Abstract
Aims
Much controversy exists concerning the efficacy of primary prophylactic implantable cardioverter-defibrillators (ICDs) in patients with low ejection fraction due to coronary artery disease (CAD) or dilated cardiomyopathy (DCM). This is also related to the bias created by function improving interventions added to ICD therapy, e.g. resynchronization therapy. The aim was to investigate the efficacy of ICD-only therapy in primary prevention in patients with CAD or DCM.
Methods and results
Public domain databases, MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials, were searched from 1980 to 2009 for randomized clinical trials of ICD vs. conventional therapy. Two investigators independently abstracted the data. Pooled estimates were calculated using both fixed-effects and random-effects models. Eight trials were included in the final analysis (5343 patients). Implantable cardioverter-defibrillators significantly reduced the arrhythmic mortality [relative risk (RR): 0.40; 95% confidence interval (CI): 0.27–0.67] and all-cause mortality (RR: 0.73; 95% CI: 0.64–0.82). Regardless of aetiology of heart disease, ICD benefit was similar for CAD (RR: 0.67; 95% CI: 0.51–0.88) vs. DCM (RR: 0.74; 95% CI: 0.59–0.93).
Conclusions
The results of this meta-analysis provide strong evidence for the beneficial effect of ICD-only therapy on the survival of patients with ischaemic or non-ischaemic heart disease, with a left ventricular ejection fraction ≤35%, if they are 40 days from myocardial infarction and ≥3 months from a coronary revascularization procedure.
Keywords: Implantable cardioverter-defibrillator, Mortality, Meta-analysis, Primary prevention, Sudden cardiac death
Introduction
Sudden cardiac and arrhythmic death (SCD) account for approximately 50% of the mortality in patients with left ventricular dysfunction.1 Life-threatening ventricular arrhythmias are involved in the majority of SCD occurrences.2 Randomized clinical trials have shown that the implantable cardioverter-defibrillator (ICD) is the most effective therapy currently available to prevent SCD by terminating ventricular arrhythmias.3–6 Therefore, the ICD has become the standard therapy for primary and secondary prevention of SCD in patients with left ventricular dysfunction.7,8 The addition of cardiac resynchronization therapy (CRT) to device therapy created not only possibilities to improve cardiac function in subgroups, but also influenced the outcome with respect to morbidity and mortality.9,10 In contrast, concerns were raised on the magnitude of the effectiveness of ICD therapy certainly in this era when the majority of infarction patients receive primary coronary intervention in a reasonable time frame.11 Some complications became more evident in the past few years, and co-morbidity became considered as limitation as it is associated with a less favourable outcome.12,13 Given these recent concerns about ICD therapy, we performed a systematic review and meta-analysis of randomized trials of primary prevention of SCD in patients with heart failure due to coronary artery disease (CAD) or dilated cardiomyopathy (DCM), which constitute the largest two subgroups of potential ICD recipients. We examined the efficacy of ICD-only therapy without CRT on rates of all-cause mortality and arrhythmic mortality. In addition, we assessed the rates of delivered ICD therapies.
Methods
Search strategy
A comprehensive search of public domain databases was performed to identify randomized clinical trials comparing ICD therapy with conventional medical therapy in patients with left ventricular dysfunction. The public domain databases MEDLINE (January 1980 to January 2009), EMBASE (1991 to the last quarter of 2008), and the Cochrane Central Register of Controlled Trials (last quarter of 2008) were searched using the terms implantable cardioverter-defibrillator, implantable defibrillator, randomized controlled trials, clinical trials, mortality, sudden death, and prevention. The search was restricted to humans and English language literature. In addition, we performed a manual search of secondary sources including references of initially identified articles and a search of reviews, editorials, commentaries, and proceedings from international cardiology meetings.
Eligibility and data abstraction
Studies considered for inclusion met the following criteria: the design was a randomized controlled clinical trial; patients were randomly assigned to ICD-only therapy excluding CRT vs. conventional medical therapy; the study population consisted of patients with left ventricular dysfunction deemed to be at high risk for sudden cardiac death or developing ventricular arrhythmias; and the main endpoints included all-cause mortality, cardiac mortality, or arrhythmic mortality. Trials in patients who survived sudden cardiac death or unstable ventricular arrhythmias (secondary prevention) were excluded. We also excluded trials in patients with inherited arrhythmic disorders, trials that did not report any of the main endpoints of interest, or trials with crossover rates greater than 50% between study groups.
