Abstract
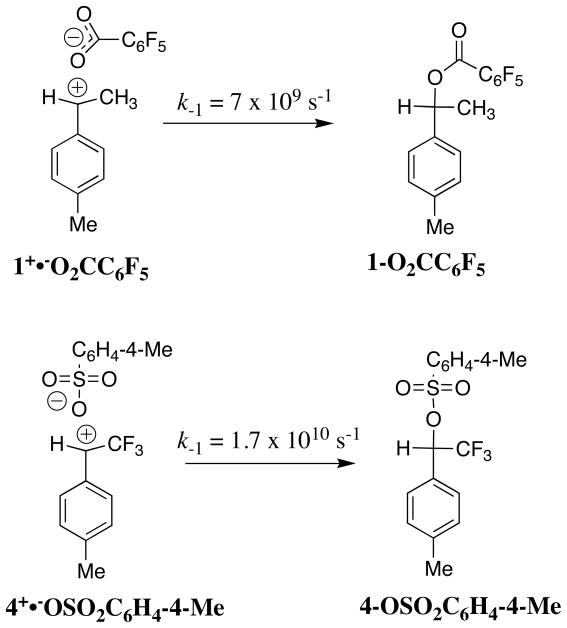
The sum of the rate constants for solvolysis and scrambling of carbon bridging and nonbridging oxygen-18 at 4-MeC6H4CH(CF3)OS(18O2)Tos in 50/50 (v/v) trifluoroethanol/water, (ksolv + kiso) = 5.4 × 10−6 s−1, is 50% larger than ksolv = 3.6 × 10−6 for the simple solvolysis reaction of the sulfonate ester. This shows that the ion pair intermediate of solvolysis undergoes significant internal return to form reactant. These data give a value of k−1 = 1.7 × 1010 s−1 for internal return of the carbocation-anion pair to the substrate. This rate constant is larger than the value of k−1 = 7 × 109 s−1 reported for internal return of an ion pair between the 1-(4-methylphenyl)ethyl carbocation and pentafluorobenzoate anion to the neutral ester (4-MeC6H4CH(CH3)O2CC6F5) in the same solvent. The partitioning of ion pairs to the 1-(4-methylphenyl)ethyl carbocation and to the highly destabilized 1-(4-methylphenyl)2,2,2-trifluoroethyl carbocation is compared and contrasted.
INTRODUCTION
We are interested in using the experimental protocols developed for the detection of the reaction of ion pair intermediates of solvolysis[1–4] to determine absolute rate constants for the very fast reactions of ion pairs;[5–7] and, to probe changes in reaction mechanism.[8–11] We have prepared several chiral or isotopically labeled substrates for solvolysis reactions, and used these to obtain data that provide estimates of absolute rate constant ratios for partitioning of ion pair intermediates between competing pathways: one pathway that leads to formation of solvolysis products, and the second to the products of racemization of a chiral benzoate ester,[5] isomerization of a thionobenzoate,[7] or exchange of oxygen-16 and oxygen-18 between bridging and nonbridging positions at an isotopically labeled benzoate or tosylate ester.[6,9] These rate constant ratios were combined with the appropriate absolute rate constant for carbocation capture by solvent to obtain estimates of absolute rate constants for reactions of carbocation ion pairs.

These earlier experiments focused on the reactions of putative ion-pair intermediates of solvolysis of ring substituted 1-phenylethyl derivatives, such as 1+•C6F5CO2−,[5] 2+•C6H5CSO−[7] and 3+•−O3SC6H4-4-Me.[9,11] We now extend this work and report the results of a study of the reorganization of the ion pair intermediate 4+•−OSO2C6H4-4-Me of solvolysis of a ring-substituted 1-phenyl-2,2,2-trifluoroethyl tosylate, where the carbocation is destabilized by the strongly electron-withdrawing α-CF3 substituent.[12–16] This work was initiated for the following reasons.
The α-CF3 for α-CH3 substitution has been shown to destabilize ring substituted 1-phenylethyl carbocations by 8–12 kcal/mol relative to neutral substrate.[14–16] We were interested in examining the effect of this strongly electron-withdrawing α-CF3 substituent on the partitioning of ion pair intermediates of solvolysis of 1-(4-methylphenyl)ethyl derivatives.
