Abstract
Hepatic grafts from non-heartbeating donors may alleviate the organ shortage, but they inherently suffer from warm ischemia. In the present study, we tested our hypothesis that augmentation of endogenous adenosine by inhibition of nucleoside transport with R75231 attenuates ischemic liver injury. Adult female beagle dogs underwent 2-hr hepatic vascular exclusion with venovenous bypass. R75231 was given to the animals by continuous intravenous infusion for 30 min before ischemia at a dose of 0.1 mg/kg (Group 2, n=6), 0.05 mg/kg (Group 3, n=6), or 0.025 mg/kg (Group 4, n=6). Nontreated animals were used as the control (Group 1, n=10). Animal survival, hepatic tissue blood flow, liver function, and histopathology were analyzed. Two-week animal survival was 30% in Group 1, 88% in Group 2, 100% in Group 3, and 100% in Group 4. Postreperfusion hepatic tissue blood flow was markedly improved by the treatment. Treatment significantly attenuated liver enzyme release, lipid peroxidation, and changes in adenine nucleotides and purine catabolites. Structural abnormality of the liver after reperfusion was markedly improved by R75231 treatment, showing better architecture and less neutrophil infiltration. Preischemic administration of a nucleoside transport inhibitor ameliorated ischemic liver injury due to the positive effects of augmented endogenous adenosine, and is applicable clinically when the liver is procured from a controlled non-heartbeating donor.
Rapid progress in and increased demand for liver transplantation have created a serious organ shortage. The problem can presently be alleviated by organs procured from non-heartbeating donors (1), split liver transplantation (2), and living-related grafting (3), while xenotransplantation would provide a permanent solution to the shortage of organs in the future (4). When transplanting livers obtained from non-heartbeating donors, the donor condition and duration of warm ischemia become major determinants in graft outcome (1). Livers procured under controlled circumstances have favorable results, but graft-related complications frequently occur if warm ischemia time is prolonged. Livers from uncontrolled non-heartbeating donors have poor outcomes. Strategies to protect the liver from damage by warm ischemia, hypothermic storage, and/or subsequent reperfusion are essential.
Liver ischemia causes progressive degradation of adenosine triphosphate (ATP),* leading to an immense accumulation of purine catabolites in ischemic tissues. Adenosine, one of the intermediary products of the degradation cascade, has been known to exert various biological actions (5–10) such as increased blood flow, vasodilation, inhibition of free radical production, suppression of neutrophil activation, and prevention of platelet aggregation. Upon reoxygenation, adenosine becomes an important substrate for ATP resynthesis. However, adenosine is rapidly deaminated into inosine during ischemia and transported out of the ischemic tissues after reperfusion, thereby depriving ischemic tissues of adenosine’s beneficial effects. In this study, in an attempt to establish a strategy for the use of livers from non-heartbeating donors, we first tested our hypothesis that augmentation of endogenous adenosine by inhibiting its transport with a nucleoside transport inhibitor, R75231, attenuates warm ischemic liver injury, using a 2-hr total hepatic vascular exclusion model.
MATERIALS AND METHODS
Animals
Adult female beagle dogs, weighing 8–12 kg, were anesthetized, after overnight fasting, with an intravenous injection of thiopental sodium, 25 mg/kg. They were intubated and the anesthesia was maintained by positive mechanical ventilation with isoflorane, nitrous oxide, and oxygen. The right cartoid artery and the right jugular vein were cannulated for arterial blood pressure monitoring and for serial blood collection.
Operative procedures
After midline laparotomy, the liver was isolated by dissecting all of the suspensory ligaments and the retro-hepatic vena cava. Hepatic vascular exclusion was achieved by cross-clamping the portal vein and the hepatic artery together with the hepatoduodenal ligament, and the suprahepatic vena cava and the infrahepatic vena cava separately. Congestion of the mesenteric and lower systemic venous beds during hepatic ischemia was decompressed by a venovenous bypass using a centrifugal pump (Biomedicus, Minetonka, MN) connected by Tygon tubings to the left femoral vein, the splenic vein and the left jugular vein (Fig. 1). For anticoagulation, 50 U/kg of heparin sodium was administered systemically 5 min before the initiation of hepatic ischemia. After 2-hr of ischemia the liver was reperfused, bypass was stopped, and a splenectomy was performed.
Figure 1.
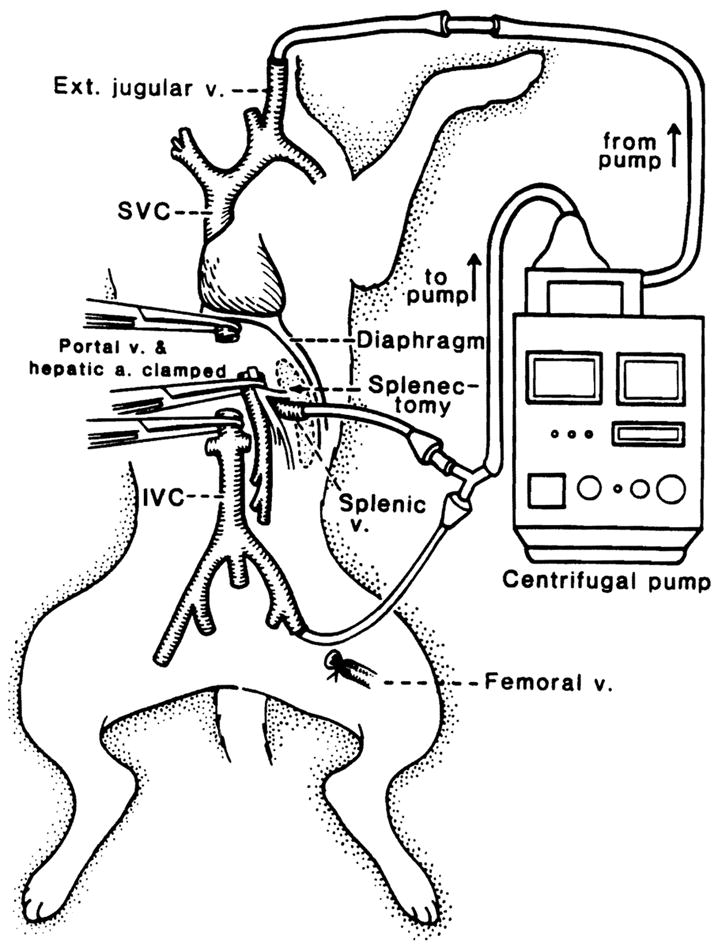
Schematic diagram of total hepatic vascular exclusion of the canine liver with venovenous bypass.