The selection of studies, quality assessment, and data abstraction were performed independently by two investigators (D.A.M.J.T. and L.J.). The criteria for quality assessment included study design aspects as randomized clinical trial, description of crossover, withdrawals and dropouts, completeness of follow-up, and objectivity of the outcome assessment.14 Data regarding detailed inclusion criteria as patient characteristics [number, mean age, gender, left ventricular ejection fraction (LVEF)], ICD device type, duration of follow-up, rates of crossover, all-cause mortality, cardiac mortality, and arrhythmic mortality (as available) were abstracted from each study. Studies were grouped according to the aetiology of cardiomyopathy.
Data analysis
A meta-analysis of summary statistics from the individual trials was performed. For each study, data regarding all-cause mortality were used to calculate the risk ratios (RRs) and 95% confidence intervals (CIs). The RRs from each included trial were pooled using both fixed- and random-effects models that used weighting based on the inverse of the variance calculated according to DerSimonian and Laird.15 Evidence of statistical heterogeneity among the trial-specific RRs was checked and quantified by the I2 statistic, and a P-value ≤0.05 was considered statistically significant. When no significant statistical heterogeneity was identified, the fixed effect was preferentially used as the summary measure. In case of statistical heterogeneity, sensitivity analysis was performed to assess the contribution of each study to the pooled estimate by excluding individual studies one at a time and the pooled estimate was recalculated for the remaining studies. When pooled analysis still resulted in a significant heterogeneity, the random-effects model was used. Data analysis was performed with Cochrane Review Manager for Windows (release 5.0.24, the Cochrane Collaboration, Copenhagen, Denmark).
Results
Search results
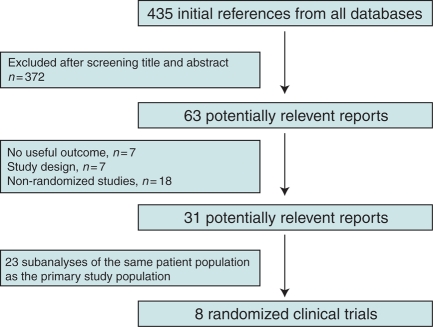
The selection of the included randomized clinical trials is shown in Figure 1. The search retrieved 435 potential relevant manuscripts. A total of 372 were excluded after the examination of the title and abstract. Of the 63 articles retrieved for further examination, 8 randomized clinical trials of ICD therapy for primary prevention were included for the analysis. No evidence of publication bias was found by funnel-plot analysis.
Figure 1.
Selection of trials included in the meta-analysis.
Qualitative findings
The included trials were randomized and controlled. The analysed primary prevention trials were the Multicenter Automatic Defibrillator Implantation Trial (MADIT),3 the Coronary Artery Bypass Graft Patch trial (CABG Patch),16 the Cardiomyopathy Trial (CAT),17 the MADIT II,5 the Amiodarone vs. Implantable Cardioverter-Defibrillator Randomized Trial (AMIOVIRT),18 the Defibrillator in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) trial,19 the Defibrillators in Acute Myocardial Infarction Trial (DINAMIT),20 and the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT).6 The Multicenter Unsustained Tachycardia Trial (MUSTT) and the Comparison of Medical Therapy, Pacing and Defibrillators in Chronic Heart Failure (COMPANION) trial were excluded from the analysis.21,22 The MUSTT trial was not a randomized controlled trial of ICD therapy, but compared with electrophysiological study guided antiarrhythmic therapy, including Class Ic drugs, in this way selecting patients for ICD therapy with empirical therapy. It is well known that Ic drugs are proarrhythmic.23,24 The COMPANION trial was not a comparison between ICD and conventional therapy, but studied the effects of CRT in a subgroup with pacing and a subgroup with ICDs, and therefore is not reporting on the effect of ICD-only therapy.
The characteristics of the included trials are presented in Table 1. In the eight trials, 5343 patients were randomly assigned to ICD therapy or conventional therapy. Of these trials, four trials evaluated patients with ischaemic heart disease (MADIT, CABG Patch, MADIT II, and DINAMIT), three trials examined patients with non-ischaemic heart disease (CAT, AMIOVIRT, and DEFINITE), and one trial (SCD-HeFT) enrolled patients with CAD and DCM.