The ion pair 1+•C6F5CO2− has been generated as an intermediate of solvolysis of 1-(4-methylphenyl)ethyl pentafluorobenzoate.[5,6] By comparison, carbocation-anion pairs to the much more unstable 1-(4-methylphenyl)-2,2,2-trifluorethyl carbocation can only be generated using a more weakly basic leaving group anion, such as tosylate anion.[12,14,15] There is little or no data for addition of tosylate anion to carbocations in aqueous solution, so that it is not clear whether addition of this weakly basic anion competes effectively with addition of the relatively nucleophilic solvent water. We were therefore interested in determining whether the ion pair intermediate of the reaction of 1-(4-methylphenyl)-2,2,2-trifluorethyl tosylate would undergo internal return of the weakly nucleophilic sulfonate anion in the nucleophilic solvent water.
EXPERIMENTAL
All organic and inorganic chemicals were reagent grade from commercial sources and were used without further purification. The oxygen-18 labeled water, 99 atom % excess, was purchased from ISOTEC, Inc. 1H and 13C NMR spectra were recorded on a JEOL AL-400 FT-NMR spectrometer operating at 400 MHz and 100.4 MHz, respectively.
Syntheses
2,2,2-Trifluoro-1-(4-methylphenyl)ethanone was synthesized by the procedure of Stewart and coworkers.[171H NMR (400 MHz, CDCl3): δ 7.93 (AB, 2H, J = 8.0 Hz, ArH), 7.34 (AB, 2H J = 8.0 Hz, ArH), 2.46 (s, 3H, CH3). 1-(4-Methylphenyl)-2,2,2-trifluoroethanol (4-OH) was synthesized by reduction of the ketone with sodium borohydride.[18] The crude alcohol was purified by silica-gel column chromatography eluting with n-hexane: yield 78 %. 1H NMR (400 MHz, CDCl3): δ 7.36 (AB, 2H, J = 8.0 Hz, ArH), 7.22 (AB, 2H, J = 8.0 Hz, ArH), 4.98 (dq, 1H, J = 4.4 Hz, 2J = 6.4 Hz, CHCF3), 2.60 (d, 1H, J = 4.4 Hz, CHOH), 2.37 (s, 3H, CH3).
[ClS(18O)2]-p-Toluenesulfonyl chloride (ClS(18O)2C6H4-4-Me) was prepared by bubbling chlorine gas through a mixture of 10 g of p-thiocresol and 5 mL of oxygen-18 labeled water for two hours.[19] The reaction products were extracted with ether, purified by silica-gel column chromatography and recrystallized from benzene/n-hexane; yield 27 %, mp. 66.0–67.5°C.
1-(4-Methylphenyl)-2,2,2-trifluoroethyl tosylate (4-OS(O)2C6H4-4-Me) was synthesized from 4-OH and p-toluenesulfonyl chloride by following a published procedure.[20] The crude reaction product was purified by recrystallization from ether/n-hexane: mp. 81.5–83.0°C. The same procedure was used to synthesize oxygen-18 labeled [(18O2)SO]-1-(4-methylbenzyl)-2,2,2-trifluoroethyl tosylate (4-OS(18O)2C6H4-4-Me) from 4-OH (1g, 5.3 mM) and ClS(18O)2-C6H4-4-Me (1g, 5.3 mM); yield 59%, mp. 84–85°C.[201H NMR (400 MHz, CDCl3): δ 7.65 (AB, 2H, J = 8.4 Hz, ArH), 7.22 (d, 4H, J = 8.0 Hz, ArH), 7.11 (AB, 2H, J = 8.4 Hz, ArH), 5.63 (q, 1H, 2J = 6.4 Hz, CHCF3), 2.40 (s, 3H, CH3), 2.33 (s, 3H, CH3); 13C NMR (100.4 MHz, CDCl3) δ 145.14, 140.33, 133.06, 126.66 (1C, quaternary), 129.57, 129.22, 127.95, 127.83 (2C, tertiary), 122.21 (q, JCF = 280.2 Hz, CF3), 78.05 (q, JCF = 34.2 Hz, CHCF3).
