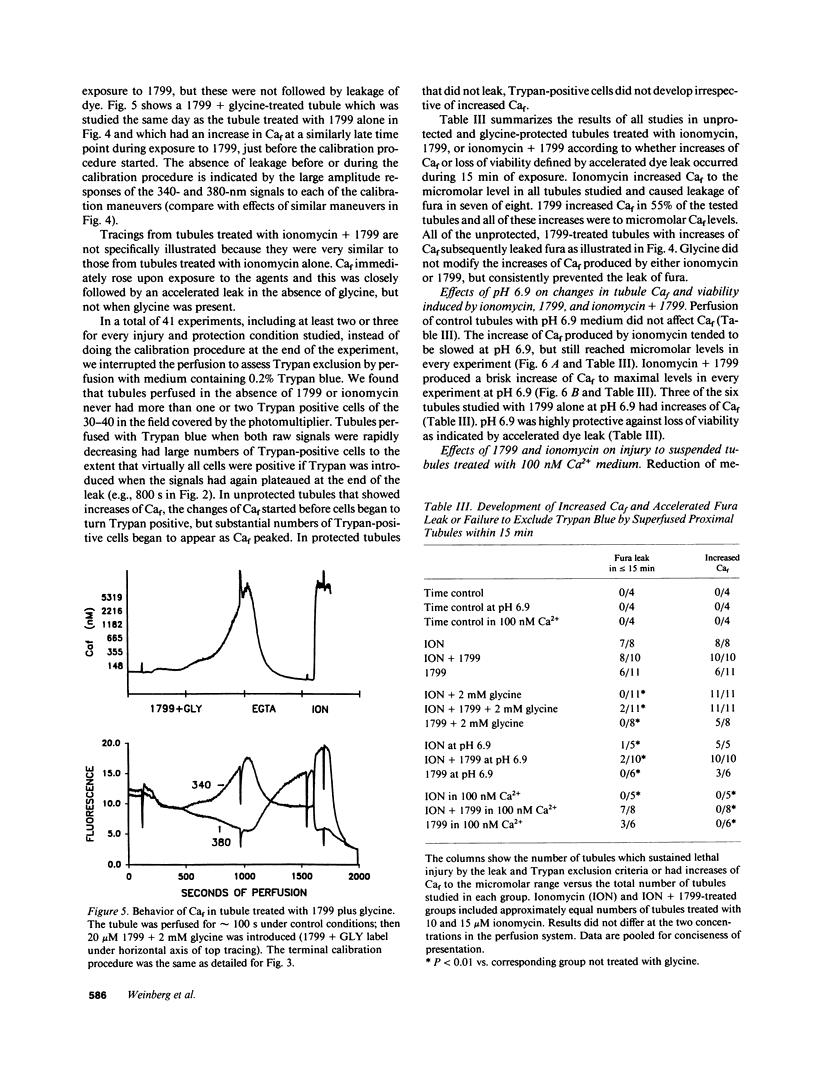
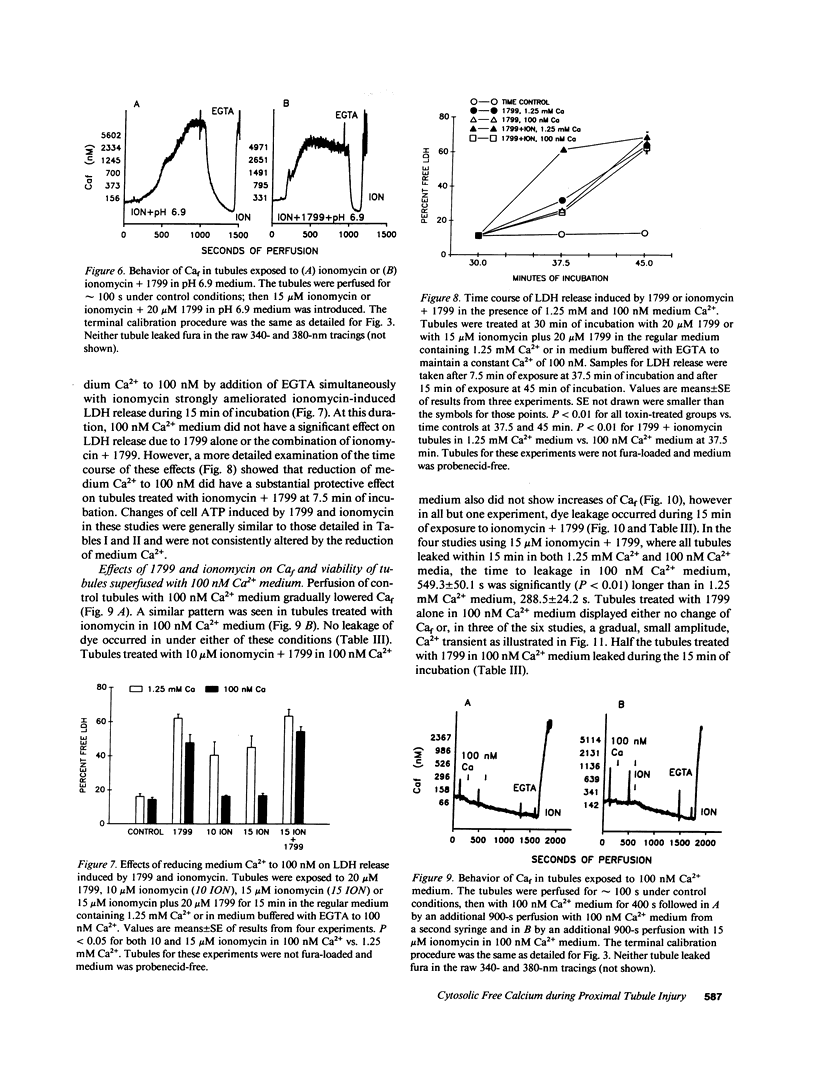
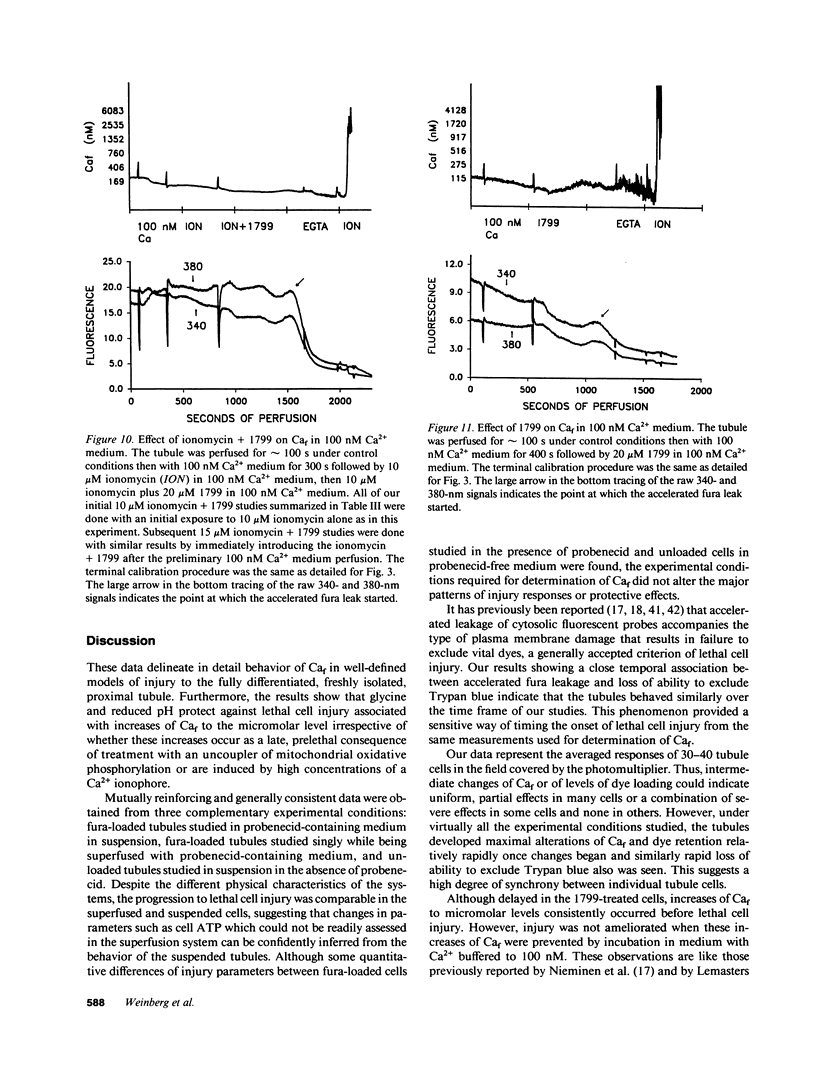
To assess the role of increased cytosolic free calcium (Caf) in the pathogenesis of acute proximal tubule cell injury and the protection afforded by exposure to reduced medium pH or treatment with glycine, fura-2-loaded tubules were studied in suspension and singly in a superfusion system. The Ca2+ ionophore, ionomycin, increased Caf to micromolar levels and rapidly produced lethal cell injury as indicated by loss of lactate dehydrogenase to the medium by suspended tubules and accelerated leak of fura and failure to exclude Trypan blue by superfused tubules. Decreasing medium Ca2+ to 100 nM prevented the ionomycin-induced increases of Caf and the injury. Reducing medium pH from 7.4 to 6.9 or adding 2 mM glycine to the medium also prevented the cell death, but did not prevent the increase of Caf to micromolar levels. Cells treated with 1799, an uncoupler of oxidative phosphorylation which produced severe adenosine triphosphate (ATP) depletion, did not develop increases of Caf until just before loss of viability. Preventing these increases of Caf with 100 nM Ca2+ medium did not protect 1799-treated cells. Reduced pH and glycine protected 1799-treated cells without ameliorating the increases of Caf. These data demonstrate the toxic potential of increased Caf in the proximal tubule and show that Caf does sharply increase prior to loss of viability in an ATP depletion model of injury, but this increase does not necessarily contribute to the outcome. The potent protective actions of decreased pH and glycine allow the cells to sustain increases of Caf to micromolar levels in spite of severe, accompanying cellular ATP depletion without developing lethal cell injury.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschuld R. A., Hostetler J. R., Brierley G. P. Response of isolated rat heart cells to hypoxia, re-oxygenation, and acidosis. Circ Res. 1981 Aug;49(2):307–316. doi: 10.1161/01.res.49.2.307. [DOI] [PubMed] [Google Scholar]
- Arnold P. E., Lumlertgul D., Burke T. J., Schrier R. W. In vitro versus in vivo mitochondrial calcium loading in ischemic acute renal failure. Am J Physiol. 1985 Jun;248(6 Pt 2):F845–F850. doi: 10.1152/ajprenal.1985.248.6.F845. [DOI] [PubMed] [Google Scholar]
