Abstract
Seasonal regression of the avian song control system, a series of discrete brain nuclei that regulate song learning and production, serves as a useful model for investigating the neuroprotective effects of steroids. In seasonally-breeding male songbirds, the song control system regresses rapidly when males are transferred from breeding to nonbreeding physiological conditions. One nucleus in particular, HVC, regresses in volume by 22% within days of castration and transfer to a nonbreeding photoperiod. This regression is primarily mediated by a 30% decrease in neuron number, a result of a caspase-dependent process of programmed cell death. Here we examine whether testosterone (T) can act locally in the brain to prevent seasonal-like neurodegeneration in HVC. We began to infuse T intracerebrally near HVC on one side of the brain in breeding-condition male white-crowned sparrows two days prior to T-withdrawal and shifting them to short day photoperiods. The birds were sacrificed three or seven days later. Local T-infusion significantly protected ipsilateral HVC from volume regression and neuron loss. In addition, T-infusion significantly reduced the number, density, and number/1000 neurons of activated caspase-3 cells and cells positive for cleaved PARP, both markers for programmed cell death, in the ipsilateral HVC. T-infusion near HVC also prevented regression of ipsilateral efferent targets of HVC neurons, including the volumes of RA and Area X, and the soma area and density of RA neurons. Thus T can act locally in the brain to have a neuroprotective effect and act transsynaptically to prevent regression of efferent nuclei.
Keywords: Apoptosis, Birdsong, Caspase, Neuroendocrine, Neuroethology, Plasticity
Introduction
Sex steroids such as testosterone (T) and its metabolites 17 β-estradiol (E2) and 5α-dihydrotestosterone (5α-DHT) protect hormone-sensitive brain areas from various forms of neurodegenerative conditions in humans. In older men, age-related declines in circulating levels of androgens are associated with cognitive decline and an increased risk of neurodegenerative disease (Beauchet, 2006). Gonadectomy exacerbates neurodegeneration due to ischemia, status epileticus, and injection of neurotoxin in various animal models (Merchenthaler et al., 2003; Ramsden et al., 2003; Veliskova et al., 2000). In addition to neuroprotection, androgens play a supportive role for several hormone-sensitive neural circuits, which is best illustrated by that fact that several hormone-sensitive brain areas regress in response to withdrawal of T (Cooke et al., 1999; Johansen et al., 2004; MacLusky et al., 2006; Panzica et al., 2001).
One of the most striking examples of regression due to androgen withdrawal is observed in the avian song control system (Bernard et al., 1997; Brown and Bottjer, 1993; Johnson and Bottjer, 1993; Thompson et al., 2007), a series of discrete brain nuclei that control song learning and production in oscine passerines (Fig 1a). Seasonally-breeding songbird species can be brought into the laboratory, where photoperiod and circulating hormones can be carefully controlled. Photosensitive adult male Gambel’s white-crowned sparrows (Zonotrichia leucophrys Gambelii) implanted with subcutaneous Silastic T-pellets and placed on long-day photoperiod (LD) are brought into breeding condition, characterized by an increase in song rate and stereotypy, and growth of the song control system (Meitzen and Thompson, 2008; Tramontin et al., 2000). Subsequent withdrawal of circulating T and shift to short-day photoperiod (SD), i.e. a transition to nonbreeding conditions, induces regression of HVC volume within 12 hours, which is followed by a loss of ~30% of neurons within four days (Thompson et al., 2007). The regression of HVC is a caspase-dependent process; intracerebral infusion of caspase inhibitors protects HVC from seasonal-like regression for at least seven days (Thompson and Brenowitz, 2008).
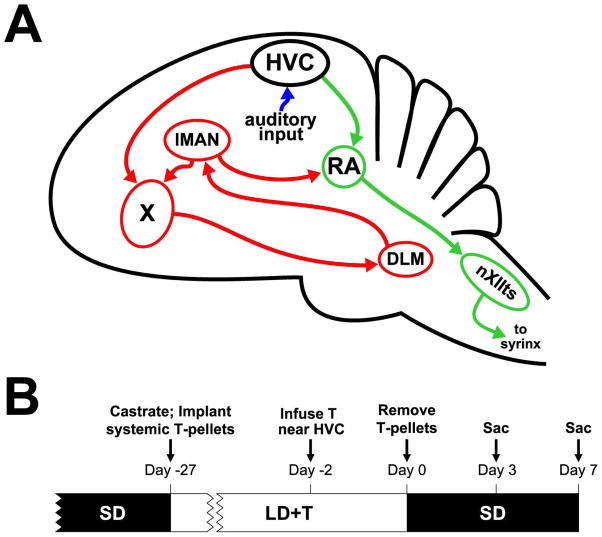
Figure 1.
The avian song control system and timeline for experimental procedures. a) Schematic sagittal diagram of the song control system showing projections of major nuclei. The green arrows indicate the descending motor pathway. The red arrows indicate the anterior forebrain pathway. The blue arrows indicate auditory input into the song control system. b) Schematic timeline of the experimental procedures. (SD: short-day photoperiod, LD+T: long-day photoperiod and high levels of circulating testosterone (T)). Groups of animals that received unilateral infusion of T were sacrificed on Days 3 and 7.
Afferent input from HVC provides transsynaptic trophic support to promote the growth of RA and Area X. Unilateral lesion of HVC blocks the growth of ipsilateral RA and Area X due to exposure to breeding conditions (Brenowitz and Lent, 2001). In addition, an intracerebral T implant placed near HVC in nonbreeding male white-crowned sparrows induces the growth of the ipsilateral HVC, RA and Area X (Brenowitz and Lent, 2002). This transsynaptic influence is driven by the action of T in HVC; a T implant placed near RA does not promote the growth of ipsilateral song control nuclei. Infusion of DHT and E2, metabolites of T, near HVC in male birds under nonbreeding conditions is sufficient to induce growth of ipsilateral RA neurons; infusion near RA has no effect (Meitzen et al., 2007). In contrast, caspase inhibitor infusion near HVC protects RA soma area from regression seven days after a transition to nonbreeding conditions but does not prevent regression of RA volume, RA neuron density, and Area X volume (Thompson and Brenowitz, 2008). This implies that though caspase inhibitors are sufficient to reduce seasonal loss of HVC neurons, rescued HVC neurons do not provide sufficient trophic support to prevent regression of RA and Area X.
We hypothesized that intracerebral administration of T near HVC would protect HVC and its efferent targets, RA and Area X, from regression due to a transition to nonbreeding conditions. To test this hypothesis we infused T unilaterally near HVC in adult male white-crowned sparrows transferred from breeding to nonbreeding conditions. We found that T-infusion near HVC protected ipsilateral HVC, RA, and Area X from neurodegeneration for at least seven days following the transition to nonbreeding conditions. In addition, T-infusion reduced activation of caspase-3 and cleaved PARP in HVC.
