Abstract
OBJECTIVE
The expression of vascular endothelial growth factor (VEGF) is elevated in diabetic macular edema (DME). Ranibizumab binds to and inhibits multiple VEGF variants. We investigated the safety and efficacy of ranibizumab in DME involving the foveal center.
RESEARCH DESIGN AND METHODS
This was a 12-month, multicenter, sham-controlled, double-masked study with eyes (age >18 years, type 1 or 2 diabetes, central retinal thickness [CRT] ≥300 μm, and best corrected visual acuity [BCVA] of 73–39 ETDRS letters [Early Treatment Diabetic Retinopathy Study]) randomly assigned to intravitreal ranibizumab (0.3 or 0.5 mg; n = 51 each) or sham (n = 49). The treatment schedule comprised three monthly injections, after which treatment could be stopped/reinitiated with an opportunity for rescue laser photocoagulation (protocol-defined criteria). After month 1, dose-doubling was permitted (protocol-defined criteria, injection volume increased from 0.05 to 0.1 ml and remained at 0.1 ml thereafter). Efficacy (BCVA and CRT) and safety were compared between pooled ranibizumab and sham arms using the full analysis set (n = 151, patients receiving ≥1 injection).
RESULTS
At month 12, mean ± SD BCVA improved from baseline by 10.3 ± 9.1 letters with ranibizumab and declined by 1.4 ± 14.2 letters with sham (P < 0.0001). Mean CRT reduction was 194.2 ± 135.1 μm with ranibizumab and 48.4 ± 153.4 μm with sham (P < 0.0001). Gain of ≥10 letters BCVA from baseline occurred in 60.8% of ranibizumab and 18.4% of sham eyes (P < 0.0001). Safety data were consistent with previous studies of intravitreal ranibizumab.
CONCLUSIONS
Ranibizumab is effective in improving BCVA and is well tolerated in DME. Future clinical trials are required to confirm its long-term efficacy and safety.
Diabetes affects >220 million people worldwide (1). Diabetic macular edema (DME) is one of the major causes of visual impairment (VI) in patients with diabetic retinopathy (2,3). With diabetes prevalence estimated to double during the next 20 years (4), in the future it is likely that DME may be responsible for substantial vision loss unless treated adequately.
Laser photocoagulation is the mainstay of DME treatment; it reduces the risk of moderate vision loss by ∼50%, with 3% of eyes showing vision improvement (≥3 lines), but a substantial proportion of treated eyes remain unresponsive (5). In a recent report of a 2-year study, focal/grid laser photocoagulation was more effective and had fewer side effects than intravitreal triamcinolone acetonide (6). Pars plana vitrectomy is another treatment modality investigated for DME; however, both intravitreal triamcinolone acetonide and pars plana vitrectomy have limited efficacy and/or significant side effects (7,8).
There is currently a significant unmet medical need for an effective DME treatment that not only stabilizes but improves and maintains vision and has a better safety profile than the available DME treatment options. Several proinflammatory cytokines including vascular endothelial growth factor (VEGF) have been shown to be extensively involved in the development and progression of DME (9). VEGF promotes neovascularization and microvascular leakage (10). Thus, inhibiting VEGF may provide an alternative therapeutic approach in DME. Anti-VEGF agents have been extensively investigated in neovascular age-related macular degeneration (nAMD). Given that anti-VEGF drugs delivered within the vitreous could pass into the systemic circulation, VEGF inhibition could in turn produce systemic adverse effects, which may be potentially serious for diabetic patients (11). Therefore, randomized clinical trials are required to establish both the efficacy and systemic adverse effects in this population.
