Abstract
OBJECTIVE
Physical activity may modify the association of adiposity with type 2 diabetes. We investigated the independent and joint association of adiposity and physical activity with fasting plasma glucose, impaired fasting glucose, and type 2 diabetes in a Chinese population.
RESEARCH DESIGN AND METHODS
Middle-aged and older Chinese (n = 28,946, ≥50 years, 72.4%women) from the Guangzhou Biobank Cohort Study were examined in 2003–2008. Multivariable regression was used in a cross-sectional analysis.
RESULTS
BMI, waist circumference, and waist-to-hip ratio (WHR) were positively associated with type 2 diabetes after multiple adjustment, most strongly for WHR with odds ratio (OR) of 3.99 (95% CI 3.60–4.42) for highest compared with lowest tertile. Lack of moderate-to-vigorous physical activity, but not walking, was associated with diabetes with an OR of 1.29 (1.17–1.41). The association of moderate-to-vigorous activity with fasting glucose varied with WHR tertiles (P = 0.01 for interaction). Within the high WHR tertile, participants who had a lack of moderate-to-vigorous activity had an OR of 3.87 (3.22–4.65) for diabetes, whereas those who were active had an OR of 2.94 (2.41–3.59).
CONCLUSIONS
In this population, WHR was a better measure of adiposity-related diabetes risk than BMI or waist circumference. Higher moderate-to-vigorous activity was associated with lower diabetes risk, especially in abdominally obese individuals.
Type 2 diabetes is a worldwide cause of morbidity and mortality. Adiposity, especially abdominal adiposity, seems to be at the core of development of hyperglycemia and type 2 diabetes (1). Increased physical activity may mitigate some of the diabetogenic impact of adiposity (2–4). Individuals who are obese but fit could even have a lower risk of mortality than those who are normal weight but unfit (5,6). However, being physically active does not completely abolish the obesity-related risk for cardiovascular disease and associated mortality (7). Adiposity is still the main risk factor for the development of type 2 diabetes (2–4,8). Although increased physical activity has been shown to be associated with reduced type 2 diabetes risk independent of adiposity, the protective effects may differ by the level of adiposity. However, the group that could benefit most from physical activity for the prevention of diabetes is still unclear (2–4,8–10).
Understanding the relationship between adiposity and physical activity is important to stratify risk groups for the development of effective diabetes prevention strategies from public health and clinical perspectives. Most of the studies relate to Caucasians (2–4,8–10), whereas Asians, including Chinese and Indians, are possibly more vulnerable to insulin resistance (11). The number of Chinese adults with type 2 diabetes was estimated to be ∼28.1 million in 2000 and may double by 2030, with China being second only to India (12). The purpose of this study was to investigate the independent and joint association of adiposity and physical activity with fasting plasma glucose, impaired fasting glucose (IFG), and type 2 diabetes in 28,946 middle-aged and older Chinese participants in the Guangzhou Biobank Cohort Study.
RESEARCH DESIGN AND METHODS
The Guangzhou Biobank Cohort Study is an ongoing collaboration among the Guangzhou Number 12 Hospital, Guangzhou, China, the University of Hong Kong, Hong Kong, and University of Birmingham, Birmingham, U.K. The study has been described in detail previously (13). All participants were drawn from the Guangzhou Health and Happiness Association for Respectable Elders, a community social and welfare association unofficially aligned with the municipal government, for which membership is open to anyone aged ≥50 years for a monthly, nominal fee of 4 yuan (50 U.S. cents). The Guangzhou Health and Happiness Association for Respectable Elders has a citywide network with ∼95,000 members who are permanent residents, constituting ∼7% of Guangzhou residents (aged ≥50 years), of whom ∼33% were selected for this study and were included if they were capable of consenting, were ambulatory, and were not receiving treatment modalities that if omitted may result in immediate life-threatening risk, such as chemotherapy or radiotherapy for cancer or dialysis for renal failure.
In total, 30,499 participants were recruited in three cross-sectional phases during 2003–2008 and received measurement of obesity indexes according to a standard protocol. A blood sample from each participant was used to assess fasting plasma glucose with a Shimadzu CL-80000 clinical chemical analyzer (Shimadzu, Kyoto, Japan). A detailed questionnaire was used to assess life-course socioeconomic position, personal disease history, and physical activity.