Table 1.
Characteristics of included studies
| Study | Inclusion criteria | Patients (n) | ICD (n) | Follow-up (months) | Main result |
|---|---|---|---|---|---|
| MADIT, 1996 | EF ≤0.35; MI ≥3 weeks before entry; NSVT; NYHA I–III | 196 | 95 | 27 | ICD therapy resulted in 54% RR reduction, P = 0.009 |
| CABG Patch, 1997 | EF ≤0.35; abnormal SAECG, scheduled for CABG; NYHA I–III | 900 | 446 | 32 | ICD therapy did not reduce mortality, P = 0.64 |
| CAT, 2002 | EF ≤0.30; new onset DCM; NYHA II–III | 104 | 50 | 66 | ICD therapy did not reduce mortality, P = 0.55 |
| MADIT II, 2002 | EF ≤0.30; MI ≥1 month before entry; NYHA I–III | 1232 | 742 | 20 | ICD therapy resulted in 31% RR reduction, P = 0.016 |
| AMIOVIRT, 2003 | EF ≤0.35; DCM; asymptomatic NSVT; NYHA I–III | 103 | 51 | 36 | ICD therapy did not reduce mortality, P = 0.80 |
| DEFINITE, 2004 | EF ≤0.35; DCM; NSVT, NYHA I–III | 458 | 229 | 29 | ICD therapy resulted in 35% RR reduction, P = 0.08 |
| DINAMIT, 2004 | EF ≤0.35; within 6–40 days of MI; NYHA I–III; abnormal HRV | 674 | 332 | 33 | ICD therapy did not reduce mortality, P = 0.66 |
| SCD-HeFT, 2005 | EF ≤0.35; 3 months optimal medical therapy; NYHA II–III | 2521 | 829 | 45.5a | ICD therapy resulted in 23% RR reduction, P = 0.007 |
AMIOVIRT, Amiodarone vs. Implantable Defibrillator Randomized Trial; CABG Patch, Coronary Artery Bypass Graft Patch trial; CAT, Cardiomyopathy Trial; DCM, dilated cardiomyopathy; DEFINITE, Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation; DINAMIT, Defibrillator in Acute Myocardial Infarction Trial; EF, left ventricular ejection fraction; HRV, heart rate variability; MADIT, Multicenter Automatic Defibrillator Implantation Trial; MI, myocardial infarction; NYHA, New York Heart Association; NSVT, non-sustained ventricular tachycardia; SAECG, signal-averaged ECG; SCD-HeFT, Sudden Cardiac Death in Heart Failure Trial.
aMedian
The baseline clinical characteristics of patients are presented in Table 2. The mean age of the trial participants was 60 years, 79% were male, and CAD was present in 73%. The mean LVEF was 25% (range 21–28%), 59% of patients had New York Heart Association (NYHA) Class II heart failure symptoms, and 26% NYHA Class III.
Table 2.
Baseline clinical characteristics of patients assigned to implantable cardioverter-defibrillator therapy
| Study | Age (years) | Men (%) | EF (%) | NYHA (%) |
CAD (%) | Pharmcological therapy (%) |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| II | III | Amiodarone | β-blocker | Digoxin | ACE/ARB | |||||
| MADIT | 62 ± 9 | 92 | 27 ± 7 | II or III, 63 | 100 | 2 | 26 | 58 | 60 | |
| CABG Patch | 64 ± 9 | 87 | 27 ± 6 | II or III, 71 | 100 | 4 | 18 | 69 | 55 | |
| CAT | 52 ± 12 | 86 | 24 ± 6 | 67 | 33 | 0 | NA | 4 | 86 | 94 |
| MADIT II | 64 ± 10 | 84 | 23 ± 5 | 35 | 25 | 100 | 13 | 70 | 72 | 68 |
| AMIOVIRT | 58 ± 11 | 67 | 22 ± 10 | 64 | 16 | 0 | NA | 52 | 71 | 85 |
| DEFINITE | 58 | 73 | 21 | 54 | 21 | 0 | 4 | 86 | 42 | 97 |
| DINAMIT | 62 ± 11 | 76 | 28 ± 5 | NA | NA | 100 | 8 | 87 | NA | 95 |
| SCD-HeFT | 60a | 76 | 25a | 71 | 29 | 52 | NA | 69 | 67 | 94 |
ACE/ARB, angiotensin-converting enzyme/angiotensin receptor blocker; AMIOVIRT, Amiodarone vs. Implantable Defibrillator Randomized Trial; CABG Patch, Coronary Artery Bypass Graft Patch trial; CAT, Cardiomyopathy Trial; DEFINITE, Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation; DINAMIT, Defibrillator in Acute Myocardial Infarction Trial; EF, left ventricular ejection fraction; MADIT, Multicenter Automatic Defibrillator Implantation Trial; MI, myocardial infarction; NYHA, New York Heart Association; SCD-HeFT, Sudden Cardiac Death in Heart Failure Trial.
