Abstract
OBJECTIVE
To identify factors that influence survival after diabetes-related amputations.
RESEARCH DESIGN AND METHODS
We abstracted medical records of 1,043 hospitalized subjects with diabetes and a lower-extremity amputation from 1 January to 31 December 1993 in six metropolitan statistical areas in south Texas. We identified mortality in the 10-year period after amputation from death certificate data. Diabetes was verified using World Health Organization criteria. Amputations were identified by ICD-9-CM codes 84.11–84.18 and categorized as foot, below-knee amputation, and above-knee amputation and verified by reviewing medical records. We evaluated three levels of renal function: chronic kidney disease (CKD), hemodialysis, and no renal disease. We defined CKD based on a glomerular filtration rate <60 ml/min and hemodialysis from Current Procedural Terminology (CPT) codes (90921, 90925, 90935, and 90937). We used χ2 for trend and Cox regression analysis to evaluate risk factors for survival after amputation.
RESULTS
Patients with CKD and dialysis had more below-knee amputations and above-knee amputations than patients with no renal disease (P < 0.01). Survival was significantly higher in patients with no renal impairment (P < 0.01). The Cox regression indicated a 290% increase in hazard for death for dialysis treatment (hazard ratio [HR] 3.9, 95% CI 3.07–5.0) and a 46% increase for CKD (HR 1.46, 95% CI 1.21–1.77). Subjects with an above-knee amputation had a 167% increase in hazard (HR 2.67, 95% CI 2.14–3.34), and below-knee amputation patients had a 67% increase in hazard for death.
CONCLUSIONS
Survival after amputation is lower in diabetic patients with CKD, dialysis, and high-level amputations.
Diabetes is the most common underlying cause of nontraumatic amputation in the U.S. and Europe (1–4). Of the 120,000 amputations performed in the U.S. every year, 40–70% are in individuals with diabetes. Among individuals with end-stage renal disease receiving dialysis, the incidence of amputation is about 10 times higher than in the general diabetic population (5).
The in-hospital and 30-day mortality after amputation in people with diabetes is higher than in people with coronary artery bypass graft surgery, breast cancer, or stroke (6–8). However, there is little published data that report the long-term survival after amputation and even less data regarding patients with chronic kidney disease (CKD). The purpose of this study was to identify differences in the proportion of amputations and survival after lower-extremity amputation in individuals with diabetes and CKD and to identify risk factors for survival after an amputation.
RESEARCH DESIGN AND METHODS
We abstracted and reviewed medical records for hospitalizations of diabetic patients requiring lower-extremity amputations from 1 January to 31 December 1993 in six metropolitan statistical areas in south Texas, including San Antonio, Corpus Christi, Brownsville, McAllen, Laredo, and Victoria. Each hospital in the study areas, including military and Veterans Administration hospital facilities, provided a list of patients that had amputations in 1993. We also abstracted medical records at the nearest state hospital facility (University of Texas Medical Branch, Galveston, Texas) to verify that indigent or uninsured patients did not leave their local community hospital to receive care at a state facility. Only three amputees were identified at the state hospital that lived in the geographic areas being evaluated.
Amputations were identified from ICD-9-CM codes and were categorized by the level as foot (84.11–84.12), below-knee (84.13–84.16), or above-knee (84.17–84.18) (9). Operative reports and medical records were reviewed to ascertain the amputation level. Foot amputations were defined as amputations below the ankle, below-knee amputations from the ankle to through-knee amputations, and above-knee amputations as amputations proximal to through-knee procedures. For individuals who had undergone multiple amputations on the same extremity, the highest level of amputation was used in the analysis.
Race was recorded from the admitting physician's history and physical, hospital admission database information, and the nurse's hospital admission notes. History of previous ipsilateral or contralateral lower-extremity amputation and the level of amputation were identified from the physician's and nurse's admission notes. A diagnosis of diabetes was verified using World Health Organization criteria (10). Only 2.9% of amputees who had diabetes by these criteria did not have a diabetes-related ICD-9-CM code included in their discharge summary.