Assessment of adiposity and physical activity
General obesity was categorized into normal weight (BMI <23 kg/m2), overweight (23 kg/m2 ≤ BMI < 25 kg/m2), and obese (BMI ≥ 25 kg/m2), as suggested for southern Chinese populations (14). Waist circumference and waist-to-hip ratio (WHR) were categorized as sex-specific tertiles. The lower limit of the high waist circumference tertile for women (>81 cm) and men (>86 cm) was comparable to the International Diabetes Federation definition of central obesity for Asians (≥80 cm for women and ≥90 cm for men).
A short version of the International Physical Activity Questionnaire (IPAQ) (15) provided information on the amount of time spent per week in vigorous, moderate, and walking activities during work, as part of house and outdoor work, transportation, recreation, and exercise. The IPAQ-Chinese showed good test-retest reliability with all intraclass correlation coefficients >0.80 (P < 0.001) in a random sample of this population. Compared with objective pedometry data, the IPAQ-Chinese showed moderate-to-high validity for total physical activity and walking (16). Weekly metabolic equivalent task scores (measured as MET hours) were calculated, assuming 8.0, 4.0, and 2.5 MET h, respectively, for vigorous, moderate, and walking activities. Moderate-to-vigorous activity was categorized into “active” (≥24 MET h/week), “moderate” (0 < and <24 METs h/week), and “inactive” (no METs). Walking and total physical activity were divided into tertiles of MET hours per week in all participants.
Ascertainment of outcomes
Glucose status was categorized as normal fasting plasma glucose (<6.1mmol/l), IFG (fasting glucose ≥6.1and <7.0 mmol/l and no glucose-lowering medication use or no history of diabetes), and type 2 diabetes (fasting glucose ≥7.0 mmol/l or treatment with glucose-lowering medications) (17). Because a categorical variable leads to loss of information, fasting glucose was also used as a continuous variable.
Statistical analysis
Multivariate multinomial logistical regression was used to estimate the odds ratios (ORs) of IFG and diabetes by adiposity and physical activity, both independently and jointly. We compared the Akaike information criterion value among models with different measures of adiposity used as continuous variables to assess the best predictor of diabetes. Multivariate censored-normal regression was used to censor the use of glucose-lowering medications and to assess the linear association of fasting glucose with WHR and physical activity independently and jointly. Whether the association of physical activity with fasting glucose or diabetes varied with adiposity was assessed from the significant interaction term by multiplying these two variables and was compared from the heterogeneity of models with and without interaction term. All P values were two-sided and obtained with STATA (version 9.2, StataCorp, College Station, TX).
RESULTS
Of 30,499 recruited participants, 28,946 participants remained after exclusion of missing data. Physical activity was associated with IFG, type 2 diabetes, and fasting glucose similarly in women and men, so women and men were combined. Table 1 shows that 22,247 (76.9% of the total) participants had normal fasting glucose, 3,064 (10.6%) had IFG, and 3,635 (12.5%) had type 2 diabetes. Participants with diabetes were less active than those with normal glucose with respect to moderate-to-vigorous activity.
Table 1.
Characteristics of 28,946 participants with normal glucose level, IFG, and type 2 diabetes
| Normal glucose | IFG | Type 2 diabetes | P value* | |
|---|---|---|---|---|
| n | 22,247 | 3,064 | 3,635 | |