Ranibizumab is a fully humanized monoclonal antibody fragment (Fab), which binds to multiple variants of VEGF-A (12), and is approved for the treatment of nAMD. In a pilot study (10 patients with DME), ranibizumab was effective and well tolerated in maintaining or improving best-corrected visual acuity (BCVA) and in reducing central retinal thickness (CRT) (13). The 6-month Ranibizumab for Edema of the Macula in Diabetes (READ-2) study (phase II) was the first to compare the efficacy of ranibizumab with laser photocoagulation or a combination of both in patients with VI due to DME; ranibizumab led to significant improvements in mean BCVA (7.2 letters) compared with laser photocoagulation (−0.4 letters) or the combination (3.8 letters) (14). Studies in DME have also been conducted with other anti-VEGF agents, pegaptanib and bevacizumab (15–19). Initial results from these studies are encouraging in some patients with DME; further prospective randomized clinical trials may confirm their effects in DME.
We report the results of the phase II RESOLVE study in patients with VI due to DME. This study evaluated the efficacy and safety of ranibizumab compared with sham treatment over 12 months.
RESEARCH DESIGN AND METHODS
Inclusion and exclusion criteria
Patients (aged >18 years) with type 1 or 2 diabetes and DME were eligible if they had a visual acuity between 20/40 and 20/160, CRT ≥300 μm, HbA1C ≤12%, decreased vision attributed to foveal thickening from DME, that was not explained by any other cause, and clinically significant DME in at least one eye confirmed by a central reading center (Bern Photographic Reading Centre, University Bern, Bern, Switzerland) using stereoscopic fundus photographs, fluorescein angiography, and optical coherence tomography (OCT) (Stratus OCT; Carl Zeiss Meditec, Jena, Germany). Eyes were deemed eligible if, in the judgment of the investigator, laser photocoagulation could be safely withheld in the study eye for at least 3 months after random assignment. Patients were excluded if they had unstable medical status including glycemic control and blood pressure or panretinal laser photocoagulation performed within 6 months before study entry, and grid/central laser photocoagulation was excluded except for patients with only mild laser burns at least 1,000 μm from the center of the fovea performed >6 months preceding day 1. Details are found in supplementary Table 1 (available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc10-0493/DC1).
Study design
Of the 207 screened patients, 151 eligible patients were randomly assigned 1:1:1 to either ranibizumab (0.3 mg, n = 51 or 0.5 mg, n = 51) or sham treatment (n = 49) (randomization details are found in the supplementary data, available in an online appendix). Before each scheduled treatment, patients were asked to self-administer a topical antibacterial agent for 3 days. Patients received three monthly ranibizumab (0.3 or 0.5 mg) or sham injections (injection volume 0.05 ml). Thereafter, treatment could be stopped or reinitiated based on treatment success, disease activity, futility, or safety criteria (supplementary Fig. 1, available in an online appendix). It is important to note that the sham arm was a nontreatment arm, and patients did not receive intraocular injections. The sham eyes were locally anesthetized, and pressure with the blunt tip of the syringe (without needle) was applied to the anesthetized surface of the eye to mimic the injection. After month 1, the ranibizumab dose (or sham) could be doubled by increasing the injection volume from 0.05 to 0.1 ml if CRT remained >300 μm or was >225 μm and the reduction in retinal edema from the previous assessment was <50 μm. Once the injection volume was increased to 0.1 ml, subsequent administrations remained at 0.1 ml (0.6 or 1.0 mg ranibizumab). If treatment had been withheld for >45 days, subsequent injections restarted with the initial injection volume of 0.05 ml. Because of this possibility of dose doubling, the ranibizumab treatment groups are referred to as “0.3–0.6 mg” and “0.5–1.0 mg.”
The study included a planned interim analysis at month 6 to facilitate early decisions on dose, treatment ratio, sample adjustments, or futility assessments (details are available in the supplementary data). Here, we present the overall pooled efficacy and safety of ranibizumab (pooled 0.3–0.6 mg with 0.5–1.0 mg) versus sham treatment (by-dose data are found in the supplementary data). The primary end point was the mean average change in BCVA from baseline to month 1 through month 12 (chosen as the primary end point because it is less sensitive to monthly variations and reflects the treatment impact over the entire treatment period). Secondary end points included mean change in BCVA and CRT from baseline to month 12, categorized BCVA outcome, and safety.
