Abstract
OBJECTIVE
Treatment guidelines for diabetes have become increasingly stringent as most research shows that more aggressive intervention reduces the risks for complications. Community data on the effect of these interventions are lacking.
RESEARCH DESIGN AND METHODS
Changes in the pharmacologic treatment of diabetes, blood pressure, and cholesterol in adults with diabetes were analyzed in a longitudinal population-based study of American Indians from 10 independent 3-year time intervals between 1975 and 2004. Trends in drug use were assessed by logistic regression models and trends in glycemia, blood pressure, and cholesterol were assessed by linear models.
RESULTS
Among the study participants, the use of any medicine for the treatment of diabetes increased from 53% in 1975–1978 to 67% in 2002–2004, Ptrend < 0.0001. The use of insulin as a single agent declined, and the use of combinations of insulin and oral agents increased. In 1990–1992, 23% of subjects had an A1C <7% and by 2002–2004, the proportion had increased to 33%, Ptrend < 0.0001. The use of anti-hypertensive medicine increased from 21% in 1975–1977 to 58% in 2002–2004, Ptrend < 0.0001, coincident with a decline in mean systolic blood pressure from 137 mmHg in 1975–1977 to 123 mmHg in 2002–2004, Ptrend < 0.0001. The use of lipid-lowering medicine also increased with an accompanying increase in HDL and a decrease in non-HDL cholesterol concentration.
CONCLUSIONS
Major changes in community treatment patterns for diabetes and related conditions coincided with improvements in glycemia, blood pressure, and cholesterol.
There have been many revisions over time in the guidelines developed for the care of diabetic patients (1). Large clinical trials such as the Diabetes Control and Complications Trial (DCCT) and the UK Prospective Diabetes Study (UKPDS) have shown the importance of tight glycemic control and lower blood pressure for the prevention of complications in subjects with diabetes (2,3). Furthermore, the proliferation of new anti-hyperglycemic drugs (such as metformin and the thiazolidinediones) and insulin analogues has led to greater use of combination therapy with a wider variety of agents. New and more potent medicines for blood pressure and lipid management are also now more widely available. The targets set for blood pressure and cholesterol are lower for people with diabetes than for those without (1,4). Efforts to achieve these targets may be enhanced by greater use of more efficacious medicines and ultimately may lead to significant improvements in care.
The Pima Indians of central Arizona have an extraordinarily high prevalence of type 2 diabetes and have participated in a longitudinal study of health for over 40 years (5). The aim of the current study was to examine changes in medicine use over 30 years in diabetic Pima Indians who participated in this longitudinal study and to determine if changes in treatment corresponded with changes in glycemia, blood pressure, and cholesterol.
RESEARCH DESIGN AND METHODS
Individuals over 5 years of age who resided in a defined area of the Gila River Indian Community were invited to participate in research examinations every 2 years, regardless of their health. Primary medical care for community residents was provided separately from the research examinations, except when clinically relevant test results (laboratory, physical examination, or other) from the research examinations, including new diagnoses of diabetes, were reported to the participants and their health care providers and appropriate referrals were made. Diabetes was diagnosed at the research examinations by World Health Organization (WHO) criteria (6) if the plasma glucose concentration 2 h after a 75-g oral glucose tolerance test was ≥200 mg/dl or if a diagnosis was made during routine clinical care. At each examination, blood was also drawn after an overnight fast for measurement of total cholesterol, A1C, and, since 1992, for HDL cholesterol and triglycerides. Subjects were weighed while dressed in light clothing and no shoes, and height was measured. Blood pressure was measured at the first and fourth Korotkoff sounds with the subject supine.