| Women (%) | 16,142 (72.5) | 2,139 (69.8) | 2,673 (73.5) | <0.001 |
| Age (years) | 61.5 ± 7.2 | 63.2 ± 6.6 | 63.7 ± 6.6 | <0.001 |
| BMI (kg/m2)† | 23.51 ± 0.04 | 24.63 ± 0.12 | 24.75 ± 0.11 | <0.001 |
| Waist circumference (cm)† | 78.8 ± 0.1 | 81.7 ± 0.3 | 82.5 ± 0.3 | <0.001 |
| WHR† | 0.860 ± 0.001 | 0.885 ± 0.002 | 0.898 ± 0.002 | <0.001 |
| Fasting plasma glucose (mmol/l)† | 5.19 ± 0.01 | 6.40 ± 0.01 | 8.70 ± 0.10 | <0.001 |
| Central obesity (%)‡ | 6,738 (30.3) | 1,427 (46.6) | 1,855 (51.1) | <0.001 |
| Overall obesity (BMI ≥25 kg/m2) | 6,692 (30.1) | 1,360 (44.4) | 1,590 (43.7) | <0.001 |
| History of cardiovascular disease | 976 (4.4) | 169 (5.5) | 269 (7.4) | <0.001 |
| Education (%) | ||||
| Primary school | 9,092 (40.9) | 1,505 (49.1) | 1,750 (48.1) | |
| Middle school | 6,082 (27.3) | 737 (24.1) | 872 (24.0) | |
| Senior technical school/college or university | 7,073 (31.8) | 822 (26.8) | 1,013 (27.9) | <0.001 |
| Occupation (%) | ||||
| Manual | 10,665 (47.9) | 1,650 (53.8) | 1,873 (51.5) | |
| Nonmanual | 6,675 (30.0) | 930 (30.4) | 1,084 (29.8) | |
| Others | 4,907 (22.1) | 484 (15.8) | 678 (18.6) | <0.001 |
| Personal income (%) | ||||
| <10,000 yuan | 7,228 (32.5) | 1,054 (34.4) | 1,240 (34.1) | |
| 10,000–15,000 yuan | 9,776 (43.9) | 1,250 (40.8) | 1,556 (42.8) | |
| >15,000 yuan | 4,188 (18.8) | 589 (19.2) | 671 (18.5) | |
| Do not know | 1,055 (4.8) | 171 (5.6) | 168 (4.6) | 0.013 |
| Smoking (%) | ||||
| Never | 17,993 (80.9) | 2,442 (79.8) | 2,956 (81.3) | |
| Former smoker | 1,946 (8.8) | 340 (11.1) | 408 (11.2) | |
| Current | 2,301 (10.3) | 280 (9.1) | 271 (7.5) | <0.001 |
| Alcohol drinking (%) | ||||
| Never | 12,918 (58.4) | 1,670 (54.9) | 2,255 (62.3) | |
| Former drinker | 2,712 (12.3) | 565 (18.6) | 525 (14.5) | |
| Current | 6,493 (29.3) | 807 (26.5) | 838 (23.2) | <0.001 |
| Moderate to vigorous physical activity categories (%) | ||||
| Active (≥24 METs h/week) | 6,020 (27.1) | 839 (27.4) | 851 (23.4) | |
| Moderate (0 < and <24 METs h/week) | 5,710 (25.7) | 851 (27.8) | 927 (25.5) | |
| Inactive (no METs) | 10,517 (47.2) | 1,374 (44.8) | 1,857 (51.1) | <0.001 |
| Walking tertiles (%) | ||||
| Active (>44 METs h/week) | 7,223 (32.5) | 1,162 (37.9) | 1,126 (31.0) | |
| Moderate (17.5 < and ≤44 METs h/week) | 5,655 (25.4) | 817 (26.7) | 994 (27.3) | |
| Inactive (≤17.5 METs h/week) | 9,369 (42.1) | 1,085 (35.4) | 1,515 (41.7) | <0.001 |
| Total physical activity tertiles (%) | ||||
| Active (≥63 METs h/week) | 7,408 (33.3) | 1,179 (38.5) | 1,095 (30.1) | |
| Moderate (31 < and <63 METs h/week) | 7,578 (34.1) | 1,061 (34.6) | 1,339 (36.8) | |
| Inactive (≤31 METs h/week) | 7,261 (32.6) | 824 (26.9) | 1,201 (33.0) | <0.001 |
Data are means ± SD or SE (continuous variables) or n (%) (categorical variables).
*P values for continuous variables were tested by multivariate linear model; P values for categorical variables were tested by the χ2 test.
†Adjusted for age and sex.
‡Central obesity is defined as waist circumference ≥80 cm for women and ≥90 cm for men.
Table 2 shows that, after adjustment for study phase, age, sex, education, occupation, income, smoking, alcohol use, history of cardiovascular disease, and total physical activity, a larger BMI, waist circumference, or WHR was associated with a higher OR of IFG and diabetes. WHR showed the strongest association with diabetes. The model using WHR fitted better than a model including waist circumference or BMI in the association with IFG and diabetes, with the smallest Akaike information criterion value (P < 0.001). WHR was strongly associated with fasting glucose (P < 0.001).
Table 2.