We identified mortality from death certificate information from the Social Security Death Index. We identified patients on hemodialysis by abstracting data from their medical records and defined CKD or renal status based on the National Kidney Disease Outcomes Quality Initiative (NKDOQI) guidelines as follows: 1) mild or no CKD, estimated glomerular filtration rate (eGFR) >60 ml/min; 2) moderate-to-severe CKD, eGFR <60 ml/min and subject is not on dialysis treatment (chronic CKD group); and 3) patients with end-stage renal disease on dialysis treatment (dialysis group). The eGFR was calculated using the Modification of Diet in Renal Disease formula based on creatinine levels immediately before amputation, sex, and race (11).
We used a χ2 test for trend to compare differences in the proportion of amputations by renal disease status (12). We used an α of 0.05 and 95% CI in the analysis. In addition, a Cox proportional hazard survival analysis was used to evaluate differences in survival based on renal disease and amputation level; renal disease and amputation level were represented as ordinal categorical variables. A Cox proportional hazard regression model (SPSS, version 17) was used to evaluate risk factors for 10-year mortality. In the analysis, multiple factors were examined using a forward stepwise procedure to evaluate which factors were appropriate for inclusion in the Cox regression. A score statistic was used to enter factors into a model, and a Wald statistic was used to withdraw factors from the model. Categorical variables for race, sex, level of amputation, marital status, level of renal disease, amputation history, peripheral vascular disease, smoking history, dialysis, cardiovascular disease, congestive heart failure, hypertension, and whether a bilateral amputation was performed were used in the stepwise procedure. Each patient's age was modeled as a continuous covariate.
RESULTS
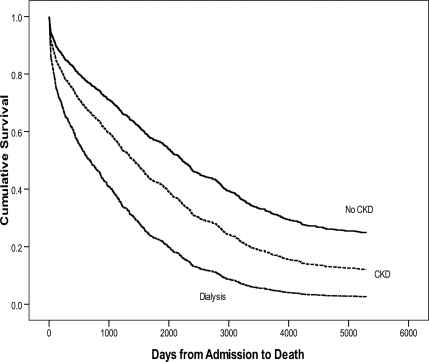
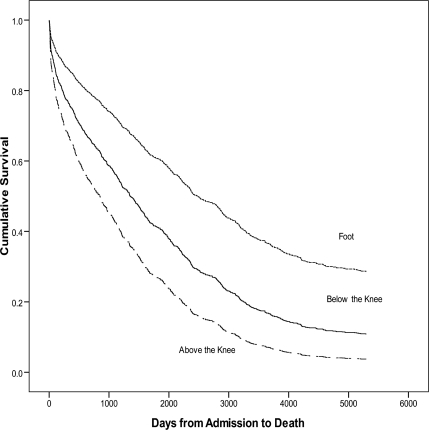
There were 1,043 individuals with diabetes and an amputation evaluated. The proportion of above-knee and below-knee amputations was significantly higher in patients with CKD and dialysis (Table 1). Survival rates after a foot, below-knee, or above-knee amputation over 10 years are also listed in Table 1. In the survival analysis, individuals with high-level amputations and individuals with renal disease displayed significantly higher mortality rates than individuals with lower-level amputations and individuals without renal disease, respectively (P < 0.001, Figs. 1 and 2).
Table 1.