aMedian.
Quantitative findings
All-cause mortality
Coronary artery disease
The MADIT, MADIT II, and SCD-HeFT trials showed significant reductions in all-cause mortality with relative risk (RR) reductions ranging from 22 to 59%. In CABG Patch and DINAMIT, no reductions in all-cause mortality were found (RR: 1.07; 95% CI: 0.81–1.42, and RR: 1.08; 95% CI: 0.76–1.55, respectively). All these trials, except DINAMIT, mandated that patients should be enrolled if they were at least 3 weeks after myocardial infarction. Defibrillators in Acute Myocardial Infarction Trial was the only study designed to test ICD therapy as primary prevention in patients recovering from an acute myocardial infarction. The MADIT, MADIT II, and SCD-HeFT trials excluded patients who underwent coronary revascularization within 1 month before enrolment, whereas in CABG Patch, patients were enroled at the time of coronary artery bypass surgery.
When the results of the five randomized trials were pooled, we found statistical evidence of heterogeneity (I2 = 74.9%; P = 0.003). To assess the impact of heterogeneity on the pooled effect estimate, we performed sensitivity analysis (Table 3). After exclusion of CABG Patch and DINAMIT, no statistical evidence of heterogeneity was present (I2 = 61.5%; P = 0.07). Pooled analysis using a fixed-effects model of the remaining studies showed a 29% RR reduction in all-cause mortality with ICD therapy (95% CI: 17–39%; P < 0.0001). Analysis with a random-effects model yielded a 33% RR reduction in all-cause mortality (95% CI: 12–49%; P = 0.004).
Table 3.
Sensitivity analysis of randomized primary prevention trials in patients with ischaemic heart disease
| Study removed | RR | 95% CI | Heterogeneity (P-value) | ICD benefit (P-value) |
|---|---|---|---|---|
| MADIT | 0.87 | 0.77–0.99 | 0.04 | 0.03 |
| CABG Patch | 0.77 | 0.68–0.88 | 0.01 | 0.0002 |
| MADIT II | 0.88 | 0.77–1.00 | 0.003 | 0.05 |
| DINAMIT | 0.80 | 0.70–0.91 | 0.005 | 0.0005 |
| SCD-HeFT | 0.86 | 0.74–1.00 | 0.002 | 0.05 |
Removal of each trial (shown in column 1) followed by re-analysis of the pooled relative risk (RR; column 2) and 95% confidence intervals (95% CIs; column 3) for the remaining trials. P-values for heterogeneity and ICD benefit are shown in columns 4 and 5.
Dilated cardiomyopathy
A tendency towards a reduction in all-cause mortality by ICD therapy was found in two trials (DEFINITE and SCD-HeFT). The DEFINITE trial showed a RR of 0.65 for all-cause mortality with ICD therapy (95% CI: 0.40–1.06; P = 0.08). The RR for all-cause mortality was 0.74 in the SCD-HeFT trial (95% CI: 0.55–1.00). The CAT and AMIOVIRT trials found no reduction in all-cause mortality with ICD therapy compared with conventional therapy. When we pooled the data, the RR for all-cause mortality was 0.74 (95% CI: 0.59–0.93; P = 0.009) both in random- and fixed-effects models. No statistical evidence for heterogeneity was present among the trials enroling patients with DCM (I2 = 0%; P = 0.98).