Adjusted ORs for the independent association of BMI categories, waist circumference, and WHR tertiles with IFG and type 2 diabetes
| IFG |
Type 2 diabetes |
|||||
|---|---|---|---|---|---|---|
| n | Model 1* | Model 2 | n | Model 1* | Model 2 | |
| BMI category† | ||||||
| Normal | 982 | 1.00 | 1.00 | 1,077 | 1.00 | 1.00 |
| Overweight | 722 | 1.35 (1.21–1.49) | 1.08 (0.97–1.22) | 968 | 1.66 (1.51–1.83) | 1.12 (1.01–1.25) |
| Obese | 1,360 | 2.05 (1.88–2.25) | 1.44 (1.27–1.64) | 1,590 | 2.19 (2.01–2.39) | 1.11 (0.99–1.25) |
| Ptrend | <0.001 | <0.001 | <0.001 | 0.11 | ||
| Waist circumference tertile‡ | ||||||
| Low | 728 | 1.00 | 1.00 | 736 | 1.00 | 1.00 |
| Medium | 991 | 1.57 (1.42–1.74) | 1.41 (1.26–1.58) | 1,140 | 1.86 (1.69–2.06) | 1.76 (1.57–1.97) |
| High | 1,343 | 2.26 (2.05–2.49) | 1.72 (1.50–1.97) | 1,755 | 3.10 (2.82–3.40) | 2.84 (2.50–3.24) |
| Ptrend | <0.001 | <0.001 | <0.001 | <0.001 | ||
| WHR tertile‡ | ||||||
| Low | 703 | 1.00 | 1.00 | 555 | 1.00 | 1.00 |
| Medium | 1,051 | 1.56 (1.41–1.73) | 1.39 (1.25–1.54) | 1,175 | 2.24 (2.02–2.50) | 2.05 (1.84–2.29) |
| High | 1,306 | 2.13 (1.93–2.35) | 1.70 (1.52–1.89) | 1,900 | 3.99 (3.60–4.42) | 3.40 (3.05–3.80) |
| Ptrend | <0.001 | <0.001 | <0.001 | <0.001 | ||
Data are ORs (95% CI).
*Model 1: adjusted for study phase, age, sex, smoking and alcohol use, history of cardiovascular disease, occupation, education, income, and total physical activity.
†Model 2: based on model 1, further adjusted for waist circumference.
‡Model 2: based on model 1, further adjusted for BMI.
Table 3 shows that lower moderate-to-vigorous physical activity was independently associated with a higher OR of diabetes but not walking. There was no significant association of physical activity with IFG. To test the possibility of participants who might change their physical activity patterns in response to ill-health leading to reverse causation, we performed additional analyses. Of the total 3,635 participants with diabetes, 60.2% had known diabetes but did not appear different in walking or moderate-to-vigorous activity than those who had newly diagnosed diabetes (data not presented).
Table 3.
Adjusted OR for the independent association of walking tertiles, moderate-to-vigorous physical activity categories, and total physical activity tertiles with IFG and type 2 diabetes
| IFG |
Type 2 diabetes |
|||||||
|---|---|---|---|---|---|---|---|---|
| n | Model 1* | Model 2 | Model 3 | n | Model 1* | Model 2 | Model 3 | |
| Walking tertile†,‡ | ||||||||
| Active | 1,162 | 1.00 | 1.00 | 1.00 | 1,126 | 1.00 | 1.00 | 1.00 |
| Moderate | 817 | 0.91 (0.82–1.01) | 0.92 (0.83–1.01) | 0.92 (0.83–1.02) | 994 | 1.07 (0.97 – 1.17) | 1.06 (0.96–1.17) | 1.08 (0.98–1.19) |
| Inactive | 1,085 | 0.92 (0.83–1.01) | 0.93 (0.84–1.02) | 0.93 (0.84–1.03) | 1,515 | 1.06 (0.96 – 1.16) | 1.07 (0.97–1.17) | 1.10 (1.00–1.21) |
| Ptrend | 0.09 | 0.12 | 0.17 | 0.27 | 0.19 | 0.05 | ||
| Moderate-to-vigorous physical activity§,‖ | ||||||||
| Active | 839 | 1.00 | 1.00 | 1.00 | 851 | 1.00 | 1.00 | 1.00 |
| Moderate | 851 | 1.03 (0.93–1.14) | 1.03 (0.92–1.14) | 1.02 (0.92–1.14) | 927 | 1.09 (0.99–1.21) | 1.08 (0.98–1.20) | 1.07 (0.97–1.19) |
| Inactive | 1,374 | 1.03 (0.94–1.14) | 1.01 (0.93–1.12) | 1.02 (0.93–1.12) | 1,857 | 1.29 (1.17–1.41) | 1.25 (1.14–1.37) | 1.25 (1.14–1.37) |
| Ptrend | 0.51 | 0.71 | 0.82 | <0.001 | <0.001 | <0.001 | ||
| Total physical activity¶,# | ||||||||
| Active | 1,179 | 1.00 | 1.00 | 1.00 | 1,095 | 1.00 | 1.00 | 1.00 |
| Moderate | 1,061 | 0.92 (0.84–1.01) | 0.92 (0.84–1.00) | 0.92 (0.84–1.01) | 1,339 | 1.15 (1.05–1.26) | 1.15 (1.05–1.26) | 1.16 (1.06–1.27) |
| Inactive | 824 | 0.93 (0.84–1.03) | 0.92 (0.83–1.02) | 0.93 (0.84–1.03) | 1,201 | 1.17 (1.06–1.29) | 1.16 (1.05–1.28) | 1.18 (1.07–1.31) |
| Ptrend | 0.13 | 0.10 | 0.14 | 0.001 | 0.003 | 0.001 | ||
Data are ORs (95% CI).