Clinical characteristics and survival data
| No CKD | CKD | Hemodialysis | Total | P | |
|---|---|---|---|---|---|
| Demographics | |||||
| n | 526 | 389 | 128 | 1,043 | |
| Age [mean ± SD (range)] | 63.85 ± 12.76 (27–96) | 67.32 ± 11.91 (35–97) | 61.24 ± 11.5 (30–84) | 64.82 ± 12.4 (27–97) | <0.001 |
| Sex (% male) | 65.8 | 52.2 | 52.3 | 59.1 | <0.001 |
| Race | 0.066 | ||||
| African American | 7.6 | 5.1 | 8.6 | 6.8 | 0.24 |
| Hispanic | 78.7 | 76.3 | 81.3 | 78.1 | 0.46 |
| Caucasian | 13.7 | 18.5 | 10.2 | 15.1 | 0.033 |
| Comorbidities | |||||
| Coronary heart disease | 31.4 | 46.0 | 44.5 | 38.4 | <0.001 |
| Hypertension | 38.4 | 56.6 | 67.2 | 48.7 | <0.001 |
| Stroke | 15.0 | 17.0 | 13.3 | 15.5 | 0.5 |
| Coronary heart failure | 12.9 | 26.5 | 36.7 | 20.9 | <0.001 |
| Diabetes medication | |||||
| Insulin | 45.8 | 55.3 | 60.9 | 51.2 | <0.001 |
| Oral agents | 41.3 | 29.6 | 20.3 | 34.3 | <0.001 |
| Diet controlled | 8.0 | 7.5 | 10.9 | 8.1 | 0.45 |
| Surgery | |||||
| Coronary bypass | 7.6 | 13.4 | 20.3 | 11.3 | <0.001 |
| Lower-extremity bypass | 17.3 | 17.0 | 22.7 | 17.8 | 0.31 |
| History of amputation | 44.3 | 49.6 | 53.9 | 47.5 | <0.001 |
| Bilateral amputation | 24.7 | 30.1 | 36.7 | 28.2 | 0.015 |
| Amputation by level | |||||
| Foot | 53.8 | 40.4 | 28.9 | 45.7 | <0.001 |
| Below knee | 27.0 | 35.7 | 43.8 | 32.3 | <0.001 |
| Above knee | 19.2 | 23.9 | 27.3 | 22.0 | <0.001 |
| Percent survival after amputation | |||||
| 1 year | 85.6 | 76.6 | 50.8 | 77.9 | <0.001 |
| 2 years | 77.0 | 67.1 | 42.2 | 69.0 | <0.001 |
| 3 years | 73.2 | 56.3 | 28.9 | 61.5 | <0.001 |
| 4 years | 66.0 | 48.6 | 20.3 | 53.9 | <0.001 |
| 5 years | 60.3 | 40.9 | 17.2 | 47.7 | <0.001 |
| 6 years | 53.8 | 34.4 | 14.8 | 41.8 | <0.001 |
| 7 years | 47.1 | 27.8 | 12.5 | 35.7 | <0.001 |
| 8 years | 43.5 | 21.9 | 10.2 | 31.4 | <0.001 |
| 9 years | 36.7 | 19.8 | 10.2 | 27.1 | <0.001 |
| 10 years | 32.9 | 17.0 | 9.4 | 24.1 | <0.001 |
Figure 1.
Survival function for CKD. There was significantly higher mortality in dialysis patients and in patients with CKD than in patients with no kidney disease.
Figure 2.
Survival function for amputation level. There was significantly higher mortality in patients with above-knee and below-knee amputations compared with foot amputations.
The Cox proportional hazard model (Table 2) indicated that the hazard of death increased by 3% for each additional year postamputation for all study subjects: the estimated hazard ratio (HR) was 1.03 (95% CI 1.02–1.04). When subjects with no renal disease were used as the reference group for comparison, the survival analysis indicated that there was a 290% increase in hazard for death for individuals on dialysis treatment (HR 3.9, 95% CI 3.07–5.0), and there was a 46% increase in hazard for death for patients with CKD (HR 1.46, 95% CI 1.21–1.77). Compared with subjects with CKD, there was a 160% increase in hazard among dialysis patients.
Table 2.
HR for risk of death based on different patient characteristics
| HR | 95% CI | P | |
|---|---|---|---|
| Age | 1.031 | 1.023–1.039 | <0.0001 |
| CKD* | 1.465 | 1.213–1.771 | <0.0001 |
| Hemodialysis* | 3.912 | 3.071–4.982 | <0.0001 |
| Below-knee amputation† | 1.669 | 1.355–2.055 | <0.0001 |
| Above-knee amputation† | 2.672 | 2.137–3.341 | <0.0001 |
*Reference group for comparison is “no renal disease.”