Plasma glucose concentration was measured using the Technicon AutoAnalyzer between 1975 and October 1991 and since then by the hexokinase method. A1C was measured by high-performance liquid chromatography (HPLC) (Bio-Rad MDMS) from 1989 until 2000 and thereafter by HPLC (Tosoh A1c 2.2 Plus). A formula derived from duplicate analyses using both methods was used to convert the newer assay to the older assay, A1C old method = −0.2792 + (1.0066 × HbA1c new method). Total cholesterol was measured until April 1992 by the ferric chloride/acetic acid-sulfuric acid technique (Technicon AutoAnalyzer) and thereafter by the enzymatic method, which was also used for measuring HDL cholesterol.
A questionnaire was used to record current medicine use. Prior to April 1992, medicine use was recorded in broad categories only (e.g., anti-hypertensive), but since April 1992 medicines were recorded by name and only by type when more specific information was unavailable.
The present analysis included all diabetic subjects over 18 years of age who had research examinations between January 1975 and December 2004 and were not pregnant at the time of examination. Data from examinations at which diabetes was first diagnosed were not analyzed, since the subjects had not yet received diabetes management at these examinations. The investigators defined 10 independent 3-year time periods. Subjects could be included in multiple time periods. When a subject had more than one examination during a time period, only the examination closest to the midpoint of that time period was included. The 3-year time periods were selected to minimize the loss of information due to the exclusion of data from repeat examinations within the same period and to be of sufficient duration that reliable prevalence estimates could be made within each period. Variables with skewed distribution (total cholesterol, HDL cholesterol, non-HDL cholesterol, and triglycerides) were log transformed for analysis.
For continuous variables, relationship with time period was examined by linear regression. A numeric variable was used to represent time periods in sequential order and the resultant P value was taken as the test for secular trend (Ptrend). For dichotomous variables, time trends were assessed using logistic regression. Because observations within an individual are not independent of one another, an assumption of conventional regression methods, both linear and logistic models, were fit with generalized estimating equations that allow for lack of independence among observations (PROC GENMOD, SAS). Statistical significance was assessed with the empirical estimate of the standard error.
RESULTS
During the study, 2,019 diabetic individuals (1,218 women, 801 men) attended examinations. Of the participants, 58% (n = 1,173) attended during only 1 or 2 time periods, whereas 1% (n = 18) attended examinations in ≥9 time periods. More people attended examinations in the final time period (n = 775) than in earlier periods in keeping with an increase in population size and overall clinic attendance. Table 1 shows clinical characteristics of the participants by time period. Mean age of the attendees declined over the course of the study, whereas mean duration of diabetes increased. There was a trend toward higher BMIs for later time periods. The proportion of clinic attendees with diabetes was steady throughout the study period.
Table 1.
Demographic and clinical characteristics by time period: Gila River Indian Community, 1975–2004
| 1975–1977 | 1978–1980 | 1981–1983 | 1984–1986 | 1987–1989 | 1990–1992 | 1993–1995 | 1996–1998 | 1999–2001 | 2002–2004 | P trend | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 459 | 452 | 462 | 492 | 480 | 632 | 623 | 464 | 575 | 775 | — |
| % of clinic attendees with diabetes | 35 | 32 | 31 | 33 | 32 | 35 | 34 | 30 | 33 | 34 | — |
| Clinical diagnosis (%) | 64 | 53 | 50 | 46 | 46 | 47 | 47 | 46 | 48 | 50 | — |
| Male sex (%) | 34 | 37 | 35 | 33 | 33 | 36 | 34 | 32 | 33 | 37 | 0.98 |
| Age (years) | 51.6 (14.1) | 50.0 (13.8) | 50.6 (14.3) | 49.7 (13.5) | 49.5 (13.2) | 50.4 (12.6) | 51.2 (13.1) | 46.3 (13.1) | 45.8 (13.0) | 45.1 (13.0) | <0.0001* |
| Diabetes duration (years) | 9.5 (6.1) | 9.8 (6.2) | 10.9 (6.7) | 11.5 (6.9) | 12.4 (7.4) | 12.7 (8.1) | 13.0 (8.7) | 11.0 (8.2) | 11.0 (8.3) | 11.4 (8.5) | <0.0001† |
| Age at diagnosis (years) | 42.0 (12.7) | 40.2 (12.3) | 39.7 (12.5) | 38.1 (11.7) | 37.0 (11.6) | 37.7 (11.3) | 38.2 (11.4) | 35.3 (11.0) | 34.8 (11.0) | 33.6 (11.4) | <0.0001† |
| BMI (kg/m2) | 30.6 (6.5) | 30.7 (6.2) | 30.8 (6.5) | 32.1 (6.9) | 32.4 (7.0) | 32.8 (7.2) | 33.3 (7.8) | 34.9 (8.5) | 36.0 (8.6) | 36.3 (9.1) | <0.0001‡ |
Except for the second row, all results pertain only to persons with diagnosed diabetes. Data are means ± SD in parentheses or frequency (%). Clinical diagnosis refers to a diagnosis of diabetes made during routine clinical care rather than at a research examination (i.e., those without a clinical diagnosis had the diagnosis made at a previous research examination). P values computed for time period. For frequencies, P values are from χ2 tests; for continuous variables, P values are computed by regression.