Materials and Methods
All procedures followed NIH animal use guidelines and were approved by the University of Washington Institutional Animal Care and Use Committee. We captured 12 male Gambel’s white-crowned sparrows (Zonotrichia leucophrys Gambelii) in eastern Washington during their post-breeding season migration. We housed the birds in indoor group aviaries for at least 10 weeks on short days (SD, 8 hrs light) before the start of the experiment to ensure that the song system and reproductive system were fully regressed and sensitive to the stimulatory effects of T and long-day photoperiods.
The song control nuclei in wild male white-crowned sparrows increase in size in response to a gradual increase in circulating T levels as day length increases and the testes grow. The timing of the increase in circulating T levels varies across individuals, however. To reduce individual variability, we exposed all birds to the same long-day photoperiod and administered T subcutaneously to rapidly elevate plasma T concentrations to those seen in breeding birds (4–12 ng/ml) (Wingfield and Farner, 1978). Even though this transition occurs more gradually in the wild, this laboratory manipulation recreates the two most important seasonal influences on white-crowned sparrows: elevated T levels and a long-day photoperiod typical of their Alaskan breeding grounds (20 h of light per day). This manipulation offers the advantage of providing a discrete starting point for time course studies of growth and regression of song circuits.
At the beginning of the experiment we transferred the birds overnight from L:D=8:16 (SD) to L:D=20:4 (LD) (Fig 1b). The next day we anesthetized each bird with isoflurane through a non-rebreathing system and castrated them. We made a small incision on the left side anterior to the caudal-most rib and dorsal to the uncinate process and aspirated the testes. Once castrated, we implanted each bird subcutaneously with a 12 mm Silastic capsule (inner diameter 1.47 mm × outer diameter 1.96 mm) filled with crystalline T (Steraloids, Newport, Rhode Island). We castrated the birds at the onset of LD to avoid subjecting birds to this additional surgery when they were later implanted with an intracerebral cannula at the transition from breeding to nonbreeding conditions. We housed the birds individually in visual and auditory contact with the other birds used in this experiment. We kept all the birds on LD+T for 28 days. This time period is sufficient to induce full growth of the song control system under these conditions (Tramontin et al., 2000).
After 26 days of LD+T conditions, we implanted a cannula attached to an osmotic pump unilaterally near HVC in each bird. We anesthetized each bird with isoflurane and placed it into a stereotaxic headholder. We made an incision in the scalp, removed the portion of the skull overlying the midsagittal sinus, and used the birfurcation of the sinus as the zero point. We randomly chose a hemisphere and lowered the cannula 0.75 mm into the telencephalon just caudal to HVC (lateral: 2.8mm, posterior 0.4 mm). We fixed the cannula to the skull with dental cement and attached an osmotic pump (Alzet Inc, model 1002, Cupertino, CA) to the cannula. Twelve birds received osmotic pumps filled with testosterone (0.01 mg/ml in 5% EtOH, 35% DMSO and 60% PEG300). We did not use a negative control for T because we previously showed that negative control infusion does not preserve the ipsilateral song control nuclei, which demonstrated that protective effects are not due to some nonspecific effect of the vehicle or the surgical implantation of the cannula (Thompson and Brenowitz, 2008). We placed the osmotic pumps into microcentrifuge tubes filled with avian saline (0.9% NaCl), sealed the tubes with paraffin and quick-drying cement, and mounted the tubes into “backpacks” custom-made for white-crowned sparrows. The osmotic pump rested between the bird’s wings and allowed for free movement. Osmotic pumps released their contents similarly to those implanted subcutaneously, and the backpack system reduced the risks of secondary infection, extrusion of pumps, and mortality that can occur with subcutaneous implant.
We began the intracerebral infusion of T two days before removal of the subcutaneous T-capsule to ensure the onset of infusion of T into HVC before systemic T-withdrawal. We shifted the birds overnight to L:D = 14:10 on the same day as the T-withdrawal and the next day shifted them overnight to SD. The intermediate photoperiod helped birds adjust to the reduction in available feeding time. We sacrificed groups of birds three days (n=6) or seven days (n=6) after withdrawal of circulating T, which we consider to be the onset of nonbreeding conditions. We excluded one bird from the three day group because of pump malfunction.
Blood draw and hormone analysis
We took blood samples from the birds at various time points throughout the experiment to measure circulating T levels. We withdrew 250 μl of blood from the alar vein in the wing into heparinized collection tubes. We immediately centrifuged the tubes to separate the plasma, which was stored at −20°C until assay. We measured plasma T concentration using the Coat-A-Count Total Testosterone radioimmunoassay kit (Diagnostic Products). The minimum detectable plasma T concentration was 0.20 ng/ml. The intra-assay coefficient of variation was ~5%, and the inter-assay coefficient of variation was 12%. Samples with undetectable levels of steroid were treated as having concentrations at the assay detection limit for statistical analysis.
Sacrifice, perfusion, tissue processing
We deeply anesthetized the birds with isoflurane inhalation and perfused them through the heart with heparinized saline followed by 4% phosphate-buffered paraformaldehyde (pH = 7.4). We post-fixed the brains in 4% paraformaldehyde for 24 hrs, embedded the brains in gelatin, and post-fixed the gelatin-embedded brains in a 20% sucrose–neutral buffered formalin solution for 48 hrs. We sectioned the brains in the coronal plane at 40 μm on a freezing microtome, mounted every third section, and stained with thionin. We kept alternate sections in saline (0.7%) for immunohistochemistry (see below).
Measurement of nuclei volume
Using an overhead projector, we traced the borders in both hemispheres throughout the full rostral-caudal extent of HVC, RA, and Area X. We scanned these tracings into a microcomputer and measured the surface area of each cross section using a customized module of NIH Image (W. Rasband, NIH). We determined the volumes of nuclei by summing the estimated volume between sections calculated with the formula for the volume of a cone frustum (Tramontin et al., 1998).
Measurement of neuronal attributes in HVC and RA
We sampled neuron size by measuring the cross-sectional area of the soma in every mounted section throughout the rostral-caudal axis of HVC and RA. We distinguished neurons from glia by their single nucleolus and uniform, nongranular cytoplasm. We used a random, systematic sampling protocol that has been previously described and validated (Tramontin et al., 1998), which yields estimates of neuronal density and size that do not differ from those obtained using the stereological optical dissector method. We measured the somatic area of neurons in HVC and RA per hemisphere of each bird in fields chosen randomly by computer in each section. In thin sections there is a likelihood of splitting of neuronal nuclei between sections, which could overestimate cell counts (Coggeshall and Lekan, 1996; West, 1993) To estimate neuron density, we therefore counted neuronal nucleoli in every field and used Konigsmark’s (Nauta et al., 1970) formula #4 to correct for nucleus splitting:
where N is the number of nucleoli present, n is the number of nucleoli counted, t is the section thickness in micrometers, r is the nucleolus radius, and k is the diameter of the uncounted fragments of nucleoli. We set k equal to 1 μm, which equaled the smallest nucleolus fragment encountered in this study. Konigsmark-corrected neuron counts were divided by the volume of the tissue sampled to obtain neuronal density.