†Reference group for comparison is “foot amputation.” HRs are estimated using Exp(B).
Taking foot level amputations as the reference group for the survival analysis, subjects with an above-knee amputation had a 167% increase in hazard compared with the reference group (HR 2.67, 95% CI 2.14–3.34). Similarly, individuals with a below-knee amputation had a 67% increase in hazard for death over baseline (HR 1.67, 95% CI 1.36–2.06). Additionally, going from a below-knee amputation to an above-knee amputation increases the hazard by 60% (HR 1.60, 95% CI 1.20–2.13).
CONCLUSIONS
The results of this study suggest that renal disease, higher level of amputation, and advancing age adversely affect survival after a lower-extremity amputation in individuals with diabetes. Several studies have identified a relationship between proximal amputation and decreased survival (13–15). Our data provide additional information highlighting that this association is more pronounced in subjects with CKD longitudinally for each year and over a 10-year period after an amputation. There are only a handful of studies that address long-term survival after amputation or evaluate the impact of renal disease among amputees.
Most of the existing work reports in-hospital or 30-day survival after amputation. In a study of 8,169 hospitalizations in California for lower-extremity amputation, we found that in-hospital mortality in individuals with diabetes was higher as amputation level increased (foot 1.5%, leg 4%, and thigh 7%) (9). Mayfield et al. (13) reported 3-year survival results demonstrating the same trend (foot 54%, leg 44%, and thigh 30%); however, neither study reported level specific rates for patients based on presence or severity of renal disease (9,13). Our first-year survival rate for the hemodialysis patients (50.8%) is in agreement with that of an observational report by McGrath and Curran (16). In this study, the authors retrospectively reviewed 47 diabetic patients who had had an amputation, 86% (32 patients) of whom were Maoris and 30% (14 patients) were on dialysis. Seven out of the 14 patients on dialysis died within 1 year after amputation, giving a first-year mortality of 50%. However, the number of patients included in this study was small.
O'Hare et al. (6) identified a 30-day postoperative amputation mortality of 16% in dialysis patients and 6% in individuals with diabetes and no renal disease, but did not evaluate level-specific rates. Patients with CKD probably have more severe diabetes-related comorbidities, so a high-level amputation and mortality may reflect the severity of peripheral vascular disease, neuropathy, cardiovascular disease, or poor response to infection (17).
It is noteworthy that mortality rates for some severe diseases are lower than those reported in patients with diabetes and end-stage renal disease postamputation. For instance, 1-, 3-, and 5-year survival rates after hospital admission for heart failure in patients with diabetes are 77, 50, and 32%, respectively (18); 5-year mortality after myocardial infarction in patients with diabetes is 72% (19); and 5-year survival after ischemic stroke has been reported to be 59.5% (20). Among dialysis patients, the 1- and 5-year survival is 79 and 68%, respectively (21); however, for patients with diabetes on hemodialysis, 5-year survival is only 54% (22). Our study similarly suggests a more dismal prognosis within dialysis amputees compared with nondialyzed subjects. One-year survival was 50.8% among dialysis amputees, 76.6% among amputees with CKD, and 85.6% among amputees without CKD.
In addition, our overall 1-year (78%) and 5-year (48%) survival rates are similar to those found by Hambleton et al. (23) (69 and 44%, respectively) in their study of Caribbean blacks in Barbados, but lower than that reported for American Indians (24). At first sight, this may appear counterintuitive because blacks, particularly in developing countries, would be expected to have higher mortality rates. However, Hambleton et al. alluded to the possibility of a different hierarchy of postamputation complications in the developing world. They suggested that infection contributed as much to mortality as cardiovascular disease, the traditional cause of postamputation mortality in the western world. This corroborates our findings that dialysis is associated with increased mortality, since individuals on dialysis are more prone to developing infections (25).
