Abstract
OBJECTIVE
We examined the effect of intrauterine diabetes exposure (IDE) on the incidence of diabetic end-stage renal disease (ESRD) in Pima Indians with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Individuals were followed from their first diabetic examination until December 2006, death, ESRD, or age of 45 years.
RESULTS
Among the 1,850 diabetic participants, 102 had IDE. ESRD developed in 57, 5 of whom had IDE. Cumulative incidence of ESRD by age 45 was 19.3% in participants with IDE and 5.1% in those without; the age- and sex-adjusted incidence rate ratio was 4.12 (95% CI 1.54–11.02). After additional adjustment for age at diabetes onset, ESRD incidence was similar in the two groups (incidence rate ratio 1.38, 95% CI 0.45–4.24).
CONCLUSIONS
IDE increases the age- and sex-adjusted incidence of ESRD fourfold in young adults with type 2 diabetes, mediated primarily by the earlier onset of type 2 diabetes in those with IDE.
The intrauterine environment plays a central role in fetal development, and an adverse environment may enhance the risk of chronic diseases in the offspring (1–4). We examined the effect of intrauterine diabetes exposure (IDE) on incidence of diabetic end-stage renal disease (ESRD) in Pima Indians with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Between 1965 and 2007, Pima Indians from the Gila River Indian Community participated in a longitudinal diabetes study. Each member of the community ≥5 years old was invited to have a research examination every 2 years that included a 75-g oral glucose tolerance test. In addition, many pregnant women also had a 75-g oral glucose tolerance test in the third trimester. Diabetes was defined by a 2-h postload plasma glucose concentration ≥11.1 mmol/l (200 mg/dl), or by a clinical diagnosis between examinations. IDE was considered present if diabetes was diagnosed in the mother before the child's birth.
Diabetic ESRD was defined as the initiation of renal replacement therapy or death from diabetic nephropathy and was ascertained independently of the research examinations. Cause of ESRD in those receiving renal replacement therapy was determined from clinical records. Death was attributed to diabetic nephropathy if ICD-9 code 250.4 was specified as the underlying cause. The study population included diabetic subjects aged 5–45 years who attended research examinations and resided in the community. The longitudinal study was approved by the review board of the National Institute of Diabetes and Digestive and Kidney Diseases. Each participant gave informed consent or assent.
Statistical analysis
Clinical features at baseline were compared by IDE with ANCOVA for continuous variables or Mantel-Haenszel χ2 for categorical variables stratified by sex. Variables with nonnormal distributions were analyzed with the Kruskal-Wallis test.
Incidence of ESRD was computed and events and person-years of follow-up were stratified in a time-dependent fashion by decades of age. Individuals were followed from their first diabetic examination after 1 January 1965 until 31 December 2006, death, onset of ESRD, or age of 45 years, whichever came first. Individuals aged ≥45 years were not included, because few people whose IDE could be determined were followed past 45 years of age. Unadjusted cumulative incidence of ESRD in diabetic subjects, stratified by IDE, was estimated by the Kaplan-Meier product-limit method either as a function of age or duration of diabetes. When calculated by age, subjects entered at the diagnosis of diabetes, and the Kaplan-Meier method was modified for left-truncated data (5). Differences in cumulative incidence were assessed by the log-rank test. Age- and sex-adjusted incidence rates of ESRD were standardized to the 1985 Pima Indian population, and rate ratios were computed from these age- and sex-adjusted rates. Incidence rate ratios adjusted for age, sex, and age at onset of diabetes were computed by Mantel-Haenszel stratification. The proportion of incident ESRD due to IDE in the diabetic population was computed as the difference in age-and sex-adjusted incidence of ESRD in the total diabetic population and in the diabetic population without IDE divided by the age- and sex-adjusted incidence in the total diabetic population.
RESULTS
Among the 1,850 diabetic subjects, 102 (5.5%) were offspring of diabetic mothers. Median follow-up from the first diabetic examination was 7.1 years (interquartile range 3.2–12.5 years). Those exposed to diabetes in utero were significantly younger at baseline than those unexposed (17.5 vs. 34.2 years; P < 0.0001), owing to the earlier onset of diabetes characteristic of this exposure, and had lower BMI, blood pressure, and serum cholesterol concentration. At the end of follow-up, median age of the unexposed participants remained significantly greater than in those with IDE (26.7 vs. 45.0 years; P < 0.0001), but the median duration of diabetes was similar (7.6 vs. 8.8 years; P = 0.22).
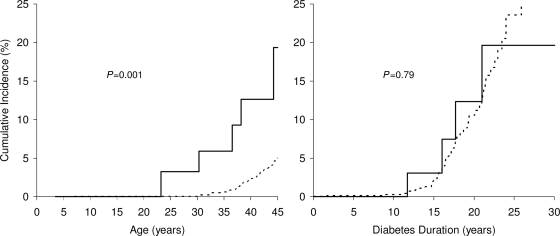
ESRD developed in 57 participants, 5 of whom were exposed to diabetes in utero. Cumulative incidence of ESRD by age was higher in participants with IDE than in those without (P = 0.001) but was equivalent in the two groups when examined by diabetes duration (P = 0.79) (Fig. 1). Unadjusted incidence was 5.61 cases/1,000 person-years in participants with IDE and 3.52 cases/1,000 person-years in those without. Age- and sex-adjusted incidence in those with IDE was 4.12 (95% CI 1.54–11.02) times that of the unexposed. After additional adjustment for age at diabetes onset, ESRD incidence was similar in the two groups (incidence rate ratio 1.38, 95% CI = 0.45–4.24). The proportion of diabetic ESRD attributable to IDE in the diabetic population aged ≤45 years was 18.9%.