*Model 1: adjusted for study phase, age, sex, smoking and alcohol use, history of cardiovascular disease, occupation, education, income and BMI.
†Model 2: based on model 1, further adjusted for waist circumference and moderate-to-vigorous physical activity.
‡Model 3: based on model 1, further adjusted for WHR and moderate-to-vigorous physical activity.
§Model 2: based on model 1, further adjusted for waist circumference and walking.
‖Model 3: based on model 1, further adjusted for WHR and walking.
¶Model 2: based on model 1, further adjusted for waist circumference.
#Model 3: based on model 1, further adjusted for WHR.
Table 4 shows the joint association of adiposity and moderate-to-vigorous activity with IFG and diabetes. Both higher WHR and lower activity were associated with diabetes (P for interaction = 0.45 between WHR and activity), and the association of WHR with diabetes was stronger than that of physical activity. Within the low WHR tertile, moderate-to-vigorous activity was not associated with diabetes (P = 0.23). Within the high WHR tertile, the active group had OR of 2.94 (95% CI 2.41–3.59), whereas the inactive group had an OR of 3.87 (3.22–4.65). Physical activity was not associated with IFG across different WHR tertiles. Repeating the analyses with a cut point of 5.6 mmol/l instead of 6.1 mmol/l did not change the findings.
Table 4.
Adjusted OR for the joint association of waist circumference or WHR tertiles and moderate-to-vigorous physical activity categories with IFG and type 2 diabetes
| IFG |
Type 2 diabetes |
|||||||
|---|---|---|---|---|---|---|---|---|
| Active | Moderate | Inactive | P trend | Active | Moderate | Inactive | P trend | |
| Waist circumference tertile | ||||||||
| Low | 1.00 | 1.19 (0.96–1.45) | 0.98 (0.82–1.19) | 0.89 | 1.00 | 0.97 (0.79–1.20) | 1.21 (1.01–1.46) | 0.05 |
| Medium | 1.48 (1.21–1.80) | 1.36 (1.11–1.67) | 1.52 (1.28–1.84) | 0.49 | 1.69 (1.38–2.06) | 1.85 (1.51–2.26) | 2.09 (1.74–2.50) | 0.005 |
| High | 1.75 (1.42–2.17) | 1.79 (1.45–2.21) | 1.81 (1.50–2.20) | 0.96 | 2.63 (2.14–3.24) | 2.98 (2.43–3.65) | 3.40 (2.82–4.11) | <0.001 |
| WHR tertile | ||||||||
| Low | 1.00 | 1.07 (0.88–1.32) | 0.98 (0.81–1.18) | 0.99 | 1.00 | 0.90 (0.71–1.14) | 1.10 (0.89–1.35) | 0.23 |
| Medium | 1.42 (1.17–1.71) | 1.44 (1.96–1.74) | 1.37 (1.14–1.63) | 0.54 | 1.81 (1.47–2.22) | 2.07 (1.69–2.53) | 2.27 (1.89–2.74) | 0.003 |
| High | 1.63 (1.34–1.98) | 1.65 (1.36–1.99) | 1.80 (1.51–2.14) | 0.30 | 2.94 (2.41–3.59) | 3.25 (2.67–3.96) | 3.87 (3.22–4.65) | <0.001 |
Data are ORs (95% CI). Adjusted for study phase, age, sex, smoking and alcohol use, history of cardiovascular disease, occupation, education, income, BMI, and walking.