Cephamandole nafate 1 g was given intraoperatively and continued for 3 postoperative days. Animals were followed for two weeks.
Experimental groups
The nucleoside transport inhibitor R75231 was supplied by the Janssen Research Foundation (Dr. H. Van Belle) as a 1 mg/ml solution. It was dissolved in 20 ml of normal saline at doses of 0.1 mg/kg (n = 6, Group 2), 0.05 mg/kg (n = 6, Group 3), and 0.025 mg/kg (n = 6, Group 4). R75231 was given to the animals as a continuous intravenous infusion for 30 min before liver ischemia. Animals given no treatment were used as the control (n = 10, Group 1). Animals were followed for two weeks postoperatively.
Determinations
The completeness of hepatic vascular interruption was determined by measuring indocyanine green (ICG) retention rate and hepatic tissue blood flow. Indocyanine green, 0.5 mg/kg, was injected intravenously 30 min after the start of hepatic ischemia and 20-min ICG retention rate was evaluated spectrophotometrically. Serial measurements of the hepatic tissue blood flow were performed by a laser doppler flow meter (Advance Laser Flowmeter, ALF21, Advance Company Ltd., Tokyo, Japan) before ischemia, during ischemia, and for 60 min after reperfusion.
Blood samples collected for measurement of aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), and total bilirubin were analyzed with a Technicon RA500 autoanalyzer (Bayer, Tarrytown, NY). Tissue and peripheral blood samples for malondialdehyde (MDA) MDA determinations were collected before ischemia, at the end of ischemia, and at 15 min and 60 min after reperfusion, and malondialdehyde concentrations were determined by the method described by Yagi et al. (11).
Wedge biopsies were repeated before ischemia, at 1 hr and 2 hr during ischemia, and 15 min and 60 min after reperfusion for the determination of adenine nucleotides (AN: adenosine triphosphate [ATP]; adenosine diphosphase [ADP]; and adenosine monophosphate [AMP]), and purine catabolites (PC: adenosine [ADO]; inosine [INO]; hypoxanthine [HX]; and xanthine [X]). The biopsies were divided into pieces, immediately frozen (within 15 sec), and stored in liquid nitrogen. Measurements were conducted as described before (12) using a Waters HPLC system (Waters Chromatography Division, Millipore Corp., Milford, MA; Model 510 pumps, Model 484 absorbance module, and Model 717 WISP system), equipped with a tunable absorbance detector (Waters Model 484). Energy charge (EC) was calculated by the equation of Atkinson et al. (13).
For histopathologic analyses, a piece of liver tissue was fixed in buffered formalin and stained with hematoxylin and eosin. The number of neutrophils that infiltrated into the hepatic tissue was counted in 10 random power fields, at a magnification of 100×, with sections stained by Leder’s neutrophil staining method (14).
Statistics
Values are expressed as mean±SEM. Animal survival was determined using the Fisher’s exact test. Intra-group analysis was performed using the unpaired Student t-test. Inter-group analysis was performed using ANOVA. When the analysis of variance showed a significant difference (P<0.05), a post-hoc test was used to determine the P values for each group.
RESULTS
Preischemic administration of R75231 caused no significant hemodynamic changes. Indocyanine green retention rate was 94.8±1.2% in Group 1, 94.1±1.6% in Group 2, 95.1±1.2% in Group 3, and 95.7± 1.5% in Group 4, indicating near total ischemia of the liver.
Immediately after unclamping, all control animals developed severe swelling and congestion of the liver, which disappeared gradually over 30 to 60 min. In contrast, livers treated with R75231 showed smooth revascularization without outflow block, except for transient and mild hepatic swelling in Groups 2 and 4. Compared with the control, hepatic tissue blood flow after reperfusion was significantly augmented in treated animals (Fig. 2). Compared with the pre-ischemic level, hepatic tissue blood flow at 5 min after reperfusion was 28.8±1.9% in Group 1, 58.1±3.8% in Group 2, 61.1 ± 2.2% in Group 3, and 56.9 ± 2.3% in Group 4.
Figure 2.
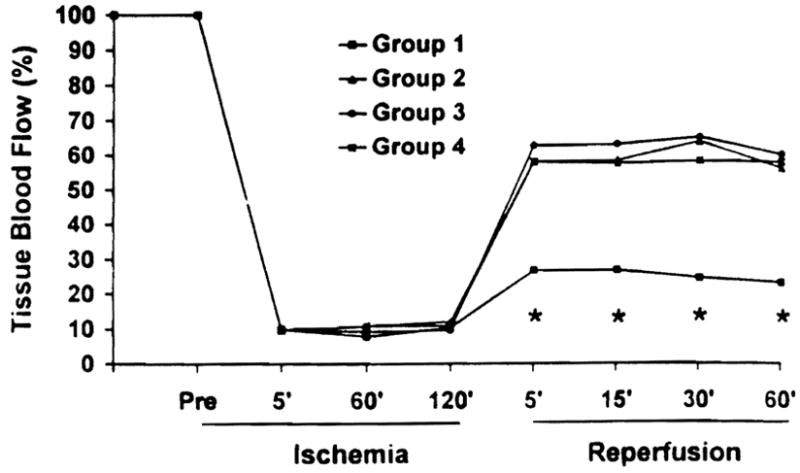
Tissue blood flow in the liver during ischemia and after reperfusion. Values are expressed as a percentage of preischemic tissue blood flow level. (*)P<0.05 versus Groups 2, 3, and 4.