We estimated neuron number by multiplying neuron density by total nucleus volume. We sampled at least 150 HVC neurons and 100 RA neurons throughout the rostral-caudal extent of each nucleus in each brain. These sample sizes are sufficient to encompass the entire range of variability in neuron density and somatic area in these nuclei, based on Tramontin et al. (Tramontin et al., 1998). All measurements were made blind to treatment and the time of sacrifice for each bird.
Primary Antibody Characterization
The rabbit polyclonal anti-activated caspase-3 antibody (1:1000, ab13847, AbCam) was raised against a synthetic peptide conjugated to KLH derived from within residues 150–250 of human active caspase-3. In Western blots, this antibody detected the activated form of caspase-3 (17 kDa) from lysates taken from Hela cells, A431 cells, and Jurkat cells. Lysates incubated with the synthetic peptide detected no activated caspase-3 (manufacturer’s datasheet). We previously showed that in vivo infusion of caspase inhibitors reduces expression of activated caspase-3 using this antibody (Thompson and Brenowitz, 2008).
The rabbit polyclonal anti-cleaved PARP antibody (1:800, G7431, Promega) was raised against p 85 fragment of the N-terminal for Human PARP that is only present after cleavage by caspases. In Western blots, this antibody detected the p85 fragment in protein extracted from Jurkat cells undergoing Fas-mediated apoptosis or caspase cleaved bovine PARP but failed to detect the full length (116 kDa) bovine PARP (manufacturer’s datasheet). To the best to our knowledge, this particular antibody has not been used in brain tissue, but the observation that the resulting label was reduced by T infusion, similar to the reduction seen in activated caspase-3 (see results below), suggests that it is specific for cleaved PARP.
Activated caspase-3 immunohistochemistry
To determine whether intracerebral T infusion decreased caspase activation in HVC, we performed immunohistochemistry for activated caspase-3, a protease that cleaves a variety of proteins that mediate programmed cell death. We rinsed floating sections containing contralateral and ipsilateral HVC in three washes of 0.1M phosphate-buffered saline with 0.1% Triton-X100 (PBS-TX; pH = 7.4) and transferred them to 90–95°C 10mM sodium citrate (pH = 6.0) for 20 min. We allowed the sections to cool to room temperature for 20 min, rinsed them in PBS-TX three times, preblocked the tissue in 10% normal goat serum for 1 hour, and incubated the tissue with a primary antibody against the activated form of caspase-3 (AbCam Inc, Cambridge, MA; 1:1000 in 10% NGS) overnight at 4°C. We washed the sections in three washes of PBS-TX, blocked endogenous peroxidase activity with 10 min of 3% H2O2 in 10% MeOH and 0.1M PBS, washed the sections in three washes of PBS-TX, and incubated the sections for 45 min in 0.01M PBS containing the secondary antibody (1:200). We visualized labeling with avidin–biotin amplification (Vectastain Elite; Vector Laboratories, Burlingame, CA) and diaminobenzidine chromagen (DAB). Negative control tissue was prepared by omitting either the primary or the secondary antibody. These sections showed no staining of cells or neuropil. We mounted the sections onto gelatin-subbed slides, let them air dry overnight, and coverslipped the slides with DPX mountant.
To quantify the activation of caspase-3, we scanned the entire cross-sectional area of HVC and counted all cells within the border of HVC that were positive for activated caspase-3. We considered cells to be positive for activated caspase-3 if they had intense DAB cytosolic label that was considerably higher than background, and had either normal somal morphology or clusters of degenerated cell fragments that are typical of cells undergoing pyknosis. Small fragments that were not observed in clusters, as well as detached fragments of dendritic and axonal arbors, were not counted. We counted cells in every section spanning the rostral-caudal extent of HVC, usually at least six sections per hemisphere. The observer was blind to treatment and day of sacrifice.
Cleaved PARP immunohistochemistry
To further determine whether T infusion effectively decreased programmed cell death, we also performed immunohistochemistry for cleaved PARP, a product of activated caspases that is typical of cells undergoing apoptosis. We rinsed floating sections containing contralateral and ipsilateral HVC in three washes of 0.1M PBS-TX, preblocked the tissue in 10% normal goat serum for 1 hour, and incubated the tissue with a primary antibody against cleaved PARP (Promega, Madison, WI; 1:800 in 10% NGS) overnight at 4°C. We washed the sections in three washes of PBS-TX, blocked endogenous peroxidase activity with 10 min of 3% H2O2 in 10% MeOH and 0.1M PBS, washed the sections in three washes of PBS-TX, and incubated the sections for 45 min in in 0.01M PBS containing the secondary antibody (1:200). We visualized labelling with avidin–biotin amplification (Vectastain Elite; Vector Laboratories, Burlingame, CA) and DAB with nickel-enhancement. Negative control tissue was prepared by omitting either the primary or the secondary antibody. These sections showed no staining of cells or neuropil. We mounted the sections onto gelatin-subbed slides, let them air dry overnight, and coverslipped the slides with DPX mountant.
To quantify cells positive for cleaved PARP, we scanned the entire cross-sectional area of HVC and counted all cells within the border of HVC that showed positive punctate staining predominantly within the nucleus. We counted cells in every section spanning the rostral-caudal extent of HVC. The observer was blind to treatment and day of sacrifice.
Statistics
We used paired t-tests to compare ipsilateral to contralateral attributes. We used two-way ANOVA followed by Bonferroni post-hoc tests to determine if there were changes in neuronal attributes within each hemisphere between three and seven days. In order to assess whether the neuronal attributes of contralateral song control nuclei regressed as expected, we compared the data presented here to previously published data (Thompson et al., 2007) using two-way ANOVA and a series of t-tests. The alpha level for all tests was 0.05. We used one-tailed tests on attributes for which there was a clear directional hypothesis, and two-tailed tests for all other comparisons.