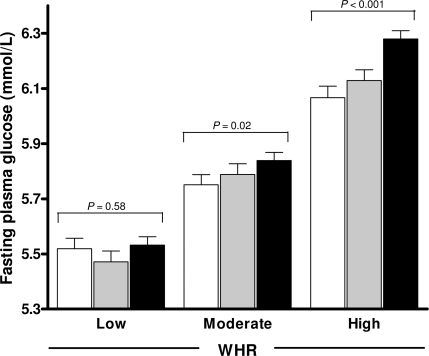
Figure 1 shows that the association of higher moderate-to-vigorous activity with lower fasting glucose varied with WHR tertiles (P for interaction = 0.01) but not with BMI (P for interaction = 0.65) or waist circumference (P for interaction = 0.30). In addition, the association of longer walking with lower fasting glucose was only found in the high WHR tertile (P = 0.02). The modification effect of walking over WHR tertiles was smaller (P for interaction = 0.05), compared with moderate-to-vigorous activity (data not presented).
Figure 1.
Fasting plasma glucose (millimoles per liter) stratified by WHR tertiles and moderate-to-vigorous physical activity categories (□, active;  , moderate; ■, inactive), adjusted for study phase, age, sex, BMI, smoking and drinking habits, history of cardiovascular disease, occupation, education, income and walking, and censored by the use of glucose-lowering medications (P = 0.01 for interaction).
, moderate; ■, inactive), adjusted for study phase, age, sex, BMI, smoking and drinking habits, history of cardiovascular disease, occupation, education, income and walking, and censored by the use of glucose-lowering medications (P = 0.01 for interaction).
CONCLUSIONS
In this cross-sectional survey, WHR was a better measure of adiposity-related type 2 diabetes risk than BMI or waist circumference. Moderate-to-vigorous physical activity but not walking duration was independently associated with type 2 diabetes. The strength of the association with WHR was greater than that with physical activity in the joint analysis. However, in the high WHR tertile, lack of moderate-to-vigorous physical activity was associated with ∼0.21mmol/l higher fasting glucose and ∼1.3-fold higher OR of diabetes, compared with those who had high moderate-to-vigorous activity (≥24 MET h/week). This association was not noticeable in the low WHR tertile group.
The present data are consistent with previous findings that central obesity, as indicated by waist circumference or WHR, is more strongly associated with type 2 diabetes than BMI (1). There are two main explanations for this observation in middle-aged and older Chinese people. First, people generally lose height with aging, so their BMI may be overestimated. Second, sarcopenia and increased body fat are associated with aging. For the same level of BMI, older adults generally have on average more fat and less muscle mass than young adults (18). Moreover, WHR showed a stronger association with diabetes than waist circumference in this population. Higher WHR can be the result of a larger waist or a smaller hip. We have previously shown that larger hips are negatively associated with diabetes in a similar southern Chinese population, but this is only significant in women after adjustment for BMI (19).
Physical activity is well known to independently decrease type 2 diabetes risk. In this study, only moderate-to-vigorous activity was negatively associated with diabetes. Even though walking, as a relatively low-intensity activity, may have a beneficial effect with daily performance over a long period, it was not associated with IFG or diabetes. Walking was, however, associated with lower fasting glucose in the high WHR tertile. Walking was the most prevalent activity in this population, probably because of its easy accessibility and minimal availability of personal mechanized transportation. We cannot exclude the possibility that random error in self-reported walking may underestimate the beneficial effect. Furthermore, participants might change their walking patterns in response to ill-health, which needs to be elucidated in a longitudinal study. Another explanation is that walking is only beneficial above a certain level of intensity. In the Nurses' Health Study, only women with a brisk walking pace (>4.8 km/h) had a significantly lower diabetes risk (2). However, we did not collect information on walking pace.
Results of previous cohort studies examining the interrelationship of adiposity and physical inactivity with diabetes are still controversial (20). Some researchers found that higher physical activity was associated with reduced diabetes risk, particularly in obese people (2–4). On the contrary, physical activity might be more beneficial for normal-weight than for obese individuals or equally beneficial (8–10). No studies reported a significant interaction between obesity and physical activity. However, those studies may not have had the power to detect such an interaction, especially when physical activity was crudely quantified or had a relatively small effect compared with obesity. A recent study showed that higher cardiorespiratory fitness was associated with a lower incidence of type 2 diabetes in overweight but not in lean individuals (21). Although cardiorespiratory fitness as an objective maker for habitual physical activity was not assessed in the present study, moderate-to-vigorous activity could partly contribute to fitness level. Our findings support the interaction of physical activity and adiposity with fasting glucose and suggest that physical activity has the potential to be more protective against elevated glucose concentrations in abdominally obese individuals, although this hypothesis needs further confirmation in intervention studies.