During a two-week follow-up, 7 control dogs died of liver failure, 6 within 24 hr and one on postoperative day 10. In the treated groups, one animal in Group 2 died of liver failure 12 hr after reperfusion. Two-week animal survival in the treated groups was significantly better than the control: 30% in Group 1 versus 83% in Group 2, 100% in Group 3, and 100% in Group 4.
In accordance with poor hepatic blood flow and animal survival, nontreated Group 1 animals had marked elevation of AST (Fig. 3), ALT, and LDH after reperfusion, reaching mean peak levels 12 hr after reperfusion at 11521±1014 U/L, 13563±1379 U/L, and 3045±633 U/L, respectively. Treatment with R75231 significantly lowered liver enzyme from control levels, but there was no statistically significant difference between treated groups.
Figure 3.
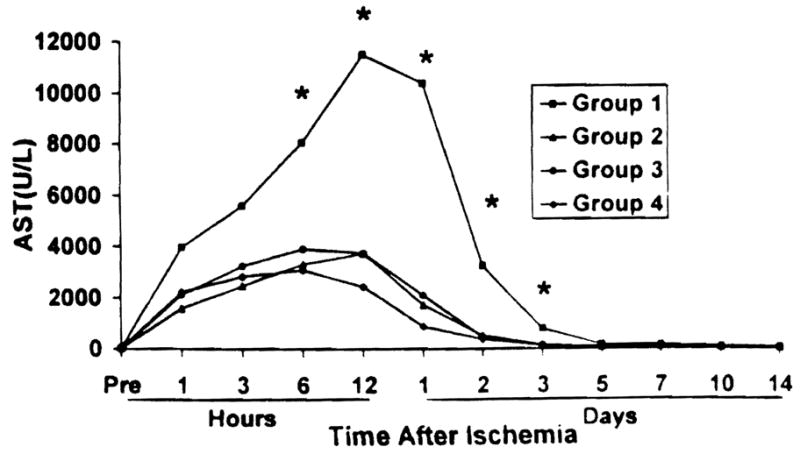
Aspartate aminotransferase levels after reperfusion. (*)P<0.05 versus Groups 2, 3, and 4.
Changes in adenine nucleotide and purine catabolite concentrations in liver tissue during ischemia and after reperfusion are shown in Table 1. Compared with preischemic values, 2 hr of hepatic vascular exclusion in control animals induced a 60% decrease in adenine nucleotides, and reciprocally, an 800% increase in purine catabolites. In particular, ischemia caused a greater than 90% reduction in ATP and 900% higher production of xanthine. Degradation of adenine nucleotides and elevation of purine catabolites by 2 hr of ischemia were markedly suppressed by the preischemic administration of R75231, especially at a dose of 0.025 mg/kg. Although tissue adenosine levels during ischemia were elevated in all treated groups, they were not statistically different from the control or between groups. While resynthesis of ATP was significantly suppressed after reperfusion in control livers, a prompt increase in ATP levels was seen with treated livers. Tissue ATP concentration in Group 4 livers was significantly higher than ATP levels of Group 1 and Group 2. Group 2 livers contained more inosine and hypoxanthine than the other treated groups.
Table 1.
Tissue concentrations of adenine nucleotides and purine catabolites in canine livers during ischemia and after reperfusion
| Group | Dose (mg/kg) | EC | Tan (nmol/mg protein) | ATP (nmol/mg protein) | ADP (nmol/mg protein) | AMP (nmol/mg protein) | ADO (nmol/mg protein) | INO (nmol/mg protein) | HX (nmol/mg protein) | X (nmol/mg protein) |
|---|---|---|---|---|---|---|---|---|---|---|
| Normal | 0.82±0.01 | 30.4±2.1 | 21.8±2.0 | 6.9±0.7 | 1. 7±0.3 | 0.99±0.02 | 0.18±0.08 | 0.84±0.12 | 0.06±0.03 | |
| 1 hr of warm ischemia: | ||||||||||
| Group 1 | 0 | 0.32±0.02 | 25.2±3.5 | 4.7±0.7 | 6.8±1.0 | 13.7±2.1 | 0.31±0.07 | 0.95±0.07 | 2.85±0.29 | 2.53±0.28 |
| Group 2 | 0.1 | 0.30±0.03 | 25.5±1.8 | 4.3±0.5 | 6.4±0.4 | 14.8±1.6 | 0.64±0.21 | 0.75±0.24 | 1.42±0.12a | 1.71±0.34 |
| Group 3 | 0.05 | 0.30±0.03 | 23.1±2.5 | 4.1±0.8 | 5.6±0.8 | 13.5±1.6 | 0.62±0.17 | 1.04±0.18 | 1.41±0.39a | 2.20±0.36 |
| Group 4 | 0.025 | 0.34±0.02 | 19.7±1.2 | 4.2±0.3 | 4.9±0.3 | 10.5±0.8 | 0.18±0.03a | 0.65±0.15 | 0.80±0.14a | 1.65±0.30a |
| 2 hr of warm ischemia: | ||||||||||
| Group 1 | 0 | 0.22±0.02 | 13.0±0.7 | 1.6±0.3 | 2.6±0.2 | 8.7±0.5 | 0.26±0.05 | 0.67±0.17 | 3.37±0.22 | 5.33±0.37 |
| Group 2 | 0.1 | 0.23±0.02 | 18.2±1.0a | 2.5±0.3 | 3.5±0.4 | 12.1±0.7a | 0.54±0.15 | 0.81±0.22 | 1.94±0.38a | 2.83±0.44a |