Figure 1.
Cumulative incidence of diabetic ESRD by age (left) and by duration of diabetes (right) according to exposure to diabetes in utero (——, exposed; – – –, unexposed). The five cases of ESRD in the exposed group result in five steps in the cumulative incidence plot by age. Only four cases are shown in the cumulative incidence plot by duration, because the fifth case occurred at a duration beyond the range of the figure.
CONCLUSIONS
Exposure to IDE was associated with a fourfold increase in the incidence of ESRD in young adults with type 2 diabetes when adjusted for age and sex. This effect was explained largely by their earlier age at onset of diabetes. To our knowledge, this study is the first to link IDE to an earlier onset of ESRD.
Recent improvements in diabetes management has reduced the overall incidence of diabetic ESRD in some populations (6,7). Less effective therapeutic management of youth and young adults with type 2 diabetes (8), however, may limit these beneficial trends in younger people (6). In the present study, estimates of the population attributable risk of ESRD ascribed to IDE suggest that interventions that delay diabetes onset in women until after the child-bearing years could ultimately eliminate 18.9% of the ESRD that would occur before the age of 45 years in the children of these women.
IDE exposure increased nearly fourfold in Pima Indians between 1967 and 1996, and the prevalence of diabetes in youth related to this exposure doubled during the same period (9). Nevertheless, because type 2 diabetes in youth is a recent phenomenon, even in the Pima Indians, the number of cases of ESRD attributable to IDE in the present study was small. The effect of this exposure, however, was large, and trends with respect to diabetes and its complications in this population have been a reliable harbinger for trends in other populations. Implementation of appropriate behavioral interventions to prevent or delay diabetes in women of child-bearing age may be an effective long-term strategy to reduce the increasing incidence of diabetic ESRD in young adults.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
No potential conflicts of interest relevant to this article were reported.
M.E.P. contributed to discussion, wrote the manuscript, and reviewed/edited the manuscript. R.L.H., W.C.K., and P.H.B. researched data, contributed to discussion, and reviewed/edited the manuscript. M.L.S. researched data and reviewed/edited the manuscript. R.G.N. researched data, contributed to discussion, wrote the manuscript, and reviewed/edited the manuscript.
Parts of this study were presented in abstract form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
We are indebted to the members of the Gila River Indian Community for participating in this investigation.
Footnotes
The findings and conclusions in this article have not been formally disseminated by the Centers for Disease Control and Prevention and should not be construed to represent any agency determination or policy.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Painter RC, Osmond C, Gluckman P, Hanson M, Phillips DI, Roseboom TJ. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG 2008;115:1243–1249 [DOI] [PubMed] [Google Scholar]
- 2. Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 2000;49:2208–2211 [DOI] [PubMed] [Google Scholar]
- 3. Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC. Congenital susceptibility to NIDDM. Role of intrauterine environment. Diabetes 1988;37:622–628 [DOI] [PubMed] [Google Scholar]
- 4. Pettitt DJ, Lawrence JM, Beyer J, Hillier TA, Liese AD, Mayer-Davis B, Loots B, Imperatore G, Liu L, Dolan LM, Linder B, Dabelea D. Association between maternal diabetes in utero and age at offspring's diagnosis of type 2 diabetes. Diabetes Care 2008;31:2126–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsai WY, Jewell NP, Wang MC. A note on the product-limit estimator under right censoring and left truncation. Biometrika 1987;74:883–886 [Google Scholar]
- 6. Pavkov ME, Knowler WC, Bennett PH, Looker HC, Krakoff J, Nelson RG. Increasing incidence of proteinuria and declining incidence of end-stage renal disease in diabetic Pima Indians. Kidney Int 2006;70:1840–1846 [DOI] [PubMed] [Google Scholar]
- 7. Burrows NR, Li Y, Geiss LS. Incidence of treatment for end-stage renal disease among individuals with diabetes in the U.S. continues to decline. Diabetes Care 2010;33:73–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petitti DB, Klingensmith GJ, Bell RA, Andrews JS, Dabelea D, Imperatore G, Marcovina S, Pihoker C, Standiford D, Waitzfelder B, Mayer-Davis E. SEARCH for Diabetes in Youth Study Group. Glycemic control in youth with diabetes: the SEARCH for Diabetes in Youth Study. J Pediatr 2009;155:668–672. e1–e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dabelea D, Hanson RL, Bennett PH, Roumain J, Knowler WC, Pettitt DJ. Increasing prevalence of type II diabetes in American Indian children. Diabetologia 1998;41:904–910 [DOI] [PubMed] [Google Scholar]