Kinetic Analyses
The reaction of 4-OS(O)2Tos in 50/50 (v/v) trifluoroethanol/water (I = 0.5, NaClO4) at 25°C was monitored by following the decrease in absorbance at 265 nm. The reaction was initiated by making a 100-fold dilution of a solution of 4-OS(O)2-C6H4-4-Me in acetonitrile into 3.0 mL of 50/50 (v/v) trifluoroethanol/water (I = 0.5, NaClO4) to give a final substrate concentration of 3 mM.
The isomerization reaction of 4-OS(18O)2C6H4-4-Me was also monitored in 50/50 (v/v) trifluoroethanol/water (I = 0.5, NaClO4). The sulfonate ester (100 mg) was dissolved in 1 mL of acetonitrile and mixed with 200 mL of 50/50 (v/v) trifluoroethanol/water (I = 0.5, NaClO4) to give a final substrate concentration of 1.4 mM. At specified reaction times the remaining substrate and reaction products were extracted into 500 mL of toluene, the organic layer was washed with water, dried over MgSO4 and the solvent removed on a rotary evaporator. The remaining residue was dissolved in 0.6 mL of C6D6 and saved for 13C-NMR analysis.
Product analyses
The products of the solvolysis and of the azide anion nucleophilic substitution reactions of 4-OS(O)2C6H4-4-Me in 50/50 (v/v) 2,2,2-trifluoroethanol/water (I = 0.5, NaClO4) were determined by HPLC analysis, as described in previous work.[15] Published methods were also used to calculate the product rate constant kas/ks (M−1) from these product yields.[15,21]
13C-NMR analyses
Proton-decoupled 13C-NMR spectra were recorded on a JEOL AL-400 FT-NMR spectrometer operating at 100.4 MHz. The spectra were centered at 78.3 ppm, and collected using a sweep width of 605.4 Hz with 8000 data points (0.076 Hz/pt), an 8 s relaxation delay time, and a pulse angle of 45°. Chemical shifts were measured in ppm relative to the peak at δ = 77.0 ppm for [13C]CDCl3.
RESULTS
The oxygen-18 labeled substrate 4-OS(18O)2C6H4-4-Me was synthesized by adaptation of published procedures (Experimental section). Earlier studies of the solvolysis and oxygen-18 scrambling of ring-substituted 1-phenylethyl derivatives used substrates enriched in carbon-13 at the benzylic carbon and oxygen-18 at the leaving group.[6,9] The substrate used in this work contains only natural abundance of carbon-13 at the benzylic carbon. This saves time in preparing the solvolysis reaction substrate, but substantially increases the time required for 13C NMR analysis of the distribution of oxygen label at the sulfonate substrate.
A first-order rate constant of ksolv = 3.6 × 10−6 s−1 was determined for solvolysis of 4-OS(O)2C6H4-4-Me in 50/50 (v/v) trifluoroethanol/water (I = 0.5, NaClO4) at 25°C by monitoring the progress of the reaction by UV spectroscopy. The values of ksolv remain constant as the azide anion concentration is increased to 0.50 M. A product rate constant ratio of kaz/ks = 1.1± 0.1 M−1 was calculated from the yields, determined by HPLC analysis, of the solvent and azide anion adducts formed in five reactions at [N3−] between 0.10 and 0.50 M. These values of ksolv and kaz/ks are in fair agreement with ksolv = 3.2 × 10−6 s−1 and kaz/ks = 0.80 M−1 reported in earlier work.