| Group 3 | 0.05 | 0.26±0.03 | 17.7±2.0a | 2.8±0.5 | 3.7±0.5a | 11.1±1.4 | 0.44±0.13 | 0.95±0.20 | 1.49±0.19a | 3.31±0.49a |
| Group 4 | 0.025 | 0.27±0.04 | 17.9±0.7a | 3.0±0.7a | 3.8±0.4a | 11.1±0.7 | 0.32±0.10 | 0.59±0.08 | 1.37±0.22a | 2.86±0.30a |
| 15 min after reperfusion: | ||||||||||
| Group 1 | 0 | 0.72±0.04 | 12.1±2.1 | 7.2±1.5 | 3.5±0.6 | 1.3±0.1 | 0.16±0.04 | 0.32±0.10 | 1.10±0.21 | 0.42±0.23 |
| Group 2 | 0.1 | 0.72±0.04 | 18.3±3.0 | 10.8±1.9 | 5.0±1.1 | 2.5±0.5 | 0.15±0.04 | 0.55±0.2 | 0.72±0.21 | 0.14±0.09 |
| Group 3 | 0.05 | 0.82±0.02a | 17.9±1.5 | 12.9±1.1a | 3.7±0.5 | 1.3±0.2 | 0.14±0.03 | 0.25±0.03 | 0.36±0.10a | 0.04±0.044a |
| Group 4 | 0.025 | 0.80±0.02 | 16.7±1.8 | 11.7±1.3a | 3.4±0.6 | 1.5±0.3 | 0.08±0.01 | 0.21±0.04 | 0.08±0.01a | 0.04±0.04a |
| 60 min after reperfusion: | ||||||||||
| Group 1 | 0 | 0.83±0.01 | 12.2±0.9 | 8.9±0.8 | 2.4±0.3 | 0.8±0.1 | 0.08±0.02 | 0.28±0.09 | 0.78±0.14 | 0.26±0.06 |
| Group 2 | 0.1 | 0.80±0.04 | 16.6±3.3 | 10.9±2.5 | 4.3±1.4 | 1.5±0.5 | 0.12±0.03 | 0.20±0.04 | 0.60±0.25 | 0.02±0.01a |
| Group 3 | 0.05 | 0.88±0.01 | 16.7±0.5 | 13.4±0.4 | 2.7±0.2 | 0.7±0.1 | 0.10±0.02 | 0.16±0.03 | 0.13±0.05a | 0.00±0.00a |
| Group 4 | 0.025 | 0.86±0.02 | 18.8±1.8a | 15.1±1.6a | 2.6±0.3 | 1.1±0.3 | 0.07±0.01 | 0.22±0.03 | 0.10±0.03a | 0.00±0.00a |
P<0.05 vs. Group 1.
Changes in MDA concentration in liver tissue and plasma during ischemia and after reperfusion are shown in Table 2. Tissue MDA levels did not show any statistically significant differences during ischemia or after reperfusion. However, plasma MDA levels increased markedly after reperfusion. The increase in plasma MDA was significantly inhibited by treatment with R75231.
Table 2.
Tissue and plasma concentration of malondialdehyde during liver ischemia and after reperfusion
| During ischemia |
After reperfusion |
|||
|---|---|---|---|---|
| 1hr | 2hr | 15 min | 60 min | |
| Tissue MDA (nmol/mg protein)a | ||||
| Group 1 | 1.375±0.364 | 0.970±0.081 | 0.488±0.113 | 0.505±0.091 |
| Group 2 | 0.875±0.083 | 0.735±0.077 | 0.517±0.135 | 0.426±0.102 |
| Group 3 | 0.905±0.086 | 1.040±0.124 | 0.757±0.107 | 0.568±0.072 |
| Group 4 | 0.607±0.059 | 0.682±0.040 | 0.479±0.063 | 0.453±0.053 |
| Plasma MDA (μmol/L)b | ||||
| Group 1 | 0.777±0.088 | 1.252±0.127 | 1.237±0.161 | |
| Group 2 | 0.844±0.072 | 0.904±0.079c | 1.006±0.160c | |
| Group 3 | 0.764±0.120 | 0.850±0.061c | 0.816±0.072c | |
| Group 4 | 0.688±0.060 | 0.728±0.049c | 0.710±0.097c | |
Normal tissue MDA level = 1.071 ± 0.098 nmol/mg protein.
Normal plasma MDA level = 0.754 ± 0.051 μmol/L.
P < 0.05 vs. Group 1.
Histopathologic examination showed no demonstrable differences between the nontreated group and treated groups at the end of ischemia. Sporadic single hepatocyte necroses were present equally in all groups. After reperfusion, however, control livers developed significant structural abnormalities, including confluenced or bridging hepatocyte necroses, ballooning, sinusoidal congestion and derangement, and endothelial cell detachment (Fig. 4A). In contrast, these alterations were rarely seen in the treated groups, which had well preserved hepatic architecture (Fig. 4B). The number of neutrophils infiltrated into postischemic livers 60 min after reperfusion was 33.1±4.4/10 fields in Group 1, 27.8±3.1/10 fields in Group 2, 14.1±2.5/10 fields in Group 3, and 10.0±4.6/l0 fields in Group 4 (P<0.05 Group 3 vs. Group 1 and Group 2, and Group 4 vs. Group 1).
Figure 4.