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments, Good Clinical Practice, and applicable regulatory requirements. The research protocol and its amendments were approved by relevant institutional review boards and ethics committees from the respective study centers, and all participants gave written informed consent.
Assessments
BCVA and CRT were assessed by certified examiners using Early Treatment Diabetic Retinopathy Study (ETDRS) standardized protocols and OCT, respectively, at scheduled visits. Safety (ocular, nonocular, or systemic) and vital signs (including blood pressure [sitting systolic and diastolic]) and serum levels of HbA1C were assessed at each scheduled monthly visit. The occurrence of adverse events (AEs) was sought by nondirective questioning of the patient at each visit, and these were also recorded when reported by the patient during or between visits or through physical examination, laboratory test, or other assessments. Serious adverse events (SAEs) were monitored continuously. Routine hematology and systemic immunoreactivity assessments (i.e., presence of serum antibodies against ranibizumab) were performed at baseline and month 12.
Statistical analysis
The plan was to screen ∼225 patients to achieve a sample size of 150 eligible patients within an estimated 12-month recruitment period. The full analysis set (FAS) was the primary efficacy analysis set. FAS comprised all patients who received the study treatment at least once (ranibizumab or sham) and who had at least one post baseline BCVA assessment. The safety population was identical to the FAS.
For the primary efficacy analysis, missing data were imputed using the last observation carried forward (LOCF) method, with alternative missing data handling procedures used for corresponding sensitivity analysis. For statistical hypothesis testing of the mean average changes from baseline in BCVA the stratified Cochran-Mantel-Haenszel test was used with observed values (permutation tests) as scores (details of the hypothesis testing are available in the supplementary data). StatXact software was used to compute the test.
In addition, the primary efficacy variable was assessed using parametric statistical methods. The two-sided 95% CI for the primary efficacy variable and the corresponding difference in means between treatments were calculated using the least squares means from an ANOVA model with treatment and categories of baseline visual acuity and baseline CRT as factors.
RESULTS
Characteristics of patients
Patients from the ranibizumab and sham arms were comparable with respect to baseline characteristics (supplementary Table 2, available in an online appendix). Less than 20% of patients in all arms had previously received laser photocoagulation for DME. There were more discontinuations in the sham arm than the ranibizumab arm (18.4 and 9.8%, respectively) (supplementary Fig. 2, available in an online appendix).
Treatment characteristics: adjustments and number of treatments
The number of patients whose treatment was interrupted or stopped was comparable between the treatment arms (37 [36.3%] and 20 [40.8%], for ranibizumab and sham, respectively). Most treatment adjustments in the sham arm were made because of a lack of efficacy (17 of 20 [85%] and 9 of 37 [24.3%] for sham and ranibizumab, respectively); conversely, for ranibizumab, they were prompted by improved BCVA and/or reduced CRT (17 of 37 [45.9%] for ranibizumab and none for sham).
The mean ± SD numbers of injections administered during 12 months were 10.2 ± 2.5 and 8.9 ± 3.5 for ranibizumab and sham, respectively. The investigators more frequently undertook dose doubling in the sham arm (45 [91.8%]) than in the ranibizumab arm (70 [68.6%]). Most instances of dose doubling occurred at month 1 (70–78%). A larger proportion of patients in the sham arm received rescue laser photocoagulation than in the ranibizumab-treated arms (17 [34.7%] and 5 [4.9%], respectively); among these patients, most received one to two laser treatments.
Efficacy
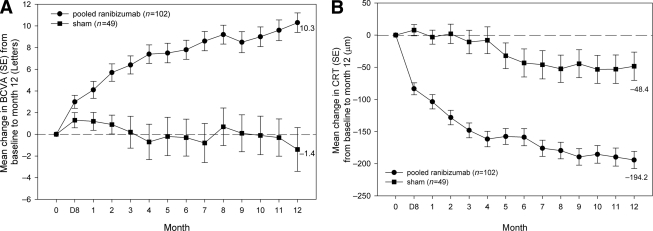
The mean average change in BCVA from baseline to month 1 through 12 (primary end point) was statistically superior with ranibizumab (7.8 letters) compared with sham (−0.1 letters) (least squares means difference 7.9 letters; P < 0.0001). At month 12, mean ± SD BCVA improved by 10.3 ± 9.1 letters from baseline with ranibizumab and declined by 1.4 ± 14.2 letters with sham (P < 0.0001) (Table 1). Ranibizumab led to a rapid and continuous improvement in mean BCVA, with superior benefits observed as early as month 1 (Fig. 1).