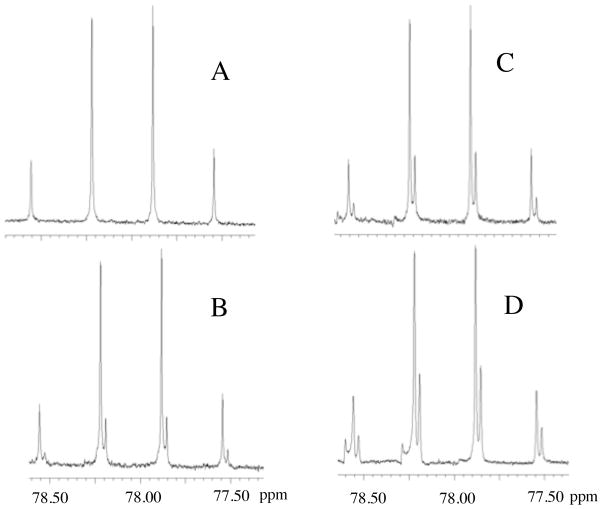
Figure 1 shows 13C NMR spectra in the region of the benzylic carbon of 4-OS(18O)2C6H4-4-Me (Figure 1A) and of the unreacted substrate recovered during solvolysis of 4-OS(18O)2C6H4-4-Me in 50/50 (v/v) trifluoroethanol/water (I = 0.5, NaClO4) at 25°C (Figures 1B–1D). The signal for the benzylic carbon at 78.342 ppm is split into a quartet by fluorines from the α-CF3 group. Figures 1B–1D show the appearance of the a second quartet for the isomerization reaction product 4-18OS(18O,16O)C6H4-4-Me, which is shifted upfield by 0.027 ppm to 78.315 when the oxygen-18 moves from a nonbridging to a bridging position.[22] Figures 1C and 1D show small peaks for an unknown impurity at ca 78.6 ppm, which is close to the most downfield peak of the quartet for the benzylic carbon. The relative area of the peaks for the isomerization reaction product 4-18OS(18O,16O) C6H4-4-Me (Aiso) and the remaining substrate 4-OS(18O)2C6H4-4-Me (AS) was therefore determined from the integrated areas of the two most upfield shifted peaks for the quartet for the benzylic carbon.
Figure 1.
Partial 13C spectra of unreacted substrate in C6D6 recovered during the solvolysis of 4-OS(18O)2C6H4-4-Me in 50/50 (v/v) trifluoroethanol/water (I = 0.5, NaClO4) at 25° C. The signal for the benzylic carbon is split by the α-fluorines (J = 34 Hz). (A) Initial spectrum of substrate 4-OS(18O)2C6H4-4-Me. (B) Spectrum of substrate recovered after a 36 hour reaction time (3220 transients). (C) Spectrum of substrate recovered after a 48 hour reaction time (3560 transients). (D) Spectrum of substrate recovered after a 69 hour reaction time (10590 transients). The origin of the small peak at ca 78.6 ppm observed in Figures 1C and 1D is not known.
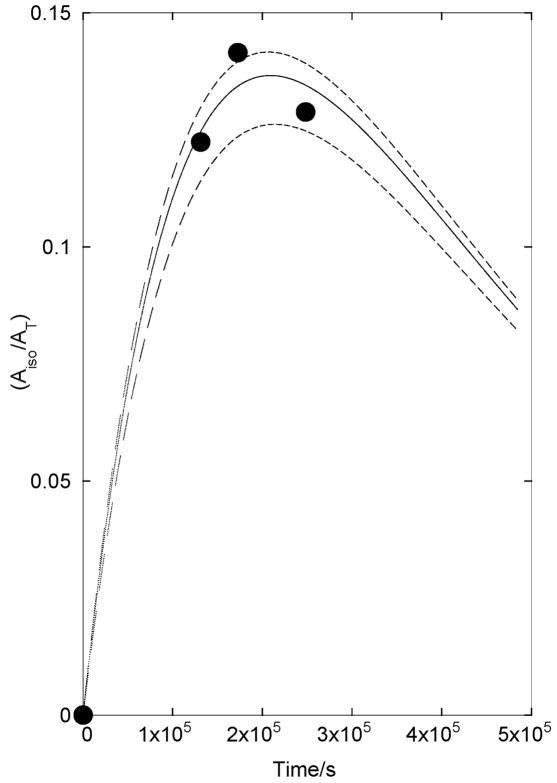
Figure 2 shows the fractional conversion of 4-OS(18O)2C6H4-4-Me to 4-18OS(18O,16O)Tos [Aiso/AT] at different reaction times t, where Aiso is the area of the carbon-13 NMR peak for the benzylic carbon of 4-18OS(18O,16O)C6H4-4-Me and AT is the total area of the peak for 4-OS(18O)2C6H4-4-Me at t = 0. Values of AT were calculated as AT = (AS+ Aiso)/exp(−ksolvt), where AS and Aiso are the observed peak areas for the benzylic carbons of 4-OS(18O)2C6H4-4-Me and 4-18OS-(18O,16O)C6H4-4-Me, respectively, and ksolv = 3.6 × 10−6 s−1.