Histopathology of the control liver (A) and R75231 treated liver (B) at 60 min after reperfusion (H&E; ×200).
DISCUSSION
Using a critical experimental model of 2-hr hepatic vascular exclusion in dogs, we demonstrated that inhibition of nucleoside transport by R75231 attenuated postreperfusion hepatic injury and improved animal survival. Preischemia administration of R75231 slowed adenine nucleotide degradation and suppressed purine catabolite accumulation in ischemic tissues. After reperfusion, treatment with R75231 enhanced ATP resynthesis, increased hepatic tissue blood flow, inhibited lipid peroxidation, and lessened neutrophil infiltration, particularly at the lower doses.
Since the first description by Drury and Szent-György in 1929 (15), adenosine has been recognized as a local hormone, or a “homeostatic metabolite,” that regulates the function of organs, tissues, and cells in the physiologic state. Over the last decade, the importance of adenosine in protecting against ischemia and reperfusion injury has caught the attention of many researchers, particularly in the field of cardiology. Cardiac preconditioning by repeated brief ischemia and reperfusion has been shown to improve cardiac function and reduce myocyte necrosis after prolonged ischemia (16). The mechanisms of protection from preconditioning include increased coronary blood flow, vasodilation, suppression of free radical generation, preservation of high energy stores, enhancement of stress protein, and inhibition of neutrophil and platelet activation (17). Most of these properties have been attributed to the biological action of adenosine (5–10) and adenosine-related molecular events, such as modulation of adenosine receptors (18), ATP sensitive potassium channel (19), and regulatory G proteins (20).
Similar to ischemic preconditioning, systemic or local administration of exogenous adenosine before ischemia, before reperfusion, or both, has been reported to attenuate ischemia and reperfusion injury (21–23); however, exogenous adenosine supplementation also has disadvantages. Exogenous adenosine disappears rapidly from the circulation, due to its extremely short half-life of a few seconds in humans (24) and a few minutes in dogs (25). Adenosine causes systemic adverse effects, including hypotension, renal impairment, and reduction of hepatic blood flow, if the dose is excessive. In contrast, augmentation of endogenous adenosine concentration by the regulation of adenosine metabolism is a safe and promising alternative, because it takes advantage of the beneficial effects of adenosine within the organ and only when it becomes ischemic, in a site-specific and event-specific manner. Regulation of adenosine metabolism can be achieved using nucleoside transport inhibitors (26), adenosine deaminase inhibitors (27), and 5-aminoimidazole-4-carboxamide riboside (AICA roboside or acadesine) (28).
R75231 is a novel nucleoside transport inhibitor, which is more potent and has a longer duration of action and a higher bioavailability than mioflazine, lidoflazine, dipyridamole, nitrobenzyl-thioinosine, and other inhibitors. The protective effect of this agent against ischemia and reperfusion injury has been reported with the heart (29–31) and the kidney (32) in humans and experimental animals. The mechanism of adenosine metabolism and drug action has been well investigated in the heart by Van Belle and associates (33, 34). According to Van Belle, AMP, which increases in ischemic tissues from the degradation of ATP and ADP, is dephosphorilized to adenosine by cytosolic 5′-nucleotidase within the myocytes, and, preferentially, by membrane-bound ecto 5′-nucleotide in the interstitial space after leaving the myocytes by diffusion. The majority of adenosine in the interstitial space is taken up, facilitated by nucleoside transport proteins, into the endothelial cells, where adenosine is rapidly converted to inosine by adenosine diaminase, and then, to hypoxanthine and xanthine. R75231 inhibits adenosine transport, suppresses further catabolism of adenosine in the endothelial cells, enhances adenosine concentration in the interstitial space, and delays adenosine wash-out after reperfusion, thereby maximizing the salutary effects of adenosine during ischemia and reperfusion.
In this study, R75231 significantly increased adenine nucleotide levels and decreased purine catabolite levels in the ischemic tissues compared to the control, but tissue adenosine levels were only slightly and insignificantly enhanced. This finding is in contrast to results obtained with hearts (29–31), which showed marked elevation of adenosine in cardiac tissues (14 times) and in the coronary effluent (7 times). Because cardiac myocytes lack adenosine deaminase (35), the transport and deamination of adenosine in the endothelial cells serves as a major route of adenosine metabolism in the heart. Thus, the inhibition of adenosine transport appears to induce a profound elevation of adenosine levels in cardiac tissues and the interstitium. However, adenosine metabolism in the liver consists of deamination in hepatocytes and adenosine transport and catabolism in endothelial cells. Isolated purified hepatocytes have been shown to produce increasing amounts of nucleosides and purine catabolites in relation to the duration of anoxia (36). In our study, R75231 treatment definitely decreased purine catabolite levels in the ischemic livers, indicating nucleoside transport inhibition and decreased production of purine catabolites in the endothelial cells. Although not measured, interstitial adenosine concentration in liver tissues would be increased by treatment. After reperfusion, R75231 administration enhanced resynthesis of high energy phosphate, possibly by increased amounts of substrate in liver tissues at the end of 2-hr ischemia. In an isolated perfusion system, Palombo et al. (37) reported enhanced high energy phosphate resynthesis by adenosine supplementation after reperfusion of rat livers preserved with UW solution.
Preischemic administration of R75231 improved hepatic tissue blood flow after reperfusion, which is thought to result from three interrelating mechanisms: vasodilation, the inhibition of neutrophil infiltration, and the preservation of microvasculatures.