*Adjusted for sex;
†adjusted for age and sex;
‡adjusted for age, sex, and diabetes duration.
Diabetes treatment
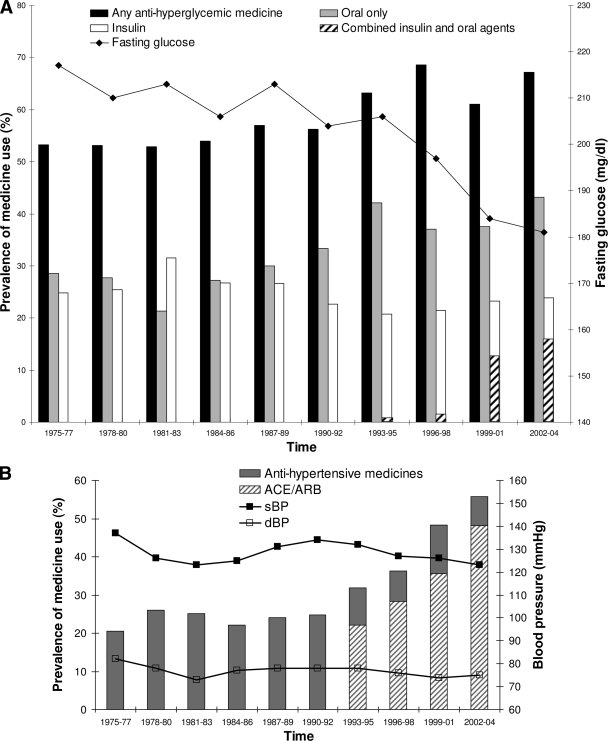
Treatment for diabetes was categorized as oral agents only, insulin only, and combined oral agents and insulin. More recently approved diabetes drugs, such as exenatide or oral dipeptdyl peptidase-IV inhibitors, were not used during this study period. Over the course of the study, the proportion of subjects receiving medicine for diabetes increased from 53% in 1975–1977 to 67% in 2002–2004. Figure 1A shows changes in the prevalence of the different modes of treatment. The use of combined oral agents and insulin increased from <1% in 1993–1995, the first time period for which accurate data are available, to 15.9% in the final time period (Ptrend < 0.0001, adjusted for age, sex, and diabetes duration). The increased use of multiple agents was accompanied by a fall in the use of insulin alone from 20% in 1993–1995 to 8% in 2002–2004 (Ptrend < 0.0001).
Figure 1.