Results
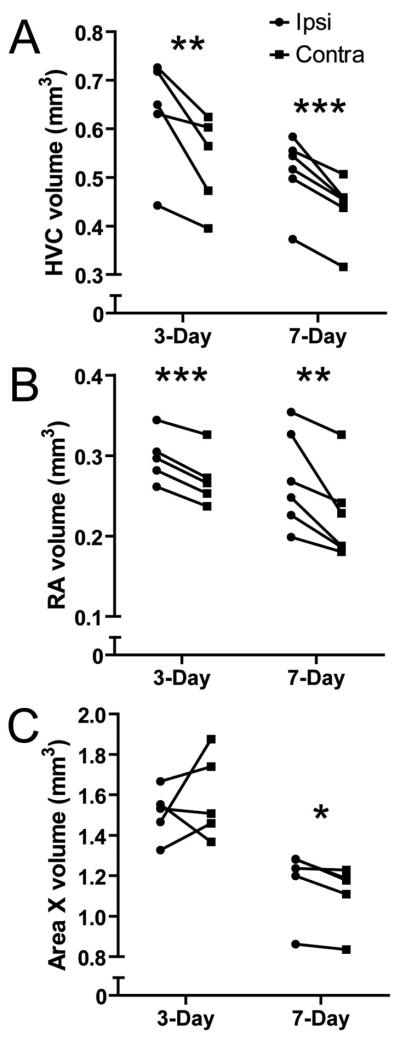
We measured the volume of song nuclei from Nissl-stained sections. Given that the projection from HVC to RA is unilateral and bilaterally symmetrical, and there are no known direct connections between the right and left HVC, unilateral infusion of T leaves the untreated contralateral hemisphere to serve as a within-animal control. To determine whether the contralateral song control system regressed normally, we compared measurements of contralateral neuronal attributes to the results of a previously published study which examined the time course of song control system regression (Thompson et al., 2007). We found that the contralateral song control system in the present study regressed as expected, based on the previously published data (Thompson et al., 2007; Thompson and Brenowitz, 2008; 2009) (These analyses are explained in detail in supplemental materials). We therefore compared measurements ipsilateral to the infusion to the contralateral hemisphere with paired t-tests (α = 0.05, one-tailed). Infusion of T significantly rescued ipsilateral HVC volume three days (p = 0.0125) and seven days (p = 0.0007) after transition to nonbreeding conditions (Fig 2a). Infusion of T significantly rescued ipsilateral RA volume three days (p = 0.0002) and seven days (p = 0.0069) after transition to nonbreeding conditions (Fig 2b). Infusion of T had no significant effect on ipsilateral Area X volume relative to contralateral Area X volume three days after transition to nonbreeding conditions (p = 0.227) but did significantly rescue ipsilateral Area X volume seven days (p = 0.0156) after transition (Fig 2c). This was expected given that significant regression of Area X volume does not occur till seven days following the transition to nonbreeding conditions (Thompson et al., 2007). Measurements for Area X from one bird in the 7-Day group were excluded due to poor histology. We used two-way ANOVA with day of sacrifice as one factor and hemisphere as the second factor to determine whether the volumes of song nuclei differed between three and seven days. We found that HVC and Area X volume differed between time points (p = 0.0101 and > 0.0001, respectively), but Bonferroni post-hoc tests failed to find significant differences within hemispheres across time. RA volume did not significantly vary across time.
Figure 2.
Infusion of T near HVC prevents regression of song nuclei volume. Infusion of T near HVC protects against the regression of A) HVC volume B) RA volume and C) Area X volume up to seven days after the transition from breeding to nonbreeding conditions. Asterisks indicate a significant difference between hemispheres (one-tailed paired t-test; * = p ≤ 0.05, ** = p ≤ 0.01, *** = p ≤ 0.005).
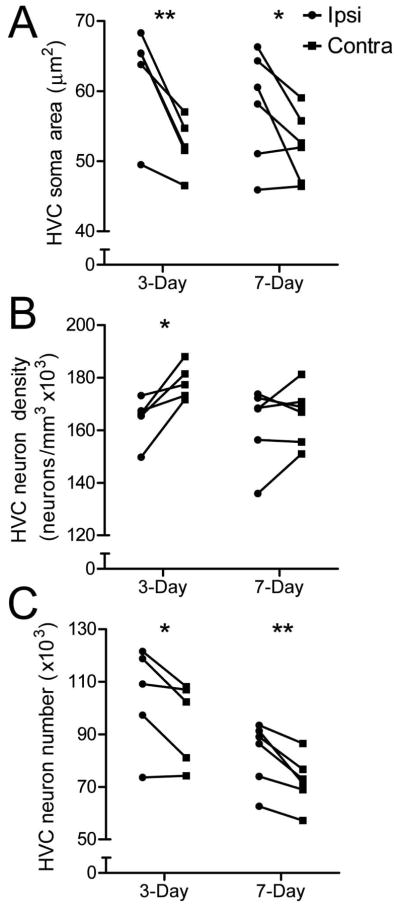
We measured HVC neuronal attributes from Nissl-stained sections using a random, systematic counting scheme (Tramontin et al., 1998). T infusion significantly preserved the soma area of HVC neurons three days (p = 0.0052) and seven days (p = 0.0324) after transition to nonbreeding conditions (Fig 3a). The regression of HVC volume due to the withdrawal of T and shift to SD photoperiod is initially mediated by an increase in neuron density over the first few days followed by a subsequent decrease in neuron number (Thompson et al., 2007). Consistent with these observations, infusion of T prevented HVC neuron density from increasing three days after the transition to nonbreeding conditions (p = 0.0108) (Fig 3b). At seven days, infusion of T had no effect on HVC neuron density (p = 0.254) (Fig 3b). T-infusion significantly protected HVC neuron number (p = 0.0294) and seven days (p = 0.0125) after the transition to nonbreeding conditions (Fig 3c). We used two-way ANOVA to determine whether HVC neuronal attributes changed over the two time points in the study and found that HVC neuron number changed significantly between three and seven days (p = 0.0023). Bonferroni post-hoc tests failed to find significant differences within hemispheres across time, however. Two-way ANOVA found that HVC neuron density and soma area did not significantly between these time points.
Figure 3.
T infusion near HVC prevents regression of HVC neuronal attributes. Infusion of T near HVC prevents regression of A) HVC soma area three and seven days after transition to nonbreeding conditions, B) HVC neuron density three days after transition to nonbreeding conditions and C) HVC neuron number seven days after the transition from breeding to nonbreeding conditions. Asterisks indicate a significant difference between hemispheres (one-tailed paired t-test; * = p ≤ 0.05, ** = p ≤ 0.01)
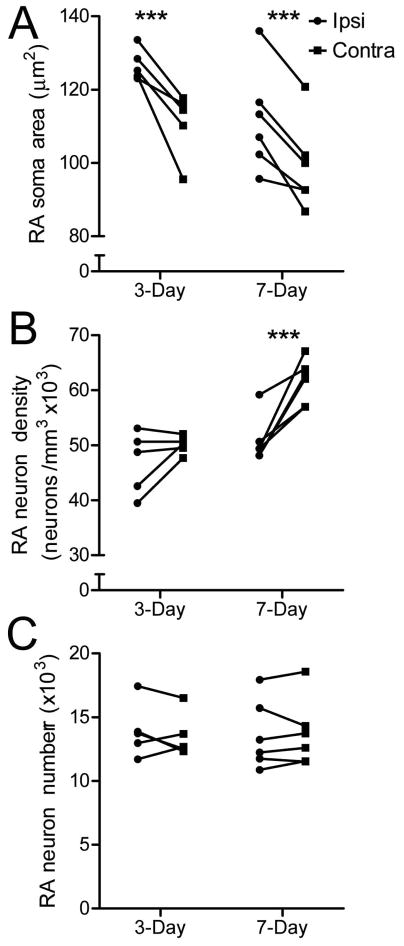
T-infusion near HVC protected several neuronal attributes of ipsilateral RA, an efferent target of HVC. Infusion of T significantly preserved RA soma area three and seven days after the transition to nonbreeding conditions (p = 0.0048 and 0.0016, respectively) (Fig 4a). Infusion of T had no effect on RA neuron density three days after the transition (p = 0.0951) but protected RA neuron density from regression seven days after the transition (p = 0.0021) (Fig 4b). T infusion had no effect on RA neuron number in the three day or seven day groups (p = 0.247 and 0.390, respectively) (Fig 4c). We used two-way ANOVA to determine whether RA neuronal attributes changed over time. We found that RA soma area and neuron density significantly differed between three and seven days (p = 0.0098 and 0.0004, respectively). Bonferroni post-hoc tests found that contralateral RA neuron density was significantly greater at seven days compared to three days. Ipsilateral RA neuron density did not change significantly over time. Bonferroni post-hoc tests failed to find significant differences within hemispheres across time for RA soma area. RA neuron number did not significantly vary over time.