- Becker G. L., Fiskum G., Lehninger A. L. Regulation of free Ca2+ by liver mitochondria and endoplasmic reticulum. J Biol Chem. 1980 Oct 10;255(19):9009–9012. [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Chenery R., George M., Krishna G. The effect of ionophore A23187 and calcium on carbon tetrachloride-induced toxicity in cultured rat hepatocytes. Toxicol Appl Pharmacol. 1981 Sep 15;60(2):241–252. doi: 10.1016/0041-008x(91)90228-7. [DOI] [PubMed] [Google Scholar]
- Cheung J. Y., Bonventre J. V., Malis C. D., Leaf A. Calcium and ischemic injury. N Engl J Med. 1986 Jun 26;314(26):1670–1676. doi: 10.1056/NEJM198606263142604. [DOI] [PubMed] [Google Scholar]
- Davis M. H., Altschuld R. A., Jung D. W., Brierley G. P. Estimation of intramitochondrial pCa and pH by fura-2 and 2,7 biscarboxyethyl-5(6)-carboxyfluorescein (BCECF) fluorescence. Biochem Biophys Res Commun. 1987 Nov 30;149(1):40–45. doi: 10.1016/0006-291x(87)91602-0. [DOI] [PubMed] [Google Scholar]
- Dominguez J. H., Rothrock J. K., Macias W. L., Price J. Na+ electrochemical gradient and Na+-Ca2+ exchange in rat proximal tubule. Am J Physiol. 1989 Oct;257(4 Pt 2):F531–F538. doi: 10.1152/ajprenal.1989.257.4.F531. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Farber J. L. Biology of disease: membrane injury and calcium homeostasis in the pathogenesis of coagulative necrosis. Lab Invest. 1982 Aug;47(2):114–123. [PubMed] [Google Scholar]
- Fariss M. W., Pascoe G. A., Reed D. J. Vitamin E reversal of the effect of extracellular calcium on chemically induced toxicity in hepatocytes. Science. 1985 Feb 15;227(4688):751–754. doi: 10.1126/science.3918345. [DOI] [PubMed] [Google Scholar]
- Garza-Quintero R., Ortega-Lopez J., Stein J. H., Venkatachalam M. A. Alanine protects rabbit proximal tubules against anoxic injury in vitro. Am J Physiol. 1990 Apr;258(4 Pt 2):F1075–F1083. doi: 10.1152/ajprenal.1990.258.4.F1075. [DOI] [PubMed] [Google Scholar]