Figure 2.
Time course for the formation of isomerized reaction product 4-18OS(18O,16O)C6H4-4-Me during solvolysis of 4-OS(18O)2C6H4-4-Me in 50/50 (v/v) TFE/H2O at 25 °C. The solid line shows the nonlinear least-squares fit of the data to eq 1 derived for Scheme 1 using ksolv = 3.6 × 10−6 s−1 and kiso = 1.8 × 10−6 s−1. The upper and lower dashed lines show the fits obtained using values of kiso that are 10% higher and 10% lower, respectively, than the value from least-squares analysis.
The solid line in Figure 2 shows the nonlinear least squares fit of the experimental data to eq 1 derived for Scheme 1 using ksolv = 3.6 × 10−6 s−1 determined by monitoring solvolysis by UV spectroscopy and kiso = 1.8 × 10−6 s−1, which is treated as a variable parameter. The upper and lower dashed lines in Figure 2 show that the experimental data is accommodated by values of kiso that lie within ±10% of the rate constant determined by least squares analysis.
Scheme 1.
| (1) |
DISCUSSION
It is surprising that the α-CF3 for α-CH3 substitution at 1+, which strongly destabilizes 4+ towards nucleophile addition, should have little effect on the rate constants for carbocation-nucleophile addition. [14–16] This shows that the increase in the reactivity of 4+ compared to 1+ toward nucleophile addition caused by the large effect of the α-CF3 substituent on the thermodynamic reaction driving force is essentially completely offset by a decrease in reactivity of 4+ due to an increase in the Marcus intrinsic barrier for carbocation-nucleophile addition.[23–25]
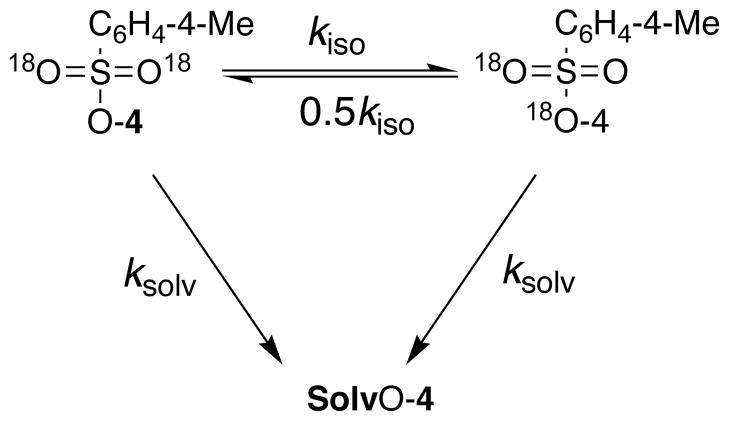
The sum of the rate constants for solvolysis and 18O-scrambling of 4-OS(18O)2C6H4-4-Me, ksolv + kiso = 5.4 × 10−6 s−1 is larger than ksolv = 3.6 × 10−6 s−1 for solvolysis of the unlabeled ester. This scrambling of oxygen-18 label between bridging and nonbridging positions may occur by rearrangement of these oxygen at an ion pair reaction intermediate followed by internal return to reactant (kr and k−1, Scheme 2), or the reaction may proceed by an uncoupled concerted pathway that avoids formation of a highly unstable reaction intermediate.[8,11,26]
Scheme 2.