Figure 4.
Infusion of T near HVC prevents regression RA neuronal attributes. T infusion near HVC prevents regression of A) RA soma area three and seven days after the transition to nonbreeding conditions, B) RA neuron density seven days after the transition to nonbreeding conditions and C) has no effect on RA neuron number. Asterisks indicate a significant difference across hemispheres (one-tailed paired t-test; *** = p ≤ 0.005)
T infusion reduced the incidence of cells in HVC positive for activated caspase-3 three days after T-withdrawal and photoshift (Fig 5a). We found that T infusion reduced the density of activated caspase-3 positive cells (p = 0.0091), total number of activated caspase-3 positive cells (p = 0.0169), and the number of activated caspase-3 positive cells per 1000 neurons (p = 0.0082) in the ipsilateral HVC (Fig 5b). Due to technical issues, we evaluated activated caspase-3 expression in only three animals sacrificed seven days after T-withdrawal and photoshift. T infusion had no further effect on density of activated caspase-3 positive cells (p = 0.181), total number of activated caspase-3 positive cells (p = 0.297), or number of activated caspase-3 positive cells per 1000 neruons (p = 0.414) (supplemental Figure 1a). There was a 48.2% reduction in the average number of activated caspase-3 positive cells in the contralateral HVC from three days to seven days.
Figure 5.
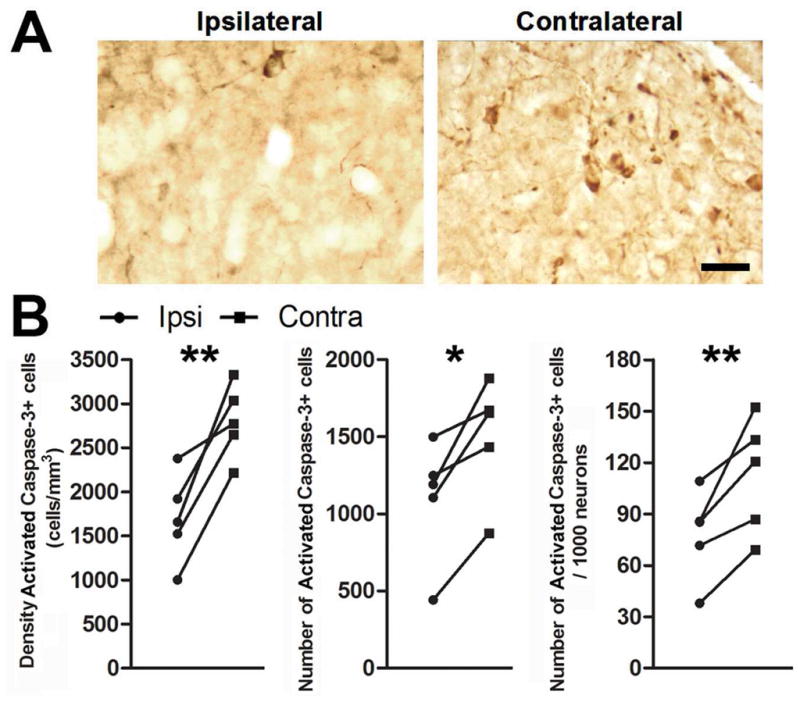
T infusion near HVC prevents activation of caspase-3 in HVC. Photomicrographs of cells positive for caspase-3 activation in HVC A) ipsilateral and B) contralateral to infusion of T. C) Infusion of T near HVC reduces the density, total number, and number/1000 neurons of activated caspase-3 positive cells in HVC three days after transition to nonbreeding conditions. Asterisks indicate a significant difference across hemispheres (one-tailed paired t-test; ** = p ≤ 0.01, *** = p ≤ 0.005). Scale bar = 20 μm
T infusion also reduced the incidence of cells positive for cleaved PARP three days after T-withdrawal and photoshift (Fig 6a). We found that T infusion reduced the density of cleaved PARP positive cells (p = 0.0072), total number of cleaved PARP positive cells (p = 0.0114), and number of cleaved PARP positive cells per 1000 neurons (p = 0.0006) in the ipsilateral HVC (Fig 6b). Poor histology in one of the animals sacrificed at seven days precluded statistical quantification of cleaved PARP positive cells (supplemental Figure 1b). The data from the remaining two animals suggest that cleaved PARP was reduced by 56.9% in the contralateral HVC seven days after the transition to nonbreeding conditions.
Figure 6.
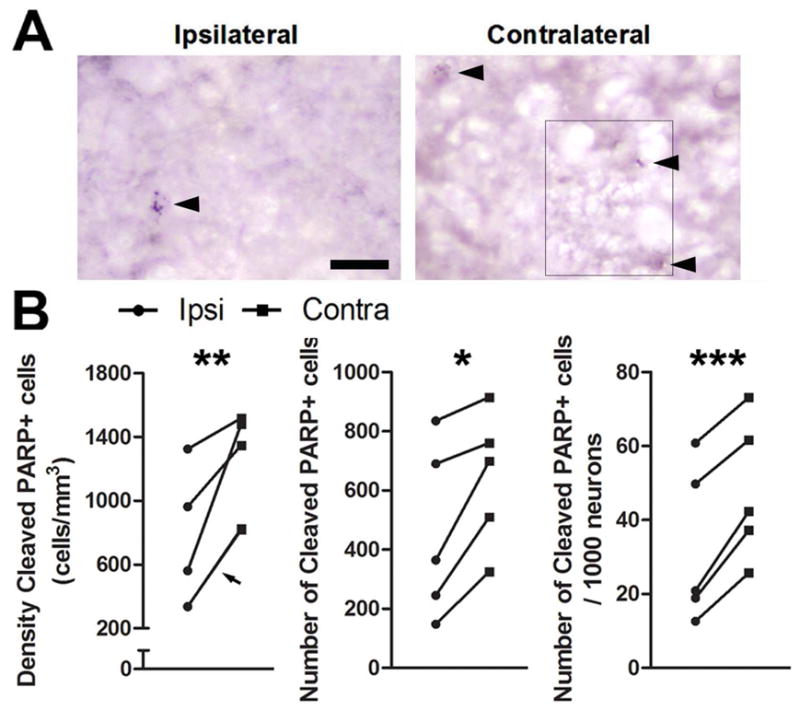
T infusion near HVC prevents an increase in cleaved PARP in HVC. Photomicrographs of cells positive for cleaved PARP in HVC A) ipsilateral and B) contralateral to infusion of T. The photo is a montage of images because cells positive for cleaved PARP were in different planes of focus. Where images were spliced is indicated by a thin black line. Arrowheads indicate cells with punctate staining over the nucleus. C) Infusion of testosterone near HVC reduces the density, total number, and number/1000 neurons of cleaved PARP positive cells in HVC in birds sacrificed three days after transition to nonbreeding conditions. Arrowhead indicates measurements for two animals that share nearly identical values and thus have overlapping symbols. Asterisks indicate a significant difference across hemispheres (one-tailed paired t-test; * = p ≤ 0.05, ** = p < 0.01) Scale bar = 20μm
We measured circulating levels of T of each bird 7–14 days after the initiation of LD+T and again at sacrifice. While birds were under breeding conditions, mean T levels were 9.08 ± 1.10 ng/ml (mean ± SEM, n = 11; one bird was excluded from analysis due to insufficient volume of plasma collected). We collected plasma at sacrifice three and seven days after withdrawal of circulating T and transition to SD photoperiod. T levels at sacrifice were significantly reduced (0.69 ± 0.16 ng/ml, n = 8, p < 0.001). This result demonstrates that while the amount of T infused from the cannula and osmotic pump was sufficient for neuroprotection in HVC, it did not elevate systemic circulating levels of T. We excluded three birds from analysis due to insufficient volume of plasma collected, but visual inspection after sacrifice confirmed that these birds had no testes and they were therefore included in the study. Level of circulating T from one animal at sacrifice was much higher than expected (7.31 ng/ml). We excluded this datum from statistical analysis of circulating T because Chauvenet’s criterion indicated that it was an outlier. Morphometric data from this animal was included, however, given that visual inspection confirmed that this bird had no testes at sacrifice and that the neuronal attributes of the contralateral song control system regressed as would be expected following transition to nonbreeding conditions.