- Gores G. J., Nieminen A. L., Wray B. E., Herman B., Lemasters J. J. Intracellular pH during "chemical hypoxia" in cultured rat hepatocytes. Protection by intracellular acidosis against the onset of cell death. J Clin Invest. 1989 Feb;83(2):386–396. doi: 10.1172/JCI113896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Harris S. I., Balaban R. S., Barrett L., Mandel L. J. Mitochondrial respiratory capacity and Na+- and K+-dependent adenosine triphosphatase-mediated ion transport in the intact renal cell. J Biol Chem. 1981 Oct 25;256(20):10319–10328. [PubMed] [Google Scholar]
- Heytler P. G. Uncouplers of oxidative phosphorylation. Methods Enzymol. 1979;55:462–442. doi: 10.1016/0076-6879(79)55060-5. [DOI] [PubMed] [Google Scholar]
- Kolber M. A., Quinones R. R., Gress R. E., Henkart P. A. Measurement of cytotoxicity by target cell release and retention of the fluorescent dye bis-carboxyethyl-carboxyfluorescein (BCECF). J Immunol Methods. 1988 Apr 6;108(1-2):255–264. doi: 10.1016/0022-1759(88)90427-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LeFurgey A., Ingram P., Mandel L. J. Heterogeneity of calcium compartmentation: electron probe analysis of renal tubules. J Membr Biol. 1986;94(2):191–196. doi: 10.1007/BF01871198. [DOI] [PubMed] [Google Scholar]
- Lemasters J. J., DiGuiseppi J., Nieminen A. L., Herman B. Blebbing, free Ca2+ and mitochondrial membrane potential preceding cell death in hepatocytes. Nature. 1987 Jan 1;325(6099):78–81. doi: 10.1038/325078a0. [DOI] [PubMed] [Google Scholar]
- Li Q., Hohl C. M., Altschuld R. A., Stokes B. T. Energy depletion-repletion and calcium transients in single cardiomyocytes. Am J Physiol. 1989 Sep;257(3 Pt 1):C427–C434. doi: 10.1152/ajpcell.1989.257.3.C427. [DOI] [PubMed] [Google Scholar]
- Liu C., Hermann T. E. Characterization of ionomycin as a calcium ionophore. J Biol Chem. 1978 Sep 10;253(17):5892–5894. [PubMed] [Google Scholar]
- Mandel L. J., Murphy E. Regulation of cytosolic free calcium in rabbit proximal renal tubules. J Biol Chem. 1984 Sep 25;259(18):11188–11196. [PubMed] [Google Scholar]
