Abstract
OBJECTIVE
We describe a maturity-onset diabetes of the young (MODY) case with mutations involving both HNF4A and HNF1A genes.
RESEARCH DESIGN AND METHODS
A male patient was diagnosed with diabetes at age 17; the metabolic control rapidly worsened to insulin requirement. At that time no relatives were known to be affected by diabetes, which was diagnosed years later in both the parents (father at age 50 years, mother at age 54 years) and the sister (at age 32 years, during pregnancy).
RESULTS
The genetic screening showed a double heterozygosity for the mutation p.E508K in the HNF1A/MODY3 gene and the novel variant p.R80Q in the HNF4A/MODY1 gene. The genetic testing of the family showed that the father carried the MODY3 mutation while the mother, the sister, and her two children carried the MODY1 mutation.
CONCLUSIONS
MODY1 and MODY3 mutations may interact by chance to give a more severe form of diabetes (younger age at presentation and early need of insulin therapy to control hyperglycemia).
Maturity-onset diabetes of the young (MODY) (MIM #606391) is a genetically and clinically heterogeneous group of disorders characterized by early onset of noninsulin-dependent diabetes and autosomal dominant inheritance. At least seven types of MODY have been identified (1). Heterozygous mutations of the gene encoding the hepatocyte nuclear factor 1-α (HNF1A/MODY3) are the most common causes of MODY in northern Europe and a frequent cause of MODY in many other populations. Mutations in the HNF4α gene (HNF4A/MODY1) are considerably less common (2,3).
We describe the clinical history of a family, including a case in which double heterozygosity for two MODY mutations was documented.
RESEARCH DESIGN AND METHODS
The proband
The patient LN was found hyperglycemic at age 17 years (fasting blood glucose [FBG] 120–150 mg/dl). Previous medical history was negative. Birth weight was 4.350 kg, and no history of neonatal hypoglycemia was reported. After a 6-month nutritional therapy, insulin was required (two NPH injections/day). After 2 years, a remission took place; the patient stopped insulin and was treated with sulfonylureas for the following 10 years, when insulin was again required. Markers of autoimmune diabetes islet cell antibody (ICA)/GAD antibody (GADA) (immunofluorescence and ELISA; Euroimmun, Lübeck, Germany) were repeatedly negative, and fasting levels of C-peptide and insulin (Electrochemiluminescent immunoassay; Roche Diagnostics, Penzberg, Germany) remained detectable throughout the observation period (C-peptide 1.1–2.4 ng/ml). The patient has been on insulin therapy since 2006 (rapid analog before breakfast and lunch, premixed analog before dinner) with good metabolic control (A1C 6.8–7.2%), without evidence of micro- and macrovascular complications.
The relatives
LN′s younger sister was referred for hyperglycemia at screening (133 mg/dl) at week 8 of her first pregnancy (age 32 years). Her medical history was negative, apart from a single record of raised FBG (115 mg/dl) 1 year earlier. The sister's birth weight was 3.8 kg, and no history of neonatal hypoglycemia was reported. A1C was 6.7% (normal values <5.9%), 60-min glucose during a 50-g glucose tolerance test was 205 mg/dl. ICA/GADA were negative; C-peptide levels (fasting/postprandial) were 1.7/2.4 ng/ml. During pregnancy, basal-bolus insulin was required. After delivery (caesarean section at week 38), FBG normalized with dietary restrictions, but a 75-g oral glucose tolerance test was positive (120-min blood glucose 216 mg/dl). A second pregnancy, 2 years later, (caesarean section at week 40), again required basal-bolus insulin. A1C and fasting and postprandial glycemia remained in the normal range (<5.9% and <140 mg/dl at 2 h) through the pregnancies. Both newborns were macrosomic (male 4.350 kg; female 4.100 kg, at week 38 and 40, respectively) and hypoglycemic (26 and 37 mg/dl at birth) and needed prolonged postnatal intravenous glucose infusion (7 and 10 days).
The siblings' father (age 57 years) had been diagnosed with type 2 diabetes at age 50, and we found high FBG in the mother (133 and 135 mg/dl) during investigations (age 54 years). Considering the whole family phenotype, the negativity of autoimmune markers in all case subjects, and LN′s maintained endogenous insulin after 19 years of diabetes, we investigated the family for the presence of monogenic diabetes.