Physical activity may have independently beneficial effects through many biological mechanisms, e.g., by reducing inflammation stress, increasing skeletal muscle mass, and improving muscle fatty acid oxidative capacity, insulin sensitivity, and glucose disposal rate (22). Its chronic effects may not be completely independent of changes in body composition. After control for the acute effect of exercise, the beneficial effects on glucose metabolism are not noticeable in obese individuals, if no certain changes in body composition are achieved, e.g., reduction in weight and visceral fat (23). Exercise-induced weight loss does not appear to result in greater improvements than dietary-induced weight loss (24). This finding indicates that in the long-term exercise may mainly interact with a reduction in body fat, especially abdominal fat, to reduce diabetes risk, rather than having an independent effect.
Our study had several strengths. We had a large sample size (∼30,000 participants) and used three measures (BMI, waist circumference, and WHR) to estimate adiposity. To our knowledge, this is the first study to look at the joint associations of adiposity and physical activity with diabetes risk in the Chinese population, and one of the few to do so in a middle-aged and older population. Limitations of this study include the cross-sectional design, which makes the examination of causal relationships impossible, and recall error describing physical activity patterns over the previous 7 days. Second, our subjects may not have been completely representative of the older population of Guangzhou. However, every adult aged ≥50 years has equal and easy accessibility to this community association, and we did not expect that those who were willing to attend had interrelationships of adiposity, physical activity, and diabetes different from those who did not attend. This population had levels of diabetes comparable to nationally representative samples of urban Chinese within sex groups and age-groups (25). Although women were overrepresented, no differences in the associations analyzed were found in men and women. Third, a low level of physical activity could be the consequence of diabetes complications. However, participants with known diabetes were not more or less active than those with newly diagnosed diabetes with regard to walking and moderate-to-vigorous activity. Therefore, our observations were not likely to be the result of reverse causation.
In summary, the present data suggested that adiposity and moderate-to-vigorous physical activity were independently associated with type 2 diabetes. WHR as a measure of adiposity-related diabetes risk performed better than BMI or waist circumference alone in this population. Although abdominal adiposity was the key risk factor, higher moderate-to-vigorous physical activity was associated with lower diabetes risk, especially in abdominally obese individuals. Future prospective studies to investigate the underlying mechanisms of chronic physical activity to diabetes risk over different categories of abdominal adiposity are needed. Our data suggest that the potential of regular physical activity should be further explored as an important and low-cost primary prevention factor for type 2 diabetes in middle-aged and older Chinese people.
Acknowledgments
This study was supported by the University of Hong Kong Foundation for Education Development and Research, the Guangzhou Public Health Bureau, the Guangzhou Science and Technology Bureau, Graduate School for Health Research at the University of Groningen (SHARE), and the University of Birmingham.
No potential conflicts of interest relevant to this article were reported.
L.Q. and E.C. researched data, wrote the manuscript, contributed to discussion, and reviewed/edited the manuscript. C.J., C.M.S., R.P.S., and T.H.L. researched data, contributed to discussion, and reviewed/edited the manuscript. G.N.T. and K.K.C. researched data and reviewed/edited the manuscript. W.Z. and G.M.L. contributed to discussion and reviewed/edited the manuscript.