There is good evidence that isomerization of esters that exchanges the position of carbon-bridging and nonbridging oxygen proceeds by a stepwise mechanism (Scheme 2), when the carbocation reaction intermediate has a lifetime of longer than 10−11 s.[8,26] The ion pair intermediate of solvolysis of 1-O2CC6F5 was shown in earlier work to undergo deprotonation to form alkene,[21] internal return to reform substrate,[6] and racemization followed by internal return to substrate.[5] A similar stepwise mechanism is strongly favored for the isomerization of 4-OS(18O)2C6H4-4-Me in 50/50 (v/v) water/trifluroethanol because the lifetimes determined for 1+ and 4+ in this solvent are similar.[21,27] The value of ks′ ≈ 6 × 109 s−1 for addition of solvent to 1+ and 4+ is estimated to be slightly larger than the value of 4 × 109 s−1 calculated using the azide ion “clock”,[27,28] because a significant fraction of the azide ion adduct is formed by a preassociation mechanism that does not involve diffusion-controlled trapping of the carbocation.[21,27]
Equations 1–3 give the relationships between the experimental rate constants ksolv and kiso and the microscopic rate constants for the individual steps in Scheme 2, where ks′ is the rate constant for direct addition of solvent to the ion pair reaction intermediate, and kd is rate constant for irreversible diffusional separation of the ion pair to the free carbocation which then undergoes addition of solvent.[6] These rate laws were derived by making the assumption that there is a single rate constant k−1 for collapse of the two ion pairs in Scheme 2, and that the reorganization of the ion pair that exchanges any of the three equivalent sulfonate oxygen (kr ≈ 1011 s−1)[7] is much faster than the other reactions of the ion pair, so that kr ≫ (ks′ + k−d), k−1′. Under these conditions both the steady state concentrations and their rates of internal return of the two ion pairs to give the neutral esters will be equal.
A value of k−1 ≈ 1.7 × 1010 s−1 for unimolecular collapse of 4+•−18OS-(18O,16O)C6H4-4-Me to form 4-18OS(18O,16O)C6H4-4-Me can be calculated (eq 3) from the experimental rate constant ratio kiso/ksolv = 0.50 and using ks′ = 6 × 109 s−1 and k−d = 1.6 × 1010 s−1. The estimated rate constant for addition of the tosylate anion to 4+ is larger than ks′ = 6 × 109 s−1 for addition of the solvent 50/50 water/trifluoroethanol. This suggests that the sulfonate anion should show a modest selectivity towards nucleophile addition to carbocations in aqueous solution.
| (1) |
| (2) |
| (3) |
To the best of our knowledge the nucleophilic selectivity of tosylate anion towards addition to carbocations in aqueous solution has not been measured because: (1) Tosylate salts are relatively insoluble in water. (2) Tosylate carbocation•anion pairs must be generated by solvolysis of a reactive substrate with a leaving group that is better than tosylate (e.g., triflate) in order to give a tosylate ester product that is sufficiently stable to isolate. However, organic triflates are only stable when attached to electron deficient carbon (e.g. methyl triflate), and their reactions in water tend to proceed by a concerted-type mechanism that avoid formation of carbocation reaction intermediates.