Combined analysis
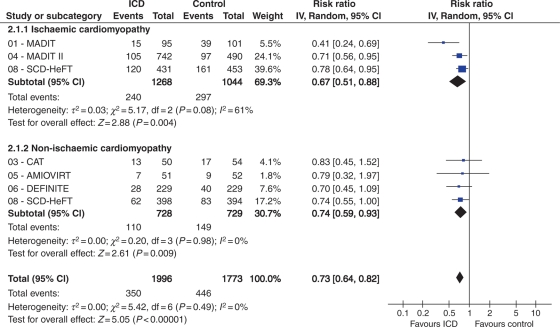
When we pooled the data of CAD and DCM, the summary of RR for all-cause mortality was 0.72 (95% CI: 0.64–0.82; P < 0.0001) with ICD therapy (Figure 2). No statistical evidence of heterogeneity was found (I2 = 0%; P = 0.49). The pooled analysis using a fixed-effects model demonstrates that ICD therapy significantly reduces the all-cause mortality, both in patients with CAD (RR: 0.71; 95% CI: 0.61–0.83) and DCM (RR: 0.74; 95% CI: 0.59–0.93). No significant differences in ICD benefit were found between ischaemic and non-ischaemic heart disease (I2 = 0%; P = 0.82).
Figure 2.
All-cause mortality among patients with ischaemic or non-ischaemic heart disease randomized to implantable cardioverter-defibrillator (ICD) vs. conventional therapy in primary prevention. For each randomized trial, the number of deaths (Events) and the number assigned (Total) are shown. The point estimates of the relative risk (RR) for individual studies are represented by squares with 95% confidence intervals (CIs) shown as bars. The midpoint of the diamond represents the overall pooled estimate of the RR, and the 95% CI is represented by the horizontal tips of the diamond. AMIOVIRT, Amiodarone vs. Implantable Defibrillator Randomized Trial; CAT, Cardiomyopathy Trial; DEFINITE, Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation; MADIT, Multicenter Automatic Defibrillator Implantation Trial; SCD-HeFT, Sudden Cardiac Death in Heart Failure Trial.
Arrhythmic mortality
Combined analysis
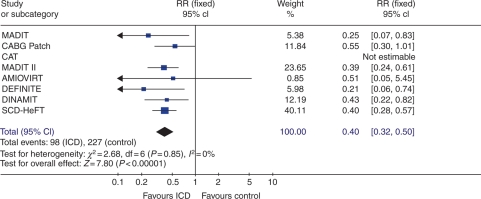
Among the 2774 patients randomized to ICD therapy, there were 98 sudden cardiac deaths compared with 227 sudden cardiac deaths among the 2569 patients randomized to conventional therapy. A pooled analysis using a fixed-effects model demonstrated a 60% relative reduction in arrhythmic mortality (RR: 0.40; 95% CI: 0.31–0.50; P < 0.0001) with ICD therapy (Figure 3). No statistical heterogeneity was found among the trials (I2 = 0%; P = 0.84). Subanalysis of SCD-HeFT demonstrated a significant reduction of arrhythmic mortality in CAD (RR: 0.43; 95% CI: 0.27–0.67) and DCM (RR: 0.34; 95% CI: 0.17–0.70).
Figure 3.
Arrhythmic mortality among primary prevention trials. For each randomized trial, the number of deaths (n) and the number assigned (N) are shown. The point estimates of the relative risk (RR) for individual studies are represented by squares with 95% confidence intervals (CIs) shown as bars. The midpoint of the diamond represents the overall pooled estimate of the RR, and the 95% CI is represented by the horizontal tips of the diamond. Abbreviations are the same as in Figure 1.
Implantable cardioverter-defibrillator therapy during follow-up
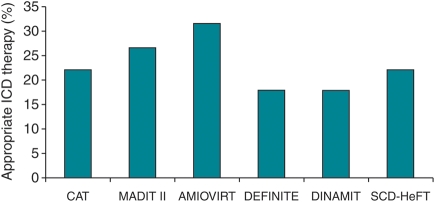
The appropriateness of ICD therapy could not be assessed reliably in the MADIT and in the CABG Patch trial, since only a small number of devices had the capacity of electrogram storage. The remaining six trials presented data on the number of patients who experienced appropriate ICD therapy delivered for ventricular tachyarrhythmias (Figure 4). The mean proportion of patients with appropriate ICD therapy was 22.9% (range 17.8–31.4%). The delivery of inappropriate ICD therapy was observed in 16.5% of the patients.
Figure 4.
Rates of appropriate implantable cardioverter-defibrillator therapy. Abbreviations are the same as in Figure 1.