Adenosine exerts vasodilatory action not only by acting directly on vascular smooth muscles, but also by modulation of vascular endothelial cells which synthesize and secrete various vasodilatory substances (such as nitric oxide and prostacyclin) and vasoconstrictive substances (such as endothelin and thromboxane A2). McKie et al. (5) reported that increased blood flow in the gastrointestinal tract and the kidney after adenosine administration was suppressed by nitro-L-arginine methy ester, a competitive inhibitor of nitric oxide synthetase. Endothelin release from ischemic myocardium of dogs was inhibited by administration of endogenous adenosine during the early reperfusion period (23). Adenosine and R75231 suppressed endothelin levels in the hepatic venous blood in separate liver ischemia experiments (unpublished data).
R75231 reduced the number of neutrophils infiltrating the postischemic livers. It has been shown that exogenous adenosine inhibits accumulation, adherence, and activation of neutrophils by attenuating the upregulation of Mac-l expression via stimulation of A2 receptor on neutrophil membranes (9) and suppresses superoxide generation (6). Thus, the “no-reflow” caused by neutrophil plugging the microvasculature of postischemic livers is prevented.
Finally, nucleoside transport inhibition appears to prevent microvasculature damage by suppressing superoxide production in the endothelial cells and Kupffer cells during ischemia, and in these cells and neutrophils after reperfusion. In this study, both hypoxanthine and xanthine were significantly elevated in ischemic control livers. Hypoxanthine is catabolized to xanthine, producing superoxide anions, by xanthine oxidase that is converted from xanthine reductase during ischemia. Recently, Brass et al. (38) have shown that in anoxic isolated rat liver cells, the conversion of xanthine dehydrogenase to xanthine oxidase occurs mostly actively in Kupffer cells, moderately in endothelial cells, and weakly in hepatocytes. Studies using ultrastructural enzyme histochemistry (39) and long-acting superoxide dismustase (40) have supported his findings. Treatment with R75231 inhibited hypoxanthine and xanthine elevations during ischemia, which suggests reduced production of superoxide in Kupffer cells and endothelial cells. Inhibition of free radical generation by adenosine in Kupffer cells and neutrophils after reperfusion has been documented (6, 8, 41).
Previous studies and this experiment indicate that free radical generation and the resulting lipid peroxidation are major causes of ischemia and reperfusion injury of the liver; however, we could not detect MDA in tissue homogenate by thiobarbituric acid (TBA) reaction during ischemia, and even after reperfusion. This may reflect a problem with sensitivity with the assay method (42). It may also suggest that lipid peroxidation occurs mainly in nonparenchymal cells rather than the hepatocytes, and that it can not be detected with tissue homogenates because more than 90% of protein in the homogenate is derived from hepatocytes. Walsh et al. (43) reported the exclusive presence of conjugated dienes in non-parenchymal cell fractions isolated from the rat livers subjected to warm ischemia.
R75231 can be used clinically for the procurement of organs from non-heartbeating donors. Non-heartbeating donors have been classified into four categories (44): dead on arrival (category 1); unsuccessful resuscitation (category 2); awaiting cardiac arrest (category 3); and cardiac arrest while brain dead (category 4). At the very least R75231 treatment would be indicated for category 3 donors. R75231 would be administered intravenously before the termination of ventilatory support, as heparin is given for anticoagulation (1). Even donors in category 2 and category 4 would benefit from R75231 treatment if treatment is started during resuscitation. This could safely be done since R75231 did not cause any significant hemodynamic changes. More important, R75231 can be added to the preservation solution to prevent further degradation of adenosine during cold storage and/or given to the recipient immediately before reperfusion to inhibit adenosine uptake by the erythrocytes and to delay its washout from ischemic tissues. The beneficial effect of the last approach has been shown in canine heart preservation and transplantation (29, 46, 47), and further study is being conducted at our laboratory using a canine liver transplantation model.
In summary, preischemic administration of the nucleoside transport inhibitor, R75231, attenuated postreperfusion liver injury, apparently from the positive effect of augmented endogenous adenosine. However, it was intriguing that we did not see much difference in the determinants depending upon the dose of R75231. This may indicated that the positive effect of adenosine is not related to the amount of adenosine in the interstitial space, but to the adenosine receptors that have different affinities to adenosine. Studies that are also currently underway with various adenosine receptor agonists and antagonists are required to elucidate the role of adenosine receptors in ischemia.
Acknowledgments
The authors are deeply indebted to Dr. H. Van Belle for his criticism and suggestions; to Mr. Steve Miller for his assistance in conducting the experiment; and to Ms. Joyce Petrow for editing and preparation of the manuscript.
Footnotes
Presented at the 22nd Annual Meeting of the American Society of Transplant Surgeons, May 29–31, 1996, Dallas, TX.
This work was aided by Research Grants from the Veterans Administration and Project Grant DK-29961 from the National Institutes of Health, Bethesda, MD.
Abbreviations: ADO, adenosine; ADP, adenosine diphosphate; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMP, adenosine monophosphate; AN, adenine nucleotides; AST, aspartate aminotransferase; ATP, adenosine triphosphate; EC, energy charge; HX, hypoxanthine; ICG, indocyanine green; INO, inosine; LDH, lactate dehydrogenase; MDA, malondialdehyde; PC, purine catabolites; POD, postoperative day; TBA, thiobarbituric acid; X, xanthine.