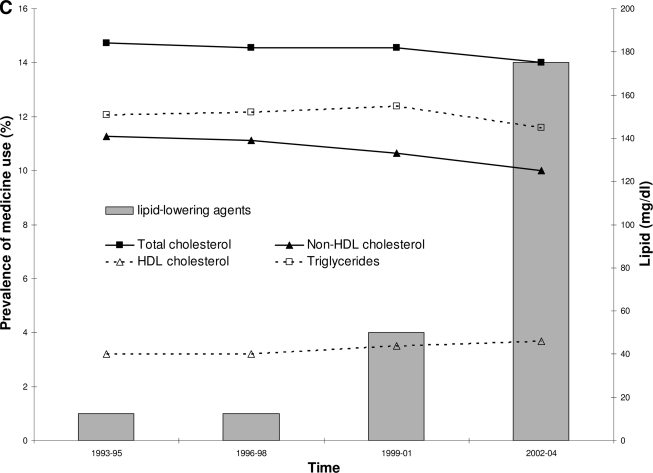
Trends in treatment over time. A: Diabetes treatment by category over time and mean fasting glucose over time. For time periods prior to 1993, combined use of oral agents and insulin was not recorded separately but included as insulin use. After 1993, data were available to show combined insulin and oral agent use. This is shown as ▨ of the insulin use column. Trend for oral agent only use, P < 0.0001. Trend for insulin only use, P < 0.0001. Trend for combined oral agents and insulin use, P < 0.0001. Trend for mean fasting glucose, change = −4.9 mg/dl per 3-year time period, P < 0.0001. All trends adjusted for age, sex, and diabetes duration. B: Anti-hypertensive use and mean sBP and dBP over time. Trend for anti-hypertensive agent use, P < 0.0001. Trend for mean sBP parameter estimate = −0.37 mmHg per 3-year time period, P = 0.0014. Trend for mean dBP parameter estimate = −0.37 mmHg, P < 0.0001. All trends adjusted for age, sex, and diabetes duration. C: Lipid-lowering medicine use and geometric mean total, HDL, non-HDL cholesterol, and triglycerides over time. Trend for lipid-lowering agent use, P < 0.0001. Trend for total cholesterol, change = 0.0004 mg/dl, P = 0.77. Trend for HDL cholesterol, change = 0.051 mg/dl, per 3-year time period <0.0001. Trend for non-HDL cholesterol, change = −0.046 mg/dl, P < 0.0001. Trend for triglycerides, change = −0.015 mg/dl, P = 0.16. All trends adjusted for age, sex, and diabetes duration.
Data for individual drug use were available in the final four time periods. The prevalence of sulfonylurea use did not change, but their use as single agents declined. The use of metformin and thiazolidinediones increased, both in total use and as single agents (Table 2).
Table 2.
Prevalence of antidiabetic oral agent use by type: Gila River Indian Community, 1993–2004
| 1993–1995 | 1996–1998 | 1999–2001 | 2002–2004 | P trend | ||
|---|---|---|---|---|---|---|
| n | 623 | 464 | 575 | 775 | ||
| Sulfonylureas | Any use | 254 (40.8%) | 166 (35.8%) | 213 (37.0%) | 299 (38.6%) | 0.93 |
| Alone | 254 (40.8%) | 136 (29.3%) | 102 (17.7%) | 90 (11.6%) | <0.0001 | |
| Metformin | Any use | 0 (0%) | 30 (6.5%) | 144 (25.0%) | 287 (37.0%) | <0.0001 |
| Alone | 0 (0%) | 1 (0.2%) | 23 (4.0%) | 73 (9.4%) | <0.0001 | |
| Thiazolidinediones | Any use | 0 (0%) | 5 (1.1%) | 46 (8.0%) | 154 (19.9%) | <0.0001 |
| Alone | 0 (0%) | 0 (0%) | 1 (0.2%) | 2 (0.3%) | 0.06 |
Data are number with percentages of all subjects in each time period in parentheses. “Any use” indicates all subjects reporting use of the drug and “Alone”indicates subjects who are taking the drug as their only therapy for diabetes. Odds ratios are the odds ratios per time period adjusted for age, sex, and diabetes duration. P value for trend adjusted for age, sex, and diabetes duration.