- Mandel L. J., Schnellmann R. G., Jacobs W. R. Intracellular glutathione in the protection from anoxic injury in renal proximal tubules. J Clin Invest. 1990 Feb;85(2):316–324. doi: 10.1172/JCI114440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy C. E., Selvaggio A. M., Alexander E. A., Schwartz J. H. Adenosine triphosphate depletion induces a rise in cytosolic free calcium in canine renal epithelial cells. J Clin Invest. 1988 Oct;82(4):1326–1332. doi: 10.1172/JCI113734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J. E., Jacob R., Hallam T. J. Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J Biol Chem. 1989 Jan 25;264(3):1522–1527. [PubMed] [Google Scholar]
- Morris A. C., Hagler H. K., Willerson J. T., Buja L. M. Relationship between calcium loading and impaired energy metabolism during Na+, K+ pump inhibition and metabolic inhibition in cultured neonatal rat cardiac myocytes. J Clin Invest. 1989 Jun;83(6):1876–1887. doi: 10.1172/JCI114094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P., Thor H., Orrenius S. Cytosolic-free Ca2+ and cell killing in hepatoma 1c1c7 cells exposed to chemical anoxia. FASEB J. 1989 Jan;3(1):59–64. doi: 10.1096/fasebj.3.1.2910738. [DOI] [PubMed] [Google Scholar]
- Nieminen A. L., Gores G. J., Wray B. E., Tanaka Y., Herman B., Lemasters J. J. Calcium dependence of bleb formation and cell death in hepatocytes. Cell Calcium. 1988 Dec;9(5-6):237–246. doi: 10.1016/0143-4160(88)90004-8. [DOI] [PubMed] [Google Scholar]
- Orrenius S., Nicotera P. On the role of calcium in chemical toxicity. Arch Toxicol Suppl. 1987;11:11–19. doi: 10.1007/978-3-642-72558-6_2. [DOI] [PubMed] [Google Scholar]
- Phelps P. C., Smith M. W., Trump B. F. Cytosolic ionized calcium and bleb formation after acute cell injury of cultured rabbit renal tubule cells. Lab Invest. 1989 May;60(5):630–642. [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Schrier R. W., Arnold P. E., Van Putten V. J., Burke T. J. Cellular calcium in ischemic acute renal failure: role of calcium entry blockers. Kidney Int. 1987 Sep;32(3):313–321. doi: 10.1038/ki.1987.211. [DOI] [PubMed] [Google Scholar]
- Snowdowne K. W., Borle A. B. Effects of low extracellular sodium on cytosolic ionized calcium. Na+-Ca2+ exchange as a major calcium influx pathway in kidney cells. J Biol Chem. 1985 Dec 5;260(28):14998–14507. [PubMed] [Google Scholar]
- Snowdowne K. W., Freudenrich C. C., Borle A. B. The effects of anoxia on cytosolic free calcium, calcium fluxes, and cellular ATP levels in cultured kidney cells. J Biol Chem. 1985 Sep 25;260(21):11619–11626. [PubMed] [Google Scholar]