There are several limitations to this study that are inherent in retrospective audits of medical records. It is unclear if our findings are generalizable to other ethnic/racial groups in the U.S. The vast majority of the study population was Hispanic. Although there is a growing Hispanic population in the U.S., the characteristics of the population and medical resources in south Texas may not reflect the mortality experience or risk factors observed in other parts of the country. The most important limitations involved missing data and inconsistent definitions of pivotal risk factors. Important explanatory variables such as the severity of vascular disease, neuropathy, glucose control, duration of diabetes, heart disease, and infection were often not identified and may have been underreported. We used the Social Security Death Index to identify mortality. Mortality data could not be verified. In addition, we may have missed study subjects who returned to Mexico before they died. These patients may not have been reported in the death index we used. However, we believe that their inclusion would have further magnified a high mortality rate that is already apparent. On the other hand, data such as the presence of diabetes and level of amputation were easily identified in the medical records because they were well-documented multiple times through laboratory reports, ICD-9-CM codes, and operative reports.
Our data provide further evidence that CKD and dialysis treatment are independent risk factors for mortality after lower-extremity amputation. We also showed that the 10-year mortality among patients on dialysis who have an amputation was three times greater than patients who require amputation without CKD. Mortality in this population is higher than many other disease processes such as coronary artery disease, stroke, and cancer (6–8). Future research specific to patients with CKD will help us determine which prevention strategies and wound therapies will improve amputation prevention in this high-risk population.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
L.A.L. researched data, wrote the manuscript, and reviewed/edited the manuscript. N.A.H. research data, contributed to the discussion, and reviewed/edited the manuscript. A.N. reviewed/edited the manuscript. D.C.L. researched data and reviewed/edited the manuscript. W.V.H. reviewed/edited the manuscript. A.J.M.B. reviewed/edited the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Lavery LA, Ashry HR, van Houtum W, Pugh JA, Harkless LB, Basu S. Variation in the incidence and proportion of diabetes-related amputations in minorities. Diabetes Care 1996;19:48–52 [DOI] [PubMed] [Google Scholar]
- 2. Boulton AJ, Vileikyte L. The diabetic foot: the scope of the problem. J Fam Pract 2000;49:S3–S8 [PubMed] [Google Scholar]
- 3. Van Houtum WH, Lavery LA. Outcomes associated with diabetes-related amputations in the Netherlands and in the state of California, USA. J Intern Med 1996;240:227–231 [DOI] [PubMed] [Google Scholar]
- 4. van Houtum WH, Lavery LA, Harkless LB. The impact of diabetes-related lower-extremity amputations in the Netherlands. J Diabetes Complications 1996;10:325–330 [DOI] [PubMed] [Google Scholar]
- 5. Eggers PW, Gohdes D, Pugh J. Nontraumatic lower extremity amputations in the Medicare end-stage renal disease population. Kidney Int 1999;56:1524–1533 [DOI] [PubMed] [Google Scholar]
- 6. O'Hare AM, Feinglass J, Reiber GE, Rodriguez RA, Daley J, Khuri S, Henderson WG, Johansen KL. Postoperative mortality after nontraumatic lower extremity amputation in patients with renal insufficiency. J Am Soc Nephrol 2004;15:427–434 [DOI] [PubMed] [Google Scholar]
- 7. Kunadian B, Dunning J, Millner RW. Modifiable risk factors remain significant causes of medium term mortality after first time Coronary artery bypass grafting. J Cardiothorac Surg 2007;2:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Puskas JD, Lattouf OM, Kilgo P, Kerendi F, Song HK, Guyton RA, Thourani VH. Elevated preoperative hemoglobin A1c level is predictive of adverse events after coronary artery bypass surgery. J Thorac Cardiovasc Surg 2008;136:631–640 [DOI] [PubMed] [Google Scholar]