Glycemia was assessed throughout the study with fasting plasma glucose and, since 1989, with A1C. Figure 1A shows a downward trend for fasting glucose with a difference in mean glucose between successive time periods of −4.91 mg/dl (Ptrend < 0.0001, adjusted for age, sex, and diabetes duration). A similar trend occurred for A1C for the last five time periods (means ± SD 9.2% ± 2.4, 9.4% ± 2.4, 8.8% ± 2.5, 8.5% ± 2.4, and 8.2% ± 2.3, respectively), with a difference in mean A1C between successive time periods of −0.319% (Ptrend < 0.0001, adjusted for age, sex, and diabetes duration). A greater proportion of subjects reached the goal of an A1C <7.0% in the last two time periods than in earlier time periods, and this was accompanied by a reduction in the percentage of subjects with an A1C ≥10%. The proportion of subjects receiving no medicine for diabetes while having an A1C ≥7% fell throughout the study from 26% in 1990–1992 to 16% in 2002–2004.
Blood pressure
Fig. 1B illustrates the increasing use of anti-hypertensive medicines, which rose from 21% in 1975–1977 to 58% in 2002–2004 (Ptrend < 0.0001, adjusted for age, sex, and diabetes duration). Since 1993, the use of ACE inhibitors and angiotensin receptor blockers (ARBs) has increased relative to other categories of anti-hypertensive medicines. ACE inhibitors and ARBs were used by 22% of individuals in 1993–1995 and by 48% of individuals in 2002–2004. In 1993–1995, diuretics, β-blockers, and calcium channel blockers were used by 6, 2, and 4% of participants, respectively; whereas in 2002–2004 the corresponding numbers were 16, 7, and 12%. In 1993–1995, 80% of subjects taking anti-hypertensive agents used only a single medicine, whereas by 2002–04 only 59% of subjects did so and 5% reported using four or more medicines for blood pressure control. The trends for use of all categories of anti-hypertensive medicines were positive and statistically significant, Ptrend < 0.0001, after adjustment for age, sex, and diabetes duration.
Mean systolic (sBP) and diastolic blood pressure (dBP) were highest in the first time period and declined throughout the 1970s and early 1980s. Both pressures increased again in the late 1980s, followed by a further decline in the 1990s. Although the use of anti-hypertensive agents increased after the initial time period, there was no reduction in use coinciding with the climb in mean sBP in the early 1990s. The percentage of subjects who met the current American Diabetes Association (ADA) goal of a blood pressure <130/80 was highest in 1981–1983 (52%), then decreased to a low of 34% in 1987–1989, and returned to 52% in the final two time periods. In 1975–1977, 56% of subjects had a blood pressure >130/80 and were using no anti-hypertensive medicines, whereas in 2002–2004 the proportion of hypertensive subjects not receiving anti-hypertensive medicines was 18%. Overall, there was a downward trend over time for mean sBP of 14 mmHg between 1975–1977 and 2002–2004 (Ptrend = 0.0014) and mean dBP of 7 mmHg (Ptrend < 0.0001) after adjustment for age, sex and diabetes duration.
Lipids
Data for lipid-lowering medicines are only available for the final four time periods. Only 1% of subjects reported the use of lipid-lowering medicines in both the 1993–1995 and 1996–1998 time periods, and the proportion increased to 14% in the final time period (Ptrend < 0.0001, adjusted for age, sex, and diabetes duration); 88% of the drugs taken for lipid lowering were statins (Fig. 1C).
Total cholesterol concentration varied throughout the study with the highest geometric mean value of 185 mg/dl in 1987–1989 and the lowest of 175 mg/dl in 1975–1977, but the variation was not statistically significant. Likewise the percentage of subjects meeting the ADA guidelines of total cholesterol <200 mg/dl was unchanged. However, although 34% of subjects had cholesterol concentrations ≥200 mg/dl and were not receiving treatment in 1993–1995, the proportion of those not receiving treatment declined to 23% by 2002–2004, and the percentage of subjects reaching their goals while on treatment increased from 0.3 to 9% over the same time period.