We thank the Clinical Trial Service Unit of the University of Oxford for their support and the Guangzhou Health and Happiness Association for the Respectable Elders for gathering the subjects.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev 2002;23:201–229 [DOI] [PubMed] [Google Scholar]
- 2. Hu FB, Sigal RJ, Rich-Edwards JW, Colditz GA, Solomon CG, Willett WC, Speizer FE, Manson JE. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA 1999;282:1433–1439 [DOI] [PubMed] [Google Scholar]
- 3. Hu G, Lindström J, Valle TT, Eriksson JG, Jousilahti P, Silventoinen K, Qiao Q, Tuomilehto J. Physical activity, body mass index, and risk of type 2 diabetes in patients with normal or impaired glucose regulation. Arch Intern Med 2004;164:892–896 [DOI] [PubMed] [Google Scholar]
- 4. Rana JS, Li TY, Manson JE, Hu FB. Adiposity compared with physical inactivity and risk of type 2 diabetes in women. Diabetes Care 2007;30:53–58 [DOI] [PubMed] [Google Scholar]
- 5. Church TS, Cheng YJ, Earnest CP, Barlow CE, Gibbons LW, Priest EL, Blair SN. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care 2004;27:83–88 [DOI] [PubMed] [Google Scholar]
- 6. Sui X, LaMonte MJ, Laditka JN, Hardin JW, Chase N, Hooker SP, Blair SN. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA 2007;298:2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med 2004;351:2694–2703 [DOI] [PubMed] [Google Scholar]
- 8. Weinstein AR, Sesso HD, Lee IM, Cook NR, Manson JE, Buring JE, Gaziano JM. Relationship of physical activity vs body mass index with type 2 diabetes in women. JAMA 2004;292:1188–1194 [DOI] [PubMed] [Google Scholar]
- 9. Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345:790–797 [DOI] [PubMed] [Google Scholar]
- 10. Meisinger C, Löwel H, Thorand B, Döring A. Leisure time physical activity and the risk of type 2 diabetes in men and women from the general population. The MONICA/KORA Augsburg Cohort Study. Diabetologia 2005;48:27–34 [DOI] [PubMed] [Google Scholar]
- 11. Dickinson S, Colagiuri S, Faramus E, Petocz P, Brand-Miller JC. Postprandial hyperglycemia and insulin sensitivity differ among lean young adults of different ethnicities. J Nutr 2002;132:2574–2579 [DOI] [PubMed] [Google Scholar]
- 12. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 13. Jiang C, Thomas GN, Lam TH, Schooling CM, Zhang W, Lao X, Adab P, Liu B, Leung GM, Cheng KK. Cohort profile: The Guangzhou Biobank Cohort Study, a Guangzhou-Hong Kong-Birmingham collaboration. Int J Epidemiol 2006;35:844–852 [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization/International Association for the Study of Obesity/International Obesity Taskforce. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Health Communications Australia, Melbourne, 2000 [Google Scholar]
- 15. International Physical Activity Questionnaire [article online], 2009. Available from http://www.ipaq.ki.se/downloads.htm. Accessed 10 June 2010
- 16. Deng HB, Macfarlane DJ, Thomas GN, Lao XQ, Jiang CQ, Cheng KK, Lam TH. Reliability and validity of the IPAQ-Chinese: the Guangzhou Biobank Cohort study. Med Sci Sports Exerc 2008;40:303–307 [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva, World Health Org., 1999. (Tech. Rep. Ser.) [Google Scholar]
- 18. Gallagher D, Visser M, Sepúlveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol 1996;143:228–239 [DOI] [PubMed] [Google Scholar]
- 19. Thomas GN, McGhee SM, Schooling M, Ho SY, Lam KS, Janus ED, Lam TH. for the Hong Kong Cardiovascular Risk Factor Prevalence Study Steering Committee. Impact of sex-specific body composition on cardiovascular risk factors: the Hong Kong Cardiovascular Risk Factor Study. Metabolism 2006;55:563–569 [DOI] [PubMed] [Google Scholar]
- 20. Qin L, Knol MJ, Corpeleijn E, Stolk RP. Does physical activity modify the risk of obesity for type 2 diabetes: a review of epidemiological data. Eur J Epidemiol 2010;25:5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee DC, Sui X, Church TS, Lee IM, Blair SN. Associations of cardiorespiratory fitness and obesity with risks of impaired fasting glucose and type 2 diabetes in men. Diabetes Care 2009;32:257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Corpeleijn E, Saris WH, Blaak EE. Metabolic flexibility in the development of insulin resistance and type 2 diabetes: effects of lifestyle. Obes Rev 2009;10:178–193 [DOI] [PubMed] [Google Scholar]
- 23. Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL, Wong SL, Nguyen-Duy TB, Lee S, Kilpatrick K, Hudson R. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res 2004;12:789–798 [DOI] [PubMed] [Google Scholar]
- 24. Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr 2006;84:1033–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gu D, Reynolds K, Duan X, Xin X, Chen J, Wu X, Mo J, Whelton PK, He J. Prevalence of diabetes and impaired fasting glucose in the Chinese adult population: International Collaborative Study of Cardiovascular Disease in Asia (InterASIA). Diabetologia 2003;46:1190–1198 [DOI] [PubMed] [Google Scholar]