RESULTS
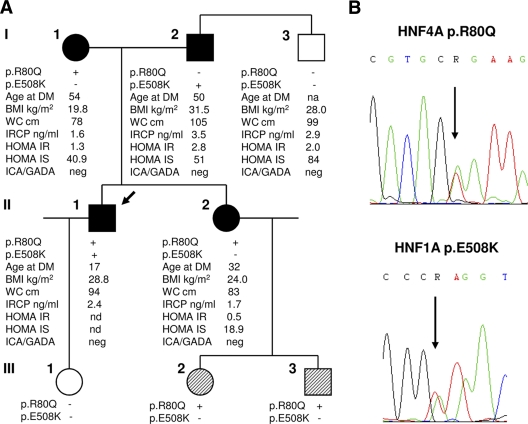
All subjects gave written informed consent. The molecular analysis of the HNF1A/MODY3 gene in the two siblings by denaturing high-performance liquid chromatography and sequencing of exons 1–10 and flanking regions identified the missense mutation p.E508K (c.1522G>A, exon 8) in the brother, which was absent in the sister. The further screening of exons 1d-10, flanking sequences, and the P2 promoter of HNF4A/MODY1 gene revealed the novel variant p.R80Q (c.239G>A, exon 2) in both siblings (Fig. 1) (supplementary Table 1 in the online appendix available at http://care.diabetesjournals.org/cgi/content/full/dc10-0561/DC1). The genetic testing of the family indicated that the father (I-2) carried the MODY3 variant, and the mother (I-1) carried the MODY1 variant, and so did both the children of the sister (III-2 and III-3). No variant was demonstrated in LN′s daughter (III-1) or in the uncle (I-3), who were both free of diabetes.
Figure 1.
A: Pedigree and genotypes of the family, showing that the proband LN (II-1) carries both the variants, while his parents (I-1, I-2), his sister EN (II-2), and the sister's children (III-2, III-3) carry a single mutation in either HNF4A or HNF1A gene. Filled symbols identify subjects with diabetes, diagonal hatching represents subjects with neonatal macrosomia and hypoglycemia, empty symbols identify healthy individuals, the arrow indicates the proband. Additional clinical data are also reported; age is at diagnosis of diabetes (DM). B: Representative chromatograms of the HNF4A p.R80Q and HNF1A p.E508K mutations identified in the family. IRCP, immunoreactive C-peptide; IS, insulin sensitivity; na, not applicable; nd, not done; WC, waist circumference.
The novel p.R80Q caused a replacement of an amino acid conserved across mammalian species. Using PolyPHEN (4) and SIFT (5) software for predicting the potential pathogenic effect, the variant was likely to be disease-causing mutations. The variant was absent in 144 normal chromosomes of Italian subjects.
CONCLUSIONS
We describe a case with double heterozygosity for mutations involving both the HNF4A/MODY1 and HNF1A/MODY3 genes. The proband carrying both variants shows a more severe form of diabetes compared with the family members carrying only one mutation.
The p.R80Q variant found in HNF4A gene has not been reported previously. A different mutation at codon 80 (p.R80W) has been recently detected in a patient with diazoxide-responsive hyperinsulinemic hypoglycemia (6). Our data confirm that this arginine is an important residue for the HNF4α function. Although no functional in vitro analysis was performed to identify the pathogenicity of this mutation, its role is supported by neonatal macrosomia and hypoglycemia (after a pregnancy with optimal glucose control) in both children inheriting the p.R80Q (7), segregation with diabetes and normal homeostasis model assessment–insulin resistance (HOMA-IR) in the family members, the absence of mutation in normal subjects, the amino acid conservation through evolution, and the bioinformatic predictions.
The p.E508K mutation detected in HNF1A gene has been previously reported as a MODY3-causing mutation. The late onset of diabetes of the proband's father carrying this mutation is consistent with the milder phenotype reported in patients with missense mutations in exons 8–10 that affect only the HNF1A(A) isoform (8). Though the coexistence of type 2 diabetes cannot be absolutely excluded in LN's father on the basis of the phenotype (overweight, waist circumference, and borderline HOMA-IR), the absence of hyperglycemia in LN's older wild-type uncle with similar phenotype is consistent with a primary role of mutation.