Since 1989, subfractions of cholesterol were measured and revealed a rise in HDL cholesterol and a fall in the non-HDL cholesterol fraction (Fig. 1C) coinciding with the increased use of cholesterol-lowering agents. Serum triglyceride concentrations were unchanged over this time period.
Goals
Specific targets are advised for patients with diabetes concerning glycemia, blood pressure, and cholesterol. Using the targets available at the close of the study, goals for treatment were A1C <7%, sBP <130 mmHg, dBP <80 mmHg (1), and total cholesterol <200 mg/dl (7). The proportion of patients meeting all three goals increased from 7.2% in 1990–1992 to 15.7% in 2002–2004 (Ptrend <0.0001, adjusted for age, sex, and diabetes duration). Female sex, younger age, and shorter duration of diabetes—but not BMI—were significantly associated with reaching all goals (P < 0.05, adjusted for time period).
CONCLUSIONS
Major changes in the treatment of diabetes and related conditions have occurred in the Gila River Indian Community over the past 30 years. These changes coincide with significant improvements in glycemia, blood pressure, and serum cholesterol concentration and with a decline in the incidence of kidney failure (8). The extent to which improvements in glycemia, blood pressure, and lipids have influenced incidence of diabetic complications in the community, however, cannot be determined from an observational study.
The number of new classes of drugs available to treat diabetes, blood pressure, and cholesterol has increased in recent years. Drugs to lower cholesterol came into common use in the community only in the last decade, and the majority of drugs prescribed for this purpose were statins. Increased use of all major classes of blood pressure agents, including established classes, was also observed, suggesting that increased use of medicines in recent years reflects not just use of newer drugs but an effort to meet more stringent treatment targets. In the most recent time period, 15.7% of subjects met ADA goals for glycemic control, blood pressure, and total cholesterol; over twice the prevalence of 7.3% among diabetic subjects in the National Health and Nutrition Examination Survey (NHANES) from 1999–2003 (9). These findings are consistent with a race/ethnicity comparison of measures of diabetes control in participants at enrollment in the Look AHEAD (Action for Health in Diabetes) clinical trial of adults with type 2 diabetes. Among American Indians in that study (some of whom are from this community), 12.2% met the goals of A1C <7%, sBP <130 mmHg, dBP <80 mmHg, and LDL cholesterol <100 mg/dl. This percentage was higher than among white, African American, or Hispanic participants (10).
Indian Health Service (IHS) audit data show a decline in mean A1C from 8.9% in 1995 to 7.9% in 2001 (11), consistent with the decline in A1C in our study, although the mean levels at both time periods in our study were higher. The IHS also reported greater improvements in dBP, total cholesterol, and triglycerides over this time period than in our study. However, mean values were all higher than in the present population. This finding may reflect demographic differences because the IHS audit participants were on average older and had a shorter mean duration of diabetes than the participants in our study. Moreover, IHS audit data are gathered from audited medical records in which dates of diagnosis are likely not as accurate and laboratory measurements not as uniform as in our study.
Other population studies have reported changes in treatment patterns for glycemia (9), blood pressure (9,10), lipids (9), and outcome measures, particularly over the last decade. A shift away from single agent insulin use has been reported, along with increased use of hypoglycemic medicine (oral or insulin), although most studies do not report improvements in glycemic control as measured by A1C (9). Increases in the use of anti-hypertensive medicines, particularly ACE inhibitors, however, are associated with reductions in mean blood pressure (9,10). The use of lipid-lowering drugs is increasing and, in some studies, this increase is accompanied by a fall in both total cholesterol and triglycerides (9).
While there was a trend toward lower blood pressure over time in the present study, the decline was not linear. What caused the earlier decline and rise is unclear. The increase in anti-hypertensive medicine use in the last 15 years is more marked and is associated with a fall in blood pressure from 1990 to 1992, returning to the lower levels seen earlier in the study when anti-hypertensive medicine was rarely used. The increased use of anti-hypertensive medication may be a response to increased blood pressure, but it may also be due to increased use of such agents to treat incipient diabetic renal disease. Our data do not permit us to distinguish between these two indications.