Discussion
Infusion of T near HVC was sufficient to preserve HVC and its efferent nuclei from degeneration due to withdrawal of circulating T and transition to SD photoperiod (nonbreeding conditions) in adult male white-crowned sparrows. Song control system nuclei normally regress rapidly in response to the transition to nonbreeding conditions (Thompson et al., 2007). We found that continuous infusion of T near HVC prevented this regression for at least seven days. T-infusion had protective effects on every neuronal attribute of HVC that we measured, including a significant preservation of neuron number. In addition, we observed that there were high numbers of activated caspase-3 and cleaved PARP positive cells in the contralateral HVC three days after the transition to nonbreeding conditions, and that in vivo infusion of T reduced the activation of caspase-3 and PARP cleavage in the ipsilateral HVC. Lastly, we showed that T-mediated protection of HVC neurons was sufficient to also protect RA and Area X from degeneration.
There is much interest in the role that sex steroids play in neuroprotection. T has neuroprotective effects in vitro (Ahlbom et al., 2001; Pike, 2001) and in some (Pike et al., 2006; Ramsden et al., 2003) but not all (Nishino et al., 1998; Yang et al., 2002) in vivo animal models of neuronal injury. Our results, however, are the first to demonstrate that T can act locally in the brain to prevent neurodegeneration. T upregulates the expression of anti-apoptotic genes such as bcl-2 in the SNB in male rats (Zup and Forger, 2002). The T metabolite E2 promotes the expression of several anti-apoptotic genes (Chiueh et al., 2003; Dubal et al., 1999; Pike, 1999; Singer et al., 1998; Stoltzner et al., 2001; Wu et al., 2005). In zebra finches, neural insult is accompanied by an increase in expression of aromatase (CYP19), an enzyme that converts T to E2 (Peterson et al., 2001; Wynne and Saldanha, 2004), and inhibition of aromatase increases apoptosis near the injury (Saldanha et al., 2005; Wynne et al., 2008). These results are consistent with the neuroprotective role of E2 in the mammalian brain (Suzuki et al., 2006), though it should be noted that E2 can have the opposite effect of activating programmed cell death in neurons under certain circumstances (Waters and Simerly, 2009). Nevertheless, the infusion of T near HVC may reduce programmed cell death by activating AR and/or ER (via aromatization to E2). We found that infusion of T reduced the number and density of activated caspase-3 and cleaved PARP positive cells in the ipsilateral HVC three days after the transition to nonbreeding conditions. These results are consistent with the hypothesis that T acts directly on HVC neurons to block programmed cell death pathways. Infusion of T near HVC may contribute to the increased survival of HVC neurons through the upregulation of anti-apoptotic genes. The reduction in neurodegeneration is no longer apparent at seven days after the transition to nonbreeding conditions. This result seems to be due primarily to a transient increase in neurodegeneration following the transition to nonbreeding conditions; the number of activated caspase-3 and cleaved PARP positive cells in the contralateral HVC are substantially reduced from three days to seven days.
We previously found that infusion of caspase inhibitors near HVC protected its neuronal attributes for up to seven days (Thompson and Brenowitz, 2008). The results of the current study show that infusion of T has a similar effect on HVC attributes; both treatments prevented the decrease in HVC volume that normally occurs within 12 hours after transition to nonbreeding conditions, prevented a significant loss of HVC neurons, and prevented the transient increase in HVC neuron density that normally happens three days after the transition to nonbreeding conditions. In addition, the infusion of T reduced the numbers and density of activated caspase-3 positive cells in the ipsilateral HVC; caspase inhibitor infusion similarly prevented the activation of caspase-3 in HVC (Thompson and Brenowitz, 2008).
We did not detect significant changes in ipsilateral neuronal attributes between three and seven days in the ANOVA analyses. This shows that our T-infusions successfully prevented regression of the ipsilateral song control system. There may be a trend for partial regression for some attributes in ipsilateral HVC, in particular volume (Fig 2a) and neuron number (Fig 3c). There are two potential explanations for this observation. First, the dose of infused T may not have been high enough to fully protect HVC from regression. We used a T dose based on the results of Meitzen et al (2007); they also saw an effect of steroid hormone infusion that was sufficient to induce changes in neuron size and electrical activity in RA but that was not completely effective. A higher concentration of T may be more effective in fully protecting HVC from regression. Second, other factors may contribute to full neuroprotection in HVC. Photoperiod and song production modulate seasonal changes in the song system (Sartor and Ball, 2005; Smith et al., 1997). In our study birds were kept on short-days and did not sing. Complete neuroprotection of HVC may require one or more of these additional factors. The role of such factors, however, is minor compared with that of T, given the lack of significant regression of attributes of the ipsilateral HVC.