- Steinberg T. H., Newman A. S., Swanson J. A., Silverstein S. C. Macrophages possess probenecid-inhibitable organic anion transporters that remove fluorescent dyes from the cytoplasmic matrix. J Cell Biol. 1987 Dec;105(6 Pt 1):2695–2702. doi: 10.1083/jcb.105.6.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. E., Reed D. J. Current status of calcium in hepatocellular injury. Hepatology. 1989 Sep;10(3):375–384. doi: 10.1002/hep.1840100322. [DOI] [PubMed] [Google Scholar]
- Trump B. F., Berezesky I. K. The role of calcium in cell injury and repair: a hypothesis. Surv Synth Pathol Res. 1985;4(3):248–256. doi: 10.1159/000156978. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M., Davis J. A., Abarzua M., Kiani T. Relationship between cell adenosine triphosphate and glutathione content and protection by glycine against hypoxic proximal tubule cell injury. J Lab Clin Med. 1989 May;113(5):612–622. [PubMed] [Google Scholar]
- Weinberg J. M., Davis J. A., Abarzua M., Rajan T. Cytoprotective effects of glycine and glutathione against hypoxic injury to renal tubules. J Clin Invest. 1987 Nov;80(5):1446–1454. doi: 10.1172/JCI113224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. M., Davis J. A., Abarzua M., Smith R. K., Kunkel R. Ouabain-induced lethal proximal tubule cell injury is prevented by glycine. Am J Physiol. 1990 Feb;258(2 Pt 2):F346–F355. doi: 10.1152/ajprenal.1990.258.2.F346. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M., Harding P. G., Humes H. D. Alterations in renal cortex cation homeostasis during mercuric chloride and gentamicin nephrotoxicity. Exp Mol Pathol. 1983 Aug;39(1):43–60. doi: 10.1016/0014-4800(83)90040-0. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M., Humes H. D. Calcium transport and inner mitochondrial membrane damage in renal cortical mitochondria. Am J Physiol. 1985 Jun;248(6 Pt 2):F876–F889. doi: 10.1152/ajprenal.1985.248.6.F876. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M. Oxygen deprivation-induced injury to isolated rabbit kidney tubules. J Clin Invest. 1985 Sep;76(3):1193–1208. doi: 10.1172/JCI112075. [DOI] [PMC free article] [PubMed] [Google Scholar]