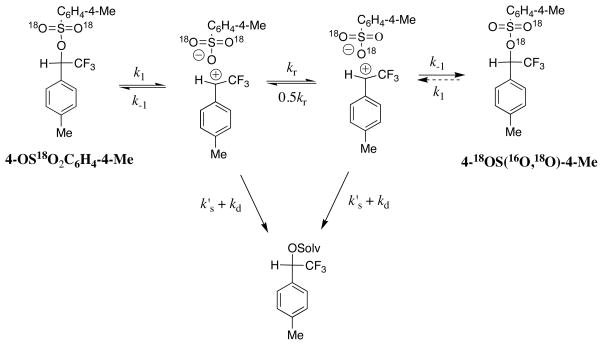
There is less internal return of the ion pair intermediate during solvolysis of 1-18OC(O)C6F5 in 50/50 trifluoroethanol/water (kiso/ksolv = 0.13)[6] compared with internal return during solvolysis of 4-OS(18O)2C6H4-4-Me in the same solvent (kiso/ksolv = 0.50). The two carbocations 1+ and 4+ show similar reactivity (ks′ = 6 × 109 s−1) toward addition of solvent.[15,21,27] The smaller rate constant ratio of kiso/ksolv = 0.13 for the reaction of 1-18OC(O)C6F5 compared with the reaction of 4-OS(18O)2C6H4-4-Me requires a smaller rate constant k−1 for internal return of the pentafluorobenzoate anion to form rearranged product 1-OC(18O)C6F5. Combining the rate constant ratio kiso/ksolv = 0.13 determined for the isomerization and solvolysis reactions of the pentafluorobenzoate ester 1-18OC(O)C6F5 with ks′ = 6 × 109 s−1 and k−d = 1.6 × 1010 s−1 gives k−1 = 7 × 109 s−1 for unimolecular collapse of the ion pair intermediate of solvolysis to the neutral benzoate ester, which is ca. 2-fold smaller than k−1 ≈ 1.7 × 1010 s−1 estimated in this work for collapse the intermediate of solvolysis of 4-OS(18O)2C6H4-4-Me (Scheme 3).[6]
Scheme 3.
The similar rate constants for collapse of the ion pairs shown in Scheme 3 is interesting. The two carbocations show the same intrinsic reactivity towards addition of nucleophlic solvent (ks′ = 6 × 109 s−1).[15,21,27] However, tosylate anion is ca 108-more reactive as a leaving group than pentafluorobenzoate anion in solvolysis reactions with rate determining bond cleavage of α-substituted 4-methoxybenzyl derivatives.[23] This leaving group is therefore expected to show a smaller nucleophilicity for reaction in the reverse bond synthesis direction. The observed similar reactivity of tosylate and pentaflurobenzoate towards addition to carbocations 1+ and 4+ which show similar intrinsic electrophilic reactivity almost certainly reflects a Hammond-type leveling in the selectivity of these anions in nucleophilic addition to reactive carbocations.[29]
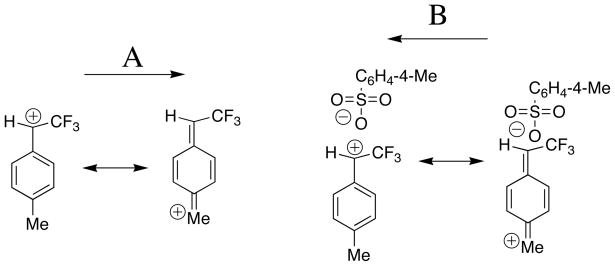
We have made the simplifying assumption that ks′ ≈ ks for addition of aqueous/trifluoroethanol to the ion pair 4+•−18OS-(18O,16O)C6H4-4-Me and to free carbocation 4+. We note, however, that there is good evidence that addition of the strongly electron-withdrawing α-CF3 group to benzylic carbocations is accompanied by an increase in delocalization of charge into the phenyl ring (Scheme 4A).[15,30] This attenuates the destabilizing inductive substituent effect by moving the center of positive charge away from the electron-withdrawing α-CF3 dipole. This increased delocalization in charge is the underlying cause of the large Marcus intrinsic barriers for nucleophile addition to ring-substituted phenyl 2,2,2-trifluoroethyl carbocations.[23,24] It is interesting to speculate that placement of a leaving group anion next to the carbocation might favor partial relocalization of charge onto the benzylic carbon (Scheme 4B). This would reduce the Marcus intrinsic reaction barrier and cause an increase in the rate constants for carbocation nucleophile addition that could partly explain the high observed reactivity of the tosylate anion in internal return to neutral substrate.
Scheme 4.
Acknowledgments
We acknowledge the National Institutes of Health (GM 39754) for generous support of this work.