The most striking differences between the infusion of caspase inhibitors and T can be seen in their effects on the efferent nuclei of HVC: RA and Area X. Caspase inhibitor infusion near HVC provided minimal protection of efferent nuclei. The only attribute rescued was RA soma area seven days after the transition to nonbreeding conditions, and even that rescue, though statistically significant, did not fully preserve RA soma area to the levels seen in birds held under breeding conditions. In contrast, T-infusion near HVC provided robust transsynaptic trophic support to RA and Area X, significantly protecting RA volume, RA neuron density, RA soma area, and Area X volume. Taken together, these results strongly suggest that T, acting directly on HVC neurons, potentiates substantial transsynaptic support to RA and Area X, whereas caspase inhibitors induce minimal trophic support to the efferent nuclei. Despite the fact that caspase inhibitors protect HVC neurons from degeneration, the transsynaptic signal that potentiates hypertrophied efferent nuclei is largely absent. T and/or its metabolites increase expression of BDNF mRNA in HVC neurons (Rasika et al., 1999; Wissman and Brenowitz, 2009), and 5α-DHT and E2 infused near HVC increase the spontaneous firing rate of RA neurons (Meitzen et al., 2007). T increases the intensity of BDNF receptor immunolabeling in the spiny motoneurons of the spinal nucleus of the bulbocavernosus (SNB) (Osborne et al., 2007), which undergoes morphological and functional degeneration following the withdrawal of circulating T (Little et al., 2008; Park et al., 2002). In addition, there is evidence that T and BDNF must interact in order to prevent the regression of SNB motoneuron dendrites (Yang et al., 2004). These and/or other factors may contribute to the transsynaptic trophic signal from HVC to its efferent nuclei.
We previously showed that HVC volume regresses by 22% within 12 hours after the withdrawal of circulating T (Thompson et al., 2007). It has been suggested that this regression was due to generalized stress induced by the brief (ca. 10 min) castration surgery rather than to T withdrawal (Charlier et al., 2008). The results of the current study, however, rule out this suggestion. Two days prior to the withdrawal of circulating T, the birds in the current study underwent a 90 minute cannula implantation surgery, a procedure which is certainly more stressful than the brief castration surgery used in Thompson et al., 2007. If stress from a surgical procedure really was the cause of the regression of song nuclei independent of T levels, then one would expect that the ipsilateral HVC in birds infused with T would be significantly regressed at three days following the transition to nonbreeding conditions. Ipsilateral HVC, as we showed above, was not significantly regressed. We therefore conclude that rapid regression of the song control system is mediated by the withdrawal of circulating T and is not the result of surgery-related stress.
We showed that constant in vivo infusion of T near HVC protects ipsilateral song system nuclei from neurodegeneration. T-infusion near HVC protected it from significant neuron loss and reduced the expression of activated caspase-3 and cleaved PARP. In addition, T-infusion near HVC protected RA and Area X neuronal attributes more substantially than did infusion caspase inhibitors. Our results indicate that seasonal-like rapid regression of the song control system serves as an excellent model to further elucidate the molecular mechanisms that underlie hormone-mediated neuroprotection.
Acknowledgments
Grants and Fellowship information: Supported by NIH MH53032 and 5 T32-GM07108.
The authors would like to thank K. Lent and P. Berberian for technical assistance.
References
- Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Research. 2001;892(2):255–262. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- Beauchet O. Testosterone and cognitive function: current clinical evidence of a relationship. Eur J Endocrinol. 2006;155(6):773–781. doi: 10.1530/eje.1.02306. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Wilson FE, Ball GF. Testis-dependent and -independent effects of photoperiod on volumes of song control nuclei in American tree sparrows (Spizella arborea) Brain Res. 1997;760(1–2):163–169. doi: 10.1016/s0006-8993(97)00277-1. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Lent K. Afferent input is necessary for seasonal growth and maintenance of adult avian song control circuits. J Neurosci. 2001;21(7):2320–2329. doi: 10.1523/JNEUROSCI.21-07-02320.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz EA, Lent K. Act locally and think globally: intracerebral testosterone implants induce seasonal-like growth of adult avian song control circuits. Proc Natl Acad Sci U S A. 2002;99(19):12421–12426. doi: 10.1073/pnas.192308799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SD, Bottjer SW. Testosterone-induced changes in adult canary brain are reversible. Journal of neurobiology. 1993;24(5):627–640. doi: 10.1002/neu.480240508. [DOI] [PubMed] [Google Scholar]
- Charlier TD, Ball GF, Balthazart J. Rapid action on neuroplasticity precedes behavioral activation by testosterone. Hormones and Behavior. 2008 doi: 10.1016/j.yhbeh.2008.03.001. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiueh C, Lee S, Andoh T, Murphy D. Induction of antioxidative and antiapoptotic thioredoxin supports neuroprotective hypothesis of estrogen. Endocrine. 2003;21(1):27–31. doi: 10.1385/endo:21:1:27. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364(1):6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc Natl Acad Sci U S A. 1999;96(13):7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM. Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J Neurosci. 1999;19(15):6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JA, Jordan CL, Breedlove SM. Steroid hormone masculinization of neural structure in rats: a tale of two nuclei. Physiol Behav. 2004;83(2):271–277. doi: 10.1016/j.physbeh.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Johnson F, Bottjer SW. Hormone-induced changes in identified cell populations of the higher vocal center in male canaries. J Neurobiol. 1993;24(3):400–418. doi: 10.1002/neu.480240311. [DOI] [PubMed] [Google Scholar]
- Little CM, Coons KD, Sengelaub DR. Neuroprotective effects of testosterone on the morphology and function of somatic motoneurons following the death of neighboring motoneurons. J Comp Neurol. 2008;512(3):359–372. doi: 10.1002/cne.21885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Prange-Kiel J, Leranth C. Androgen modulation of hippocampal synaptic plasticity. Neuroscience. 2006;138(3):957–965. doi: 10.1016/j.neuroscience.2005.12.054. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Moore IT, Lent K, Brenowitz EA, Perkel DJ. Steroid Hormones Act Transsynaptically within the Forebrain to Regulate Neuronal Phenotype and Song Stereotypy. J Neurosci. 2007;27(44):12045–12057. doi: 10.1523/JNEUROSCI.3289-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Thompson CK. Seasonal-like growth and regression of the avian song control system: Neural and behavioral plasticity in adult male Gambel’s white-crowned sparrows. Gen Comp Endocrinol. 2008 doi: 10.1016/j.ygcen.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I, Dellovade TL, Shughrue PJ. Neuroprotection by estrogen in animal models of global and focal ischemia. Ann N Y Acad Sci. 2003;1007:89–100. doi: 10.1196/annals.1286.009. [DOI] [PubMed] [Google Scholar]