- 9. Lavery LA, van Houtum WH, Armstrong DG, Harkless LB, Ashry HR, Walker SC. Mortality following lower extremity amputation in minorities with diabetes mellitus. Diabetes Res Clin Pract 1997;37:41–47 [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization. WHO Expert Committee on Diabetes Mellitus. Second Report. Geneva, World Health Org., 1980. (Tech Rep Ser., no. 646) [PubMed] [Google Scholar]
- 11. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 12. Florkowski CM, Scott RS, Graham PJ, Han DY, Moir CL. Cause-specific and total mortality in the Canterbury (New Zealand) insulin-treated diabetic registry population: a 15-year follow-up study. Diabet Med 2003;20:191–197 [DOI] [PubMed] [Google Scholar]
- 13. Mayfield JA, Reiber GE, Maynard C, Czerniecki JM, Caps MT, Sangeorzan BJ. Survival following lower-limb amputation in a veteran population. J Rehabil Res Dev 2001;38:341–345 [PubMed] [Google Scholar]
- 14. Lavery LA, Van Houtum WH, Armstrong DG. Institutionalization following diabetes-related lower extremity amputation. Am J Med 1997;103:383–388 [DOI] [PubMed] [Google Scholar]
- 15. Izumi Y, Satterfield K, Lee S, Harkless LB, Lavery LA. Mortality of first-time amputees in diabetics: a 10-year observation. Diabetes Res Clin Pract 2009;83:126–131 [DOI] [PubMed] [Google Scholar]
- 16. McGrath NM, Curran BA. Recent commencement of dialysis is a risk factor for lower-extremity amputation in a high-risk diabetic population. Diabetes Care 2000;23:432–433 [DOI] [PubMed] [Google Scholar]
- 17. Ndip A, Lavery LA, Lafontaine J, Rutter MK, Vardhan A, Vileikyte L, Boulton AJ. High levels of foot ulceration and amputation risk in a multiracial cohort of diabetic patients on dialysis therapy. Diabetes Care 2010;33:878–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tribouilloy C, Rusinaru D, Mahjoub H, Tartière JM, Kesri-Tartière L, Godard S, Peltier M. Prognostic impact of diabetes mellitus in patients with heart failure and preserved ejection fraction: a prospective 5-year study. Heart 2008;94:1450–1455 [DOI] [PubMed] [Google Scholar]
- 19. Gandhi GY, Roger VL, Bailey KR, Palumbo PJ, Ransom JE, Leibson CL. Temporal trends in prevalence of diabetes mellitus in a population-based cohort of incident myocardial infarction and impact of diabetes on survival. Mayo Clin Proc 2006;81:1034–1040 [DOI] [PubMed] [Google Scholar]
- 20. Gunarathne A, Patel JV, Potluri R, Gammon B, Jessani S, Hughes EA, Lip GY. Increased 5-year mortality in the migrant South Asian stroke patients with diabetes mellitus in the United Kingdom: the West Birmingham Stroke Project. Int J Clin Pract 2008;62:197–201 [DOI] [PubMed] [Google Scholar]
- 21. Johnson JG, Gore SM, Firth J. The effect of age, diabetes, and other comorbidity on the survival of patients on dialysis: a systematic quantitative overview of the literature. Nephrol Dial Transplant 1999;14:2156–2164 [DOI] [PubMed] [Google Scholar]
- 22. Lee CC, Sun CY, Wu MS. Long-term modality-related mortality analysis in incident dialysis patients. Perit Dial Int 2009;29:182–190 [PubMed] [Google Scholar]
- 23. Hambleton IR, Jonnalagadda R, Davis CR, Fraser HS, Chaturvedi N, Hennis AJ. All-cause mortality after diabetes-related amputation in Barbados: a prospective case-control study. Diabetes Care 2009;32:306–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Resnick HE, Carter EA, Lindsay R, Henly SJ, Ness FK, Welty TK, Lee ET, Howard BV. Relation of lower-extremity amputation to all-cause and cardiovascular disease mortality in American Indians: the Strong Heart Study. Diabetes Care 2004;27:1286–1293 [DOI] [PubMed] [Google Scholar]
- 25. Powe NR, Jaar B, Furth SL, Hermann J, Briggs W. Septicemia in dialysis patients: incidence, risk factors, and prognosis. Kidney Int 1999;55:1081–1090 [DOI] [PubMed] [Google Scholar]