References
- 1.Casavilla A, Ramirez C, Shapiro R, et al. Experience with liver and kidney allografts from non-heartbeating donors. Transplantation. 1995;59:197. doi: 10.1097/00007890-199501000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pichlmayr R, Ringe B, Gubematis G, Hauss J, Bunzendahl H. Transplantation einer Spenderleber auf zwei Empfanger (Splitting-Transplantation)–Eine neue Methode in der Weiter-entwicklung der Lebersegmenttransplantation. Langenbecks Arch Chir. 1988;373:127. [PubMed] [Google Scholar]
- 3.Yamaoka Y, Morimoto T, Inamoto T, et al. Safety of the donor in living-related liver transplantation–an analysis of 100 parental donors. Transplantation. 1995;59:224. [PubMed] [Google Scholar]
- 4.Starzl TE, Fung J, Tzakis A, et al. Baboon to human liver transplantation. Lancet. 1993;341:65. doi: 10.1016/0140-6736(93)92553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKie LD, Bass BL, Dunkin BJ, Harmon JW. Nitric oxide mediates the blood flow response to intravenous adenosine in the rabbit. Circulatory Shock. 1994;43:103. [PubMed] [Google Scholar]
- 6.Ward PA, Cunningham TW, McCulloch KK, Johnson KJ. Regulatory effects of adenosine and adenine nucleotides on oxygen radical responses of neutrophils. Lab Invest. 1988;58:438. [PubMed] [Google Scholar]
- 7.Kitakaze M, Hori M, Sato H, Takashima S, Inoue M, Kitabatake A, Kamada T. Endogenous adenosine inhibits platelet aggregation during myocardial ischemia in dogs. Circ Res. 1991;69:1402. doi: 10.1161/01.res.69.5.1402. [DOI] [PubMed] [Google Scholar]
- 8.De La Harpe J, Nathan CF. Adenosine regulates the respiratory burst of cytokine-triggered human neutrophils adherent to biologic surfaces. J Immunol. 1989;143:596. [PubMed] [Google Scholar]
- 9.Wollner A, Wollner S, Smith JB. Acting via A2 receptors, adenosine inhibits the upregulation of Mac-1 (CD11b/CD18) expression on FMLP-stimulated neutrophils. Am J Respir Cell Mol Bioi. 1993;9:179. doi: 10.1165/ajrcmb/9.2.179. [DOI] [PubMed] [Google Scholar]
- 10.Mullane K, Bullough D. Harnessing an endogenous cardioprotective mechanism: Cellular sources and sites of action of adenosine. J Mol Cell Cardiol. 1995;27:1041. doi: 10.1016/0022-2828(95)90073-x. [DOI] [PubMed] [Google Scholar]
- 11.Yagi K. A simple flurometric assay for lipoperoxide in blood plasma. Biochem Med. 1976;15:212. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- 12.Hamamoto I, Takaya S, Todo S, et al. Can adenine nucleotides predict primary nonfunction of the human liver homograft? Transplant Int. 1994;7:89. doi: 10.1007/bf00336468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atkinson DE. The energy charge of the adenylate pool as a regulatory parameter, interaction with feed back modifiers. Biochemistry. 1968;7:4030. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- 14.Stevens A. Enzyme histochemistry: diagnostic applications. In: Bancroft JD, Stevens A, editors. Theory and practice of histologic techniques. New York: Churchill Livingstone; 1990. p. 401. [Google Scholar]
- 15.Drury AN, Szent-György A. The physiological activity of adenine compounds with special reference to their action upon the mammalian heart. J Physiol (Land) 1929;68:213. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins DP, Baxter GF, Yellon DM. The pathophysiology of ischemic preconditioning. Pharmacol Res. 1995;31:219. doi: 10.1016/1043-6618(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson KA, Trivedi BK, Churchill PC, Williams M. Novel therapeutics acting via purine receptors. Biochem Pharmacol. 1991;41:1399. doi: 10.1016/0006-2952(91)90555-j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cason BA, Gordon HJ, Avery EG, Hickey RF. The role of ATP sensitive postassium channels in myocardial protection. J Cardiac Surg. 1995;10:441. doi: 10.1111/j.1540-8191.1995.tb00675.x. [DOI] [PubMed] [Google Scholar]
- 20.De Jonge HW, Van Heugten HA, Lamers JM. Signal transduction by the phosphatidylinositol cycle in myocardium. J Mol Cell Cardiol. 1995;27:93. doi: 10.1016/s0022-2828(08)80010-7. [DOI] [PubMed] [Google Scholar]
- 21.Babbitt DG, Virmani R, Forman MB. Intracoronary adenosine administered after reperfusion limits vascular injury after prolonged ischemia in the canine model. Circulation. 1989;80:1388. doi: 10.1161/01.cir.80.5.1388. [DOI] [PubMed] [Google Scholar]
- 22.Norton ED, Jackson EK, Virmani R, Forman MB. Effect of intravenous adenosine on myocardial reperfusion injury in a model with low myocardial blood flow. Am Heart J. 1991;122:1283. doi: 10.1016/0002-8703(91)90567-2. [DOI] [PubMed] [Google Scholar]
- 23.Velasco CE, Jackson EK, Morrow JA, Vitola JV, Inagami T, Forman MB. Intravenous adenosine suppresses cardiac release of endothelin after myocardial ischemia and reperfusion. Cardiovasc Res. 1993;27:121. doi: 10.1093/cvr/27.1.121. [DOI] [PubMed] [Google Scholar]
- 24.Kolassa N, Pfleger K. Adenosine uptake by erythrocytes of man, rat, and guinea pig and its inhibition by hexobendine and dipyridamole. Biochem Pharmacol. 1975;24:154. doi: 10.1016/0006-2952(75)90331-7. [DOI] [PubMed] [Google Scholar]
- 25.Van Belle H. Differential inactivation of adenosine by human and canine blood. FEBS Lett. 1969;3:133. doi: 10.1016/0014-5793(69)80116-x. [DOI] [PubMed] [Google Scholar]