- Nauta WJH, Ebbesson SOE. Contemporary research methods in neuroanatomy. New York: Springer-Verlag; 1970. National Institute of Neurological Diseases and Stroke., University of Puerto Rico (Râio Piedras Campus) p. viii.p. 386. [Google Scholar]
- Nishino H, Nakajima K, Kumazaki M, Fukuda A, Muramatsu K, Deshpande SB, Inubushi T, Morikawa S, Borlongan CV, Sanberg PR. Estrogen protects against while testosterone exacerbates vulnerability of the lateral striatal artery to chemical hypoxia by 3-nitropropionic acid. Neuroscience Research. 1998;30(4):303–312. doi: 10.1016/s0168-0102(98)00010-8. [DOI] [PubMed] [Google Scholar]
- Osborne MC, Verhovshek T, Sengelaub DR. Androgen regulates trkB immunolabeling in spinal motoneurons. J Neurosci Res. 2007;85(2):303–309. doi: 10.1002/jnr.21122. [DOI] [PubMed] [Google Scholar]
- Panzica G, Viglietti-Panzica C, Balthazart J. Sexual dimorphism in the neuronal circuits of the quail preoptic and limbic regions. Microsc Res Tech. 2001;54(6):364–374. doi: 10.1002/jemt.1149. [DOI] [PubMed] [Google Scholar]
- Park JJ, Zup SL, Verhovshek T, Sengelaub DR, Forger NG. Castration reduces motoneuron soma size but not dendritic length in the spinal nucleus of the bulbocavernosus of wild-type and BCL-2 overexpressing mice. J Neurobiol. 2002;53(3):403–412. doi: 10.1002/neu.10103. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Saldanha CJ, Schlinger BA. Rapid Upregulation of Aromatase mRNA and Protein Following Neural Injury in the Zebra Finch (Taeniopygia guttata) Journal of Neuroendocrinology. 2001;13(4):317–323. doi: 10.1046/j.1365–2826.2001.00647.x. [DOI] [PubMed] [Google Scholar]
- Pike CJ. Estrogen modulates neuronal Bcl-xL expression and beta-amyloid-induced apoptosis: relevance to Alzheimer’s disease. J Neurochem. 1999;72(4):1552–1563. doi: 10.1046/j.1471-4159.1999.721552.x. [DOI] [PubMed] [Google Scholar]
- Pike CJ. Testosterone attenuates [beta]-amyloid toxicity in cultured hippocampal neurons. Brain Research. 2001;919(1):160–165. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Rosario ER, Nguyen TV. Androgens, aging, and Alzheimer’s disease. Endocrine. 2006;29(2):233–241. doi: 10.1385/ENDO:29:2:233. [DOI] [PubMed] [Google Scholar]
- Ramsden M, Shin TM, Pike CJ. Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience. 2003;122(3):573–578. doi: 10.1016/j.neuroscience.2003.08.048. [DOI] [PubMed] [Google Scholar]
- Rasika S, Alvarez-Buylla A, Nottebohm F. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22(1):53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Rohmann KN, Coomaralingam L, Wynne RD. Estrogen provision by reactive glia decreases apoptosis in the zebra finch (Taeniopygia guttata) J Neurobiol. 2005;64(2):192–201. doi: 10.1002/neu.20147. [DOI] [PubMed] [Google Scholar]
- Sartor JJ, Ball GF. Social suppression of song is associated with a reduction in volume of a song-control nucleus in European starlings (Sturnus vulgaris) Behav Neurosci. 2005;119(1):233–244. doi: 10.1037/0735-7044.119.1.233. [DOI] [PubMed] [Google Scholar]
- Singer CA, Rogers KL, Dorsa DM. Modulation of Bcl-2 expression: a potential component of estrogen protection in NT2 neurons. Neuroreport. 1998;9(11):2565–2568. doi: 10.1097/00001756-199808030-00025. [DOI] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Wingfield JC. Roles of photoperiod and testosterone in seasonal plasticity of the avian song control system. J Neurobiol. 1997;32(4):426–442. doi: 10.1002/(sici)1097-4695(199704)32:4<426::aid-neu6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Stoltzner SE, Berchtold NC, Cotman CW, Pike CJ. Estrogen regulates bcl-x expression in rat hippocampus. Neuroreport. 2001;12(13):2797–2800. doi: 10.1097/00001756-200109170-00009. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29(2):209–215. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Bentley GE, Brenowitz EA. Rapid seasonal-like regression of the adult avian song control system. Proc Natl Acad Sci U S A. 2007;104(39):15520–15525. doi: 10.1073/pnas.0707239104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Brenowitz EA. Caspase inhibitor infusion protects an avian song control circuit from seasonal-like neurodegeneration. J Neurosci. 2008;28(28):7130–7136. doi: 10.1523/JNEUROSCI.0663-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Brenowitz EA. Neurogenesis in an adult avian song nucleus is reduced by decreasing caspase-mediated apoptosis. J Neurosci. 2009;29(14):4586–4591. doi: 10.1523/JNEUROSCI.5423-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin AD, Hartman VN, Brenowitz EA. Breeding conditions induce rapid and sequential growth in adult avian song control circuits: a model of seasonal plasticity in the brain. J Neurosci. 2000;20(2):854–861. doi: 10.1523/JNEUROSCI.20-02-00854.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin AD, Smith GT, Breuner CW, Brenowitz EA. Seasonal plasticity and sexual dimorphism in the avian song control system: stereological measurement of neuron density and number. J Comp Neurol. 1998;396(2):186–192. doi: 10.1002/(sici)1096-9861(19980629)396:2<186::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Veliskova J, Velisek L, Galanopoulou AS, Sperber EF. Neuroprotective effects of estrogens on hippocampal cells in adult female rats after status epilepticus. Epilepsia. 2000;41(Suppl 6):S30–35. doi: 10.1111/j.1528-1157.2000.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Waters EM, Simerly RB. Estrogen Induces Caspase-Dependent Cell Death during Hypothalamic Development. J Neurosci. 2009;29(31):9714–9718. doi: 10.1523/JNEUROSCI.0135-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993;14(4):275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Farner DS. The annual cycle of plasma irLH and steroid hormones in feral populations of the white-crowned sparrow, Zonotrichia leucophrys gambelii. Biol Reprod. 1978;19(5):1046–1056. doi: 10.1095/biolreprod19.5.1046. [DOI] [PubMed] [Google Scholar]
- Wissman AM, Brenowitz EA. The role of neurotrophins in the seasonal-like growth of the avian song control system. J Neurosci. 2009;29(20):6461–6471. doi: 10.1523/JNEUROSCI.0638-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TW, Wang JM, Chen S, Brinton RD. 17Beta-estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience. 2005;135(1):59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Wynne RD, Saldanha CJ. Glial aromatization decreases neural injury in the zebra finch (Taeniopygia guttata): influence on apoptosis. J Neuroendocrinol. 2004;16(8):676–683. doi: 10.1111/j.1365-2826.2004.01217.x. [DOI] [PubMed] [Google Scholar]
- Wynne RD, Walters BJ, Bailey DJ, Saldanha CJ. Inhibition of injury-induced glial aromatase reveals a wave of secondary degeneration in the songbird brain. Glia. 2008;56(1):97–105. doi: 10.1002/glia.20594. [DOI] [PubMed] [Google Scholar]
- Yang LY, Verhovshek T, Sengelaub DR. Brain-derived neurotrophic factor and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinology. 2004;145(1):161–168. doi: 10.1210/en.2003-0853. [DOI] [PubMed] [Google Scholar]
- Yang S-H, Perez E, Cutright J, Liu R, He Z, Day AL, Simpkins JW. Testosterone increases neurotoxicity of glutamate in vitro and ischemia-reperfusion injury in an animal model. J Appl Physiol. 2002;92(1):195–201. doi: 10.1152/jappl.2002.92.1.195. [DOI] [PubMed] [Google Scholar]
- Zup SL, Forger NG. Testosterone regulates BCL-2 immunoreactivity in a sexually dimorphic motor pool of adult rats. Brain Res. 2002;950(1–2):312–316. doi: 10.1016/s0006-8993(02)03190-6. [DOI] [PubMed] [Google Scholar]