Despite the improvements in glycemia, blood pressure, and cholesterol fractions, BMI increased throughout the study. This increase may simply reflect the increasing trend for BMI seen in the general population (9), but it could also be linked to the medicines used to treat diabetes (12) and to the improvements in glycemic control (3). IHS audit data show an increase in mean BMI in diabetic American Indians between 1995 and 2004 (13), and NHANES reported an increase in obesity among diabetic subjects between 1988–1994 and 1999–2000 without any overall improvement in glycemia (9).
Along with changes in available medicines and treatment goals, there have also been changes in the diagnostic criteria for diabetes that may influence our findings. In 1985, WHO published new criteria for the diagnosis of diabetes, advocating diagnostic cut points of ≥140 mg/dl for fasting glucose and ≥200 mg/dl for 2-h glucose (6). In 1997, ADA proposed a move away from the oral glucose tolerance test to a reliance on fasting glucose, using the lower cut point of ≥126 mg/dl (14). WHO also lowered the fasting cut point to ≥126 mg/dl but continues to advocate for the 2-h glucose measurement (15). (More recent proposals to base the diagnosis of diabetes on A1C occurred after the present study [16,17].) Throughout this study, we used the 2-h glucose ≥200 mg/dl to diagnose diabetes. Not all subjects, however, are diagnosed in the research setting; some are diagnosed as part of routine medical care. Therefore, differences in diagnostic criteria may have occurred over the course of the study. When subjects were divided according to method of diagnosis, those diagnosed on the basis of a 2-h postload glucose of ≥200 mg/dl had lower mean fasting glucose and A1C than those diagnosed on clinical grounds alone.
The DCCT showed the importance of tight glycemic control for the prevention of microvascular complications in subjects with type 1 diabetes (2), and the UKPDS reported similar findings in type 2 diabetes (3). However, overly intensive glycemic control in patients with long-standing type 2 diabetes may also have risks. Three recent clinical trials that sought to reduce the target A1C to levels below <7% (i.e., A1C <6–6.5%), found no benefit on cardiovascular outcomes and one, the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial, found higher death and cardiovascular event rates with more aggressive attempts to normalize blood glucose (18–20). Each of these recent trials also reported a significantly increased risk of severe hypoglycemia in the groups with the lower A1C goals. As blood pressure control is important for reducing both renal and cardiovascular complications (21), lower goals for blood pressure are advocated for people with diabetes (1,4). The benefits of lowering blood pressure and lipids, however, appear to be limited, as recently reported by the ACCORD clinical trial (22,23). Additionally, in order to reduce the high risk of cardiovascular disease in people with diabetes (24), a greater use of aspirin (1) has been encouraged. We did not report data on aspirin use in the present study since data on aspirin use may be less accurate than data on other medicines because aspirin is nonprescription.
The changes reported in this study could represent, in part, a change in the research clinic attendance pattern as opposed to a change in actual practice if, for instance, the research clinic attendees have become more likely to attend hospital clinics or have fewer complications of diabetes than those who do not attend clinics. However, neither the recruitment policy for the study clinic nor the proportion of attendees with diagnosed diabetes at the clinic changed over the course of the study. The mean age of clinic attendees with diabetes and the mean age at onset of diabetes were younger in the more recent time periods in keeping with a shift to an onset of diabetes at younger ages (8,25). Medicine use was assessed by self-report rather than from pharmacy records. This approach may have led to the underreporting of medicines, but it may also have reduced the likelihood that prescribed but untaken medicines were recorded.
In a recent 30-year period, the goals for treatment and the medicines available to meet those goals have changed considerably. The present study indicates that efforts to meet ADA treatment goals for the control of glycemia, blood pressure, and cholesterol have met with some success since the prevalence of attaining these goals was over two times as high in diabetic Pima Indians in 2002–2004 as in 1990–1992. These improvements have been accompanied by an increase in mean BMI. Nevertheless, improvements in the other parameters suggest that members of this community have benefited substantially from progress in diabetes care over the last three decades.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
No potential conflicts of interest relevant to this article were reported.