References
- 1.Harris JM. Prog Phys Org Chem. 1974;11:89–173. [Google Scholar]
- 2.Raber DJ, Harris JM, Schleyer PvR. In: Ions and Ion Pairs in Organic Reactions. Szwarc M, editor. John Wiley & Son; New York: 1974. p. 2. [Google Scholar]
- 3.Tsuji Y, Kim SH, Saeki Y, Yatsugi KI, Fujio M, Tsuno Y. Tetrahedron Lett. 1995;36:1465–8. [Google Scholar]
- 4.Allen AD, Fujio M, Tee OS, Tidwell TT, Tsuji Y, Tsuno Y, Yatsugi KI. J Am Chem Soc. 1995;117:8974–8981. [Google Scholar]
- 5.Tsuji Y, Mori T, Toteva MM, Richard JP. J Phys Org Chem. 2003;16:484–490. [Google Scholar]
- 6.Tsuji Y, Mori T, Richard JP, Amyes TL, Fujio M, Tsuno Y. Org Lett. 2001;3:1237–1240. doi: 10.1021/ol015706s. [DOI] [PubMed] [Google Scholar]
- 7.Richard JP, Tsuji Y. J Am Chem Soc. 2000;122:3963–3964. [Google Scholar]
- 8.Amyes TL, Toteva MM, Richard JP. In: Reactive Intermediate Chemistry. Moss RA, Platz MS, MJ Jr, editors. John Wiley & Sons; Hoboken, NJ: 2004. pp. 41–68. [Google Scholar]
- 9.Tsuji Y, Toteva MM, Amyes TL, Richard JP. Org Lett. 2004;6:3633–3636. doi: 10.1021/ol0484409. [DOI] [PubMed] [Google Scholar]
- 10.Tsuji Y, Richard JP. Chem Rec. 2005;5:94–106. doi: 10.1002/tcr.20038. [DOI] [PubMed] [Google Scholar]
- 11.Tsuji Y, Richard JP. J Am Chem Soc. 2006;128:17139–17145. doi: 10.1021/ja066235d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen AD, Ambidge C, Che C, Michael H, Muir RJ, Tidwell TT. J Am Chem Soc. 1983:2343–2350. [Google Scholar]
- 13.Tidwell TT. Angew Chem, Int Ed Engl. 1984;23:20–32. [Google Scholar]
- 14.Richard JP. J Am Chem Soc. 1986;108:6819–20. [Google Scholar]
- 15.Richard JP. J Am Chem Soc. 1989;111:1455–65. [Google Scholar]
- 16.Richard JP, Amyes TL, Bei L, Stubblefield V. J Am Chem Soc. 1990;112:9513–19. [Google Scholar]
- 17.Stewart R, Teo KC. Can J Chem. 1980;58:2491–2496. [Google Scholar]
- 18.Allen AD, Ambidge IC, Che C, Micheal H, Muir RJ, Tidwell T. J Am Chem Soc. 1983;105:2343–2350. [Google Scholar]
- 19.Oae S, Kitao T, Kitaoka Y. Tetrahedron. 1963;19:827–832. [Google Scholar]
- 20.Allen AD, Jansen MP, Koshy KM, Mangru NN, Tidwell TT. J Am Chem Soc. 1982;104:207–211. [Google Scholar]
- 21.Richard JP, Jencks WP. J Am Chem Soc. 1984;106:1373–1383. [Google Scholar]
- 22.Risley JM, Etten RLV. J Am Chem Soc. 1979;101:252–253. [Google Scholar]
- 23.Amyes TL, Stevens IW, Richard JP. J Org Chem. 1993;58:6057–6066. [Google Scholar]
- 24.Richard JP, Amyes TL, Toteva MM. Acc Chem Res. 2001;34:981–988. doi: 10.1021/ar0000556. [DOI] [PubMed] [Google Scholar]
- 25.Richard JP. Tetrahedron. 1995;51:1535–73. [Google Scholar]
- 26.Jencks WP. Chem Soc Rev. 1981;10:345–375. [Google Scholar]
- 27.Richard JP, Rothenberg ME, Jencks WP. J Am Chem Soc. 1984;106:1361–1372. [Google Scholar]
- 28.Richard JP, Jencks WP. J Am Chem Soc. 1982;104:4689–4691. [Google Scholar]
- 29.Hammond GS. J Am Chem Soc. 1955;77:334–338. [Google Scholar]
- 30.Lee I, Dong SC, Hak JJ. Tetrahedron. 1994;50:7981–7986. [Google Scholar]