- 26.Van Belle H. Nucleoside transport inhibition: a therapeutic approach to cardioprotection via adenosine? Cardiovasc Res. 1993;27:68. doi: 10.1093/cvr/27.1.68. [DOI] [PubMed] [Google Scholar]
- 27.Sandhu GS, Burrier AC, Janero DR. Adenosine deaminase inhibitors attenuate ischemic injury and preserve energy balance in isolated guinea pig heart. Am J Physiol. 1993;265:H1249. doi: 10.1152/ajpheart.1993.265.4.H1249. [DOI] [PubMed] [Google Scholar]
- 28.Galinanes M, Mullane KM, Bullough D, Hearse DJ. Acadesine and myocardial protection: studies of time of administration and dose-response relations in the rat. Circulation. 1992;86:598. doi: 10.1161/01.cir.86.2.598. [DOI] [PubMed] [Google Scholar]
- 29.Masuda M, Chang-Chun C, Mollhoff T, Van Belle H, Flameng W. Effects of nucleoside transport inhibition on long-term ex vivo preservation of canine hearts. J Thorac Cardiovasc Surg. 1992;104:1610. [PubMed] [Google Scholar]
- 30.Masuda M, Demeulemeester A, Chang-Chun C, Hendrikx M, Van Belle H, Flameng W. Cardioprotective effects of nucleoside transport inhibition in rabbit hearts. Ann Thorac Surg. 1991;52:1300. doi: 10.1016/0003-4975(91)90017-k. [DOI] [PubMed] [Google Scholar]
- 31.Flameng W, Sukehiro S, Mollhoff T, Van Belle H, Janssen P. A new concept of long-term donor heart preservation: nucleoside transport inhibition. J Heart Lung Transplant. 1991;10:990. [PubMed] [Google Scholar]
- 32.Booster MH, Yin M, Maessen JG, Stubenitsky BM, Wijnen RM, Kootstra G. Protection of canine renal grafts by renin-angio-tensin inhibition through nucleoside transport blockade. Transplant Int. 1995;8:207. doi: 10.1007/BF00336539. [DOI] [PubMed] [Google Scholar]
- 33.Van Belle H, Goossens F, Wynants J. Formation and release of purine catabolites during hypoperfusion, anoxia, and ischemia. Am J Physiol. 1987;252:H886. doi: 10.1152/ajpheart.1987.252.5.H886. [DOI] [PubMed] [Google Scholar]
- 34.Van Belle H, Wynants J, Xhonneux R, Flameng W. Changes in creatine phosphate, inorganic phosphate, and the purine pattern in dog hearts with time of coronary artery occlusion and effect thereon of mioflazine, a nucleoside transport inhibitor. Cardiovasc Res. 1986;20:658. doi: 10.1093/cvr/20.9.658. [DOI] [PubMed] [Google Scholar]
- 35.Nees S. The adenosine hypothesis of metabolic regulation of coronary flow in the light of newly recognized properties of the coronary endothelium. Z Kardiol. 1989;78:42. [PubMed] [Google Scholar]
- 36.Bontemps F, Vincent MF, Van Den Berghe G. Mechanisms of elevation of adenosine levels in anoxic hepatocytes. Biochem J. 1993;290:671. doi: 10.1042/bj2900671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palombo JD, Pomposelli JJ, Fechner KD, Blackburn GL, Bistrian BR. Enhanced restoration of adenine nucleotides in rat liver following extended preservation in UW solution by provision of adenosine during reperfusion. Transplantation. 1991;51:867. doi: 10.1097/00007890-199104000-00025. [DOI] [PubMed] [Google Scholar]
- 38.Wiezorek JS, Brown DH, Kupperman DE, Brass CA. Rapid conversion to high xanthine oxidase activity in viable Kupffer cells during hypoxia. J Clin Invest. 1994;94:2224. doi: 10.1172/JCI117584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Angermuller S, Schunk M, Kusterer K. Alteration of xanthine oxidase activity in sinusoidal endothelial cells and morphological changes of Kupffer cells in hypoxic and reoxygenated rat liver. Hepatology. 1995;21:1594. doi: 10.1002/hep.1840210618. [DOI] [PubMed] [Google Scholar]
- 40.Koo A, Komatsu H, Tao G, Inoue M, Guth PH, Kaplowitz N. Contribution of no-reflow phenomenon to hepatic injury after ischemia-reperfusion: evidence for a role for superoxide anion. Hepatology. 1991;15:507. doi: 10.1002/hep.1840150325. [DOI] [PubMed] [Google Scholar]
- 41.Gao, Hijioka T, Lindert KA, Caldwell-Kenkel JC, Lemasters JJ, Thurman RG. Evidence that adenosine is a key component in Carolina rinse responsible for reducing graft failure after orthotopic liver transplantation in the rat. Transplantation. 1991;52:992. doi: 10.1097/00007890-199112000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Bonnes-Taourel D, Guerin MC, Torreilles J. Is malonaldehyde a valuable indicator of lipid peroxidation? Biochem Pharmacol. 1992;44:985. doi: 10.1016/0006-2952(92)90132-3. [DOI] [PubMed] [Google Scholar]
- 43.Walsh TR, Rao PN, Makowka L, et al. Lipid peroxidation is a nonparenchymal cell event with reperfusion after prolonged liver ischemia. J Surg Res. 1990;49:18. doi: 10.1016/0022-4804(90)90104-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daemen JWHG, de Wit RJ, Heineman E, Kootstra G. Kidney transplantation from non-heartbeating donors. Transplant Rev. 1995;9:159. [Google Scholar]
- 45.Flameng W, Sukehiro S, Mollhoff T, Van Belle H, Janssen P. A new concept of long-term donor heart preservation: nucleoside transport inhibition. J Heart Lung Transplant. 1991;10:990. [PubMed] [Google Scholar]
- 46.Mollhoff T, Sukehiro S, Van Belle H, Van Aken H, Flameng W. Successful transplantation after long-term preservation of dog hearts. Anesthesiology. 1992;77:291. doi: 10.1097/00000542-199208000-00012. [DOI] [PubMed] [Google Scholar]