Discussion
Arrhythmic death and implantable cardioverter-defibrillator interventions
Previous meta-analyses demonstrated that the ICD is associated with a 50% RR reduction for arrhythmic death in secondary prevention patients.25,26 In primary prevention, the risk reduction for arrhythmic death is similar. In SCD-HeFT, as was confirmed in this analysis, the benefit of ICD therapy in reducing arrhythmic death is similar, regardless of whether a patient had left ventricular dysfunction caused by CAD or DCM.27 The proportion of ICD interventions in the follow-up cannot be used as a surrogate for its efficacy in preventing mortality, but shows that ICDs are effective in terminating ventricular arrhythmias.
All-cause mortality
In contrast to arrhythmic mortality, the results on all-cause mortality are heterogeneous. We confirmed with this meta-analysis that there is a substantial benefit (a risk reduction of 27%), which is comparable with former analysis.28 Former meta-analysis did not exclude DINAMIT and CABG Patch, which both contributed significantly in the heterogeneity of the pooled analysis in CAD. This resulted in a homogeneous CAD population in our observation. The observed benefit for the entire group and for DCM remains present in spite of the inclusion of small negative trials studying DCM, and additional exclusion of trials such as MUSTT, comparing ICD therapy with potentially proarhythmic drug therapy as control.23,24 Further, in contrast to previous meta-analyses,26,28–30 we excluded trials with a potential benefit of CRT (as COMPANION), as CRT alone already has a benefit on survival.9,10 This is part of the explanation that ICD therapy in non-ischaemic cardiomyopathy only received a recommended Class I-B indication in the 2006 and 2008 international guidelines. 7,8 The benefit of CRT is most evident in patients with DCM and left bundle branch block,31 but its impact on mortality in comparison with ICD was not evident in a recent study in patients with mild heart failure,32 confirming our position that the benefit of both intervention modalities (ICD and CRT) should be further clarified.
Implantable cardioverter-defibrillator-only benefit
A huge variation exists in the utilization of ICDs with or without CRT in different social and medical environments.33 Uncertainty of the effect of the ICD alone in the present era of infarction therapy, and doubts on the value of ICDs for DCM, e.g. in the Dutch guidelines,34 prompted us to perform this meta-analysis, which was performed for the first time with unpublished data on SCD-HeFT (separate mortality data for ischaemic and non-ischaemic cardiomyopathy).
The timing of device implantation after myocardial infarction remains debated. The role of ICD therapy in the early post-myocardial infarction period was examined by the DINAMIT study.20 No benefit of ICD therapy in reducing all-cause mortality was observed when device implantation occurred within 40 days post-acute myocardial infarction. A recent study, Immediate Risk Stratification Improves Survival (IRIS), that included patients very early after infarction with additional risk factors confirmed this finding.35 Time-dependent benefit of ICD therapy was observed in the MADIT II study.36 Benefit was present for remote events more than 18 months after myocardial infarction, as could be expected from other observations.2
Thus, this analysis confirms that ICD-only therapy reduces the RR for all-cause mortality by 27% for patients with a LVEF ≤35%, if they are 40 days from myocardial infarction and ≥3 months from a coronary revascularization procedure, without a previous cardiac arrest or symptomatic ventricular arrhythmias. This beneficial effect of ICD-only therapy on survival exists regardless of whether a patient has left ventricular dysfunction due to CAD or DCM.
Study limitations
Our analysis has several limitations. First, we could not obtain individual patient data, which offers the possibility to explore subgroups which may benefit more or less from ICD therapy. The conclusions of this analysis are limited by the available data. Another possible limitation of our analysis is the influence of publication bias. This type of bias was minimized by an extensive search and through the inclusion of unpublished data in our analysis. We performed funnel-plot analysis which did not indicate publication bias, although the power is limited due to the small number of included studies.
Conclusion
The results of our meta-analysis provide strong evidence supporting the beneficial effect of ICD-only therapy on survival of patients with LVEF ≤35% due to ischaemic or non-ischaemic heart disease, if they are 40 days from myocardial infarction and at least 3 months from a coronary revascularization procedure.
Conflict of interest: D.A.M.J.T. received research grants from Biotronik, Boston Scientific, and St Jude Medical, and he is a consultant to Cameron Health (USA). L.J. received research grants and speaker fees from Biotronik, Boston Scientific, Medtronic, Sorin, and St Jude Medical. G.H.B. received research grants from St Jude Medical and the National Institute of Health (USA), and he has equity, intellectual property, and a board position in Cameron Health (USA). T.S. and M.G.M.H. have no conflicts of interest to declare.