H.C.L. researched the data, wrote the manuscript, contributed to the discussion, and edited the manuscript. J.K. researched the data, contributed to the discussion, and edited the manuscript. V.A. researched the data and contributed to the discussion. K.K. researched the data and contributed to the discussion. R.G.N. researched the data, contributed to the discussion, and edited the manuscript. W.C.K. researched the data, contributed to the discussion, and edited the manuscript. R.L.H. researched the data, contributed to the discussion, and edited the manuscript.
We are grateful to the volunteers for their participation in this study and to the National Institutes of Health clinic staff.
References
- 1. American Diabetes Association. Standards of Medical Care in Diabetes-2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 3. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 4. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National Heart, Lung, and Blood Institute, National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–1252 [DOI] [PubMed] [Google Scholar]
- 5. Bennett PH, Burch TA, Miller M. Diabetes mellitus in American (Pima) Indians. Lancet 1971;2:125–128 [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization Study Group. Diabetes mellitus. In World Health Organization Technical Report Series 727. Geneva, Switzerland, World Health Organization, 1985, p. 1–113 [PubMed] [Google Scholar]
- 7. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 8. Pavkov ME, Knowler WC, Bennett PH, Looker HC, Krakoff J, Nelson RG. Increasing incidence of proteinuria and declining incidence of end-stage renal disease in diabetic Pima Indians. Kidney Int 2006;70:1840–1846 [DOI] [PubMed] [Google Scholar]
- 9. Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 2004;291:335–342 [DOI] [PubMed] [Google Scholar]
- 10. Bertoni AG, Clark JM, Feeney P, Yanovski SZ, Bantle J, Montgomery B, Safford MM, Herman WH, Haffner S. Look AHEAD Research Group. Suboptimal control of glycemia, blood pressure, and LDL cholesterol in overweight adults with diabetes: the Look AHEAD Study. J Diabetes Complications 2008;22:1–9 [DOI] [PubMed] [Google Scholar]
- 11. Wilson C, Gilliland S, Cullen T, Moore K, Roubideaux Y, Valdez L, Vanderwagen W, Acton K. Diabetes outcomes in the Indian health system during the era of the Special Diabetes Program for Indians and the Government Performance and Results Act. Am J Public Health 2005;95:1518–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Looker HC, Knowler WC, Hanson RL. Changes in BMI and weight before and after the development of type 2 diabetes. Diabetes Care 2001;24:1917–1922 [DOI] [PubMed] [Google Scholar]
- 13. Wilson C, Gilliland S, Moore K, Acton K. The epidemic of extreme obesity among American Indian and Alaska native adults with diabetes. Prev Chronic Dis 2007;4:A06. [PMC free article] [PubMed] [Google Scholar]
- 14. The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. In Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Geneva, Switzerland, World Health Organization, Department of Noncommunicable Disease Surveillance, 1999 [Google Scholar]
- 16. International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2543–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 20. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 21. Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, Holman RR. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 1998;316:823–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. ACCORD Study Group, Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC, Jr, Cushman WC, Simons-Morton DG, Byington RP. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ACCORD Study Group, Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nelson RG, Sievers ML, Knowler WC, Swinburn BA, Pettitt DJ, Saad MF, Liebow IM, Howard BV, Bennett PH. Low incidence of fatal coronary heart disease in Pima Indians despite high prevalence of non-insulin-dependent diabetes. Circulation 1990;81:987–995 [DOI] [PubMed] [Google Scholar]
- 25. Pavkov ME, Hanson RL, Knowler WC, Bennett PH, Krakoff J, Nelson RG. Changing patterns of type 2 diabetes incidence among Pima Indians. Diabetes Care 2007;30:1758–1763 [DOI] [PubMed] [Google Scholar]