Table 1.
Mean BCVA and CRT at month 12
| Ranibizumab pooled | Sham | |
|---|---|---|
| N | 102 | 49 |
| BCVA (ETDRS letters) | ||
| Baseline | 60.2 ± 9.9 | 61.1 ± 9.0 |
| Mean average change from baseline to month 1 through month 12 | ||
| Average month 1 to month 12 | 68.0 ± 11.7 | 61.0 ± 13.9 |
| Average change from baseline | 7.8 ± 7.7 | −0.1 ± 9.8 |
| Comparison vs. sham | ||
| Difference in least squares means | 7.9 | — |
| 95% CI for difference | 5.0 to 10.9 | — |
| P value | <0.0001 | — |
| Mean change from baseline to month 12 | ||
| Month 12 | 70.5 ± 12.1 | 59.7 ± 17.3 |
| Change from baseline | 10.3 ± 9.1 | −1.4 ± 14.2 |
| Comparison vs. sham | ||
| Difference in least squares means | 11.9 | — |
| 95% CI for difference | 8.1 to 15.7 | — |
| P value | <0.0001 | — |
| CRT (μm) | ||
| Baseline | 455.4 ± 114.2 | 448.9 ± 102.8 |
| Month 12 | 261.2 ± 81.9 | 400.5 ± 139.2 |
| Comparison vs sham | ||
| Change from baseline | −194.2 ± 135.1 | −48.4 ± 153.4 |
| Difference in least squares means | −155.0 | — |
| 95% CI for difference | −195.4 to −114.6 | — |
| P value | <0.0001 | — |
| Categorized BCVA outcome, n (%) | ||
| Gain of ≥1 letters* | 92 (90.2) | 27 (55.1) |
| Gain of ≥10 letters* | 62 (60.8) | 9 (18.4) |
| Loss of ≥10 letters* | 5 (4.9) | 12 (24.5) |
| Gain of ≥15 letters† | 33 (32.4) | 5 (10.2) |
| Loss of ≥15 letters† | 3 (2.9) | 10 (20.4) |
Data are means ± SD unless otherwise indicated. Ranibizumab dose and by group data (A, B, and A + B) are presented in supplementary Tables 3 and 4 (available in an online appendix).
*P < 0.0001;
†P = 0.0001. (Test for treatment difference [ranibizumab vs. sham], Cochran-Mantel-Haenszel test is of “general association.” Stratified analysis includes baseline visual acuity [≤60 or >60 letters] and baseline CRT [≤400 μm or >400 μm].)
Figure 1.
Mean change from baseline to month 12 in (A) BCVA and (B) CRT of the study eye: data for pooled ranibizumab doses (0.3–0.6 and 0.5–1.0 mg) versus sham. Full analysis set, LOCF. (Ranibizumab by-dose data are found in supplementary Fig. 4A and B, available in an online appendix.)
The mean BCVA improvement with ranibizumab treatment over time was paralleled by improvement in mean CRT (Fig. 1). The mean change in CRT from baseline to month 12 was significantly higher in the ranibizumab arm than in the sham arm (−194.2 vs. −48.4 μm, respectively; difference in least squares means, −155 μm, P < 0.0001) (Table 1). The impact of therapy on macular edema (fundus photographs and OCT) is illustrated for ranibizumab (0.3–0.6 mg) and sham in the supplementary data. At month 12, 60.8% of the patients receiving ranibizumab gained ≥10 letters of BCVA from baseline compared with 18.4% in the sham arm (P < 0.0001). A similar difference was seen in all the other categories (Table 1).