Funding
This study was supported by ‘College voor zorgverzekeringen’ filed under project number OP08/642/07. Funding to pay the Open Access publication charges was provided by Thoraxcentrum Research BV in Rotterdam.
References
- 1.Solomon SD, Zelenkofske S, McMurray JJ, Finn PV, Velazquez E, Ertl G, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352:2581–8. doi: 10.1056/NEJMoa043938. doi:10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]
- 2.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–82. doi: 10.1056/NEJMra000650. doi:10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–40. doi: 10.1056/NEJM199612263352601. doi:10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 4.The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–83. doi: 10.1056/NEJM199711273372202. doi:10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. doi:10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 6.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. doi:10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 7.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace. 2006;8:746–837. doi: 10.1093/europace/eul108. doi:10.1093/europace/eul108. [DOI] [PubMed] [Google Scholar]
- 8.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1–62. doi: 10.1016/j.jacc.2008.02.032. doi:10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 9.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. doi:10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 10.Rivero-Ayerza M, Theuns DA, Garcia-Garcia HM, Boersma E, Simoons M, Jordaens LJ. Effects of cardiac resynchronization therapy on overall mortality and mode of death: a meta-analysis of randomized controlled trials. Eur Heart J. 2006;27:2682–88. doi: 10.1093/eurheartj/ehl203. doi:10.1093/eurheartj/ehl203. [DOI] [PubMed] [Google Scholar]
- 11.Tung R, Zimetbaum P, Josephson ME. A critical appraisal of implantable cardioverter-defibrillator therapy for the prevention of sudden cardiac death. J Am Coll Cardiol. 2008;52:1111–21. doi: 10.1016/j.jacc.2008.05.058. doi:10.1016/j.jacc.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 12.Lee DS, Tu JV, Austin PC, Dorian P, Yee R, Chong A, et al. Effect of cardiac and noncardiac conditions on survival after defibrillator implantation. J Am Coll Cardiol. 2007;49:2408–15. doi: 10.1016/j.jacc.2007.02.058. doi:10.1016/j.jacc.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 13.Hauser RG, Hayes DL. Increasing hazard of Sprint Fidelis implantable cardioverter-defibrillator lead failure. Heart Rhythm. 2009;6:605–10. doi: 10.1016/j.hrthm.2009.02.024. doi:10.1016/j.hrthm.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 14.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. doi:10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. doi:10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Bigger JT., Jr. Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery. Coronary Artery Bypass Graft (CABG) Patch Trial Investigators. N Engl J Med. 1997;337:1569–75. doi: 10.1056/NEJM199711273372201. doi:10.1056/NEJM199711273372201. [DOI] [PubMed] [Google Scholar]
- 17.Bansch D, Antz M, Boczor S, Volkmer M, Tebbenjohanns J, Seidl K, et al. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT) Circulation. 2002;105:1453–58. doi: 10.1161/01.cir.0000012350.99718.ad. doi:10.1161/01.CIR.0000012350.99718.AD. [DOI] [PubMed] [Google Scholar]
- 18.Strickberger SA, Hummel JD, Bartlett TG, Frumin HI, Schuger CD, Beau SL, et al. Amiodarone versus implantable cardioverter-defibrillator: randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia—AMIOVIRT. J Am Coll Cardiol. 2003;41:1707–12. doi: 10.1016/s0735-1097(03)00297-3. doi:10.1016/S0735-1097(03)00297-3. [DOI] [PubMed] [Google Scholar]