The combined effect of two variants may explain the more severe diabetes in the proband. Similar effects were shown in subjects with double mutations in HNF1A and the A3243G in mitochondrial DNA (9). HNF4α has a key role in regulating the islet transcriptional networks and, in combination with HNF1α, has been proposed to form a functional regulatory loop in adult β-cell (10). The clinical features of our patient with double heterozigosity support this hypothesis.
Supplementary Material
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
G.F. and V.M. researched data, contributed to the discussion, and wrote the manuscript. S.Z. researched data, contributed to the discussion, and reviewed/edited the manuscript. A.D.R. and E.M. performed genetic tests and reviewed/edited the manuscript. R.D.L. and M.S. researched data and reviewed/edited the manuscript. G.R. contributed to the discussion and reviewed/edited the manuscript. G.M. contributed to the discussion and wrote the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Fajans SS, Bell GI, Polonsky KS. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med 2001;345:971–980 [DOI] [PubMed] [Google Scholar]
- 2. Frayling TM, Bulamn MP, Ellard S, Appleton M, Dronsfield MJ, Mackie AD, Baird JD, Kaisaki PJ, Yamagata K, Bell GI, Bain SC, Hattersley AT. Mutations in the hepatocyte nuclear factor-1alpha gene are a common cause of maturity-onset diabetes of the young in the U.K. Diabetes 1997;46:720–725 [DOI] [PubMed] [Google Scholar]
- 3. Ellard S, Colclough K. Mutations in the genes encoding the transcription factors hepatocyte nuclear factor 1 alpha (HNF1A) and 4 alpha (HNF4A) in maturity-onset diabetes of the young. Hum Mutat 2006;27:854–869 [DOI] [PubMed] [Google Scholar]
- 4. Sunyaev S, Ramensky V, Koch I, Lathe W, 3rd, Kondrashov AS, Bork P. Prediction of deleterious human alleles. Hum Mol Genet 2001;10:591–597 [DOI] [PubMed] [Google Scholar]
- 5. Ng PC, Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acid Res 2003;31:3812–3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flanagan SE, Kapoor RR, Mali G, Cody D, Murphy N, Schwahn B, Siahanidou T, Banerjee I, Akcay T, Rubio-Cabezas O, Shield JP, Hussain K, Ellard S. Diazoxide-responsive hyperinsulinemic hypoglycemia caused by HNF4A gene mutations. Eur J Endocrinol 2010;162:987–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pearson ER, Boj SF, Steele AM, Barrett T, Stals K, Shield JP, Ellard S, Ferrer J, Hattersley AT. Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4A gene. PLoS Med 2007;4:e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bellanné-Chantelot C, Carette C, Riveline JP, Valéro R, Gautier JF, Larger E, Reznik Y, Ducluzeau PH, Sola A, Hartemann-Heurtier A, Lecomte P, Chaillous L, Laloi-Michelin M, Wilhem JM, Cuny P, Duron F, Guerci B, Jeandidier N, Mosnier-Pudar H, Assayag M, Dubois-Laforgue D, Velho G, Timsit J. The type and the position of HNF1A mutation modulate age at diagnosis of diabetes in patients with maturity-onset diabetes of the young (MODY)-3. Diabetes 2008;57:503–508 [DOI] [PubMed] [Google Scholar]
- 9. Cervin C, Liljeström B, Tuomi T, Heikkinen S, Tapanainen JS, Groop L, Cilio CM. Cosegregation of MIDD and MODY in a pedigree: functional and clinical consequences. Diabetes 2004;53:1894–1899 [DOI] [PubMed] [Google Scholar]
- 10. Odom DT, Zizlsperger N, Gordon DB, Bell GW, Rinaldi NJ, Murray HL, Volkert TL, Schreiber J, Rolfe PA, Gifford DK, Fraenkel E, Bell GI, Young RA. Control of pancreas and liver gene expression by HNF transcription factors. Science 2004;303:1378–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.