In terms of the ETDRS severity score, the observed change from baseline to month 12 could be analyzed in ∼50% of FAS patients. Deterioration within the categories mild-moderate-severe (0–35, 43–47, and ≥53) was observed in 3.9% (n = 2 of 51) ranibizumab-treated patients, compared with 18% (n = 4 of 22) sham patients. Corresponding relevant improvements were seen in 21.6% (n = 11 of 51) ranibizumab patients, whereas no such improvement occurred in the sham patients.
Safety
There were no imbalances in the rates of ocular and nonocular SAEs or AEs between the ranibizumab and sham arms (Table 2). The proportion of patients with ocular SAEs in the study eye was comparable between the treatment arms (ranibizumab: 4 [3.9%]; sham: 1 [2.0%]). Most of the SAEs were nonocular in origin (ranibizumab: 14 [13.7%]; sham: 8 [16.3%]). There was one occurrence of myocardial infarction (nonocular SAE) with ranibizumab that was suspected to be related to the study drug. One death from urinary bladder cancer was reported with ranibizumab, which was not suspected to be related to the study drug or procedure. Endophthalmitis (n = 2 of 102) and myocardial infarction (n = 1 of 102) led to study drug discontinuation in three patients. The most frequently reported ocular AEs (ranibizumab and sham) were conjunctival hemorrhage, intraocular pressure increase, and eye pain (Table 2). The proportion of patients reporting nonocular AEs was comparable between the ranibizumab and sham arms (64 [62.7%] and 32 [65.3%], respectively).
Table 2.
Most frequent SAEs and AEs over 12 months
| Ranibizumab pooled | Sham | |
|---|---|---|
| N | 102 | 49 |
| SAEs | ||
| Ocular SAEs | ||
| Total | 4 (3.9) | 1 (2.0) |
| Vitreous hemorrhage* | 1 (1.0) | 0 (0.0) |
| Retinal ischemia | 1 (1.0) | 0 (0.0) |
| Retinal artery occlusion* | 1 (1.0) | 0 (0.0) |
| Endophthalmitis* | 2 (2.0) | 0 (0.0) |
| Retinal detachment | 0 (0.0) | 1 (2.0) |
| Nonocular SAEs | ||
| Total | 14 (13.7) | 8 (16.3) |
| Infections and infestations† | 2 (2.0) | 3 (6.1) |
| Urinary bladder cancer | 1 (2.0) | 0 (0.0) |
| AEs | ||
| Ocular AEs | ||
| Total | 80 (78.4) | 28 (57.1) |
| Conjunctival hemorrhage* | 23 (22.5) | 7 (14.3) |
| Eye pain* | 18 (17.6) | 10 (20.4)‡ |
| Nonocular AEs | ||
| Total | 64 (62.7) | 32 (65.3) |
| Nasopharyngitis | 10 (9.8) | 1 (2.0) |
| Hypertension | 7 (6.9) | 4 (8.2) |
| Potentially related to systemic VEGF inhibition | ||
| Total | 14 (13.7) | 6 (12.2) |
| Arterial thromboembolic events§ | 3 (2.9) | 2 (4.1) |
| Hypertension | 9 (8.8) | 5 (10.2) |
| Nonocular hemorrhage | 2 (2.0) | 0 (0.0) |
Data are n (%). Additional safety data are presented in supplementary Tables 3, 5, and 6 (available in an online appendix).
*Suspected to be related to study drug/procedure.
†Infections and infestations include gastroenteritis viral, infected epidermal cyst, cellulitis, diabetic gangrene, and gastroenteritis.
‡One event documented after start of treatment with nonstudy medication (marketed ranibizumab).
§Myocardial infarction (1 in sham and ranibizumab), retinal artery occlusion (1 in ranibizumab), transient ischemic attack (1 in ranibizumab), and angina pectoris (1 in sham).
The incidence of hypertension and arterial thromboembolic events, both possibly due to VEGF inhibition, were comparable between ranibizumab and sham arms (hypertension: 9 [8.8%] and 5 [10.2%]; arterial thromboembolic events: 3 [2.9%] and 2 [4.1%], respectively).