- 19.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–8. doi: 10.1056/NEJMoa033088. doi:10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 20.Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–8. doi: 10.1056/NEJMoa041489. doi:10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 21.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–90. doi: 10.1056/NEJM199912163412503. doi:10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 22.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. doi:10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 23.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–8. doi: 10.1056/NEJM199103213241201. doi:10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg HM, Dwyer EM, Jr, Hochman JS, Steinberg JS, Echt DS, Peters RW. Interaction of ischaemia and encainide/flecainide treatment: a proposed mechanism for the increased mortality in CAST I. Br Heart J. 1995;74:631–5. doi: 10.1136/hrt.74.6.631. doi:10.1136/hrt.74.6.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee DS, Green LD, Liu PP, Dorian P, Newman DM, Grant FC, et al. Effectiveness of implantable defibrillators for preventing arrhythmic events and death: a meta-analysis. J Am Coll Cardiol. 2003;41:1573–82. doi: 10.1016/s0735-1097(03)00253-5. doi:10.1016/S0735-1097(03)00253-5. [DOI] [PubMed] [Google Scholar]
- 26.Ezekowitz JA, Rowe BH, Dryden DM, Hooton N, Vandermeer B, Spooner C, et al. Systematic review: implantable cardioverter defibrillators for adults with left ventricular systolic dysfunction. Ann Intern Med. 2007;147:251–62. doi: 10.7326/0003-4819-147-4-200708210-00007. [DOI] [PubMed] [Google Scholar]
- 27.Packer DL, Prutkin JM, Hellkamp AS, Mitchell LB, Bernstein RC, Wood F, et al. Impact of implantable cardioverter-defibrillator, amiodarone, and placebo on the mode of death in stable patients with heart failure. Analysis from the Sudden Cardiac Death in Heart Failure Trial. Circulation. 2009;120:2170–6. doi: 10.1161/CIRCULATIONAHA.109.853689. doi:10.1161/CIRCULATIONAHA.109.853689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Khatib SM, Sanders GD, Mark DB, Lee KL, Bardy GH, Bigger JT, et al. Implantable cardioverter defibrillators and cardiac resynchronization therapy in patients with left ventricular dysfunction: randomized trial evidence through 2004. Am Heart J. 2005;149:1020–34. doi: 10.1016/j.ahj.2005.02.005. doi:10.1016/j.ahj.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Desai AS, Fang JC, Maisel WH, Baughman KL. Implantable defibrillators for the prevention of mortality in patients with nonischemic cardiomyopathy: a meta-analysis of randomized controlled trials. JAMA. 2004;292:2874–9. doi: 10.1001/jama.292.23.2874. doi:10.1001/jama.292.23.2874. [DOI] [PubMed] [Google Scholar]
- 30.Nanthakumar K, Epstein AE, Kay GN, Plumb VJ, Lee DS. Prophylactic implantable cardioverter-defibrillator therapy in patients with left ventricular systolic dysfunction: a pooled analysis of 10 primary prevention trials. J Am Coll Cardiol. 2004;44:2166–72. doi: 10.1016/j.jacc.2004.08.054. doi:10.1016/j.jacc.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 31.Reuter S, Garrigue S, Barold SS, Jais P, Hocini M, Haissaguerre M, et al. Comparison of characteristics in responders versus nonresponders with biventricular pacing for drug-resistant congestive heart failure. Am J Cardiol. 2002;89:346–50. doi: 10.1016/s0002-9149(01)02240-8. doi:10.1016/S0002-9149(01)02240-8. [DOI] [PubMed] [Google Scholar]
- 32.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–38. doi: 10.1056/NEJMoa0906431. doi:10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 33.Dickstein K, Bogale N, Priori S, Auricchio A, Cleland JG, Gitt A, et al. The European cardiac resynchronization therapy survey. Eur Heart J. 2009;30:2450–60. doi: 10.1093/eurheartj/ehp359. doi:10.1093/eurheartj/ehp359. [DOI] [PubMed] [Google Scholar]
- 34.van Erven L, van Dessel PFHM, Simmers TA, Gelder IC, Hauer RNW, Wever EFD, et al. Guidelines ICD implantation an update. 2005. http://www.nvvc.nl/media/richtlijn/25/Guidelines.ICD.implantation.2005-an.update.pdf . [Google Scholar]
- 35.Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffmann E, Wojciechowski D, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;361:1427–36. doi: 10.1056/NEJMoa0901889. doi:10.1056/NEJMoa0901889. [DOI] [PubMed] [Google Scholar]
- 36.Wilber DJ, Zareba W, Hall WJ, Brown MW, Lin AC, Andrews ML, et al. Time dependence of mortality risk and defibrillator benefit after myocardial infarction. Circulation. 2004;109:1082–4. doi: 10.1161/01.CIR.0000121328.12536.07. doi:10.1161/01.CIR.0000121328.12536.07. [DOI] [PubMed] [Google Scholar]