There were no clinically significant differences between treatment arms at baseline for mean serum levels of HbA1C and mean blood pressure (supplementary Table 7, available in an on online appendix). The urine dipstick protein test was performed in 60% of the FAS patients. The deterioration within the categories (categories 1+, 2+, 3+, or greater) was comparable between the ranibizumab and sham groups (22.9% [n = 14 of 61] and 28.5% [n = 8 of 21], respectively), none reported as AEs by the investigators.
The formation of antibodies to ranibizumab was reported in three patients post baseline. One patient in the sham arm showed positive immunoreactivity both at baseline and post baseline.
CONCLUSIONS
Ranibizumab led to significant and continuous improvements in both BCVA and CRT over 12 months compared with sham treatment in patients with VI due to DME. The safety profile of ranibizumab appears to be similar to that reported for its registered use in nAMD. Over the 12-month study period, ranibizumab-treated patients had a mean average gain in BCVA of 7.8 letters compared with baseline, whereas sham patients had a mean average decrease of 0.1 letter. At the end of the 12-month assessment period, ranibizumab led to a mean gain of 10.3 letters from baseline compared with a decline of 1.4 letters in the sham patients. The proportion of patients who gained ≥10 letters as well as ≥15 letters was threefold higher in the ranibizumab arm compared than in the sham arm.
More patients receiving ranibizumab had their dose adjusted because of disease improvement (BCVA and/or CRT), whereas more patients in the sham arm had their dose adjusted because of lack of efficacy. Ranibizumab was well tolerated over 12 months with a safety profile comparable to that observed in prior nAMD studies (20–22). There were no new AEs reported in patients with DME compared with patients with nAMD. The incidence of ocular and nonocular AEs and SAEs was low. There were two cases of endophthalmitis (SAEs) reported in the ranibizumab treatment group (2%). In one patient, this event resulted in study discontinuation, and the event was considered by the investigator to be related to the study procedure. In the second patient, endophthalmitis was considered by the investigator to be related to the study medication (because it recurred on rechallenge). However, this case of endophthalmitis resolved and at study end the patient had a 7-letter BCVA increase compared with baseline. The incidence of endophthalmitis (SAE) in the RESOLVE study (2%) was slightly higher compared with that reported in prior AMD trials (0–1.0%) (23). However, the underlying sample size and the number of events are too small to allow for conclusions regarding the risk of endophthalmitis in patients with DME. A recent analysis of safety in diabetic and nondiabetic patients with AMD from the AMD trials Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in AMD (ANCHOR), Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular AMD (MARINA), Phase IIIb, Multicenter, Randomized, Double-Masked, Sham Injection-Controlled Study of the Efficacy and Safety of Ranibizumab in Subjects with Subfoveal Choroidal Neovasularization with or without Classic CNV Secondary to Age-Related Macular Degeneration (PIER), the Safety Assessment of Intravitreal Lucentis for AMD (SAILOR), EXCITE, and EXTEND I (N = 3,736) has revealed that the incidences of endophthalmitis were comparable between the diabetic (0.4% [2 of 523]) and nondiabetic patients (0.5% [17 of 3,213]) with AMD, with no indication of increased risk for the diabetic population (24). As in prior nAMD studies eye pain, conjunctival hemorrhage, and transient intraocular pressure increase were the most frequently reported ocular AEs, and these were suspected to be related to study procedure (ocular injection).
One of the limitations of this study was that there was no laser control arm, but laser photocoagulation was permitted as rescue therapy (starting month 3). Approximately 5% ranibizumab and 35% sham patients received laser photocoagulation during the study. Furthermore, the impact of rescue laser treatment on BCVA outcome was not assessed.
When patients were enrolled, we were aware that the study required deferral of laser treatment for 3 months or discontinuation of patients who needed laser photocoagulation within the first 3 months of the study. This is at the border between requiring observation and immediate treatment and we recognize that it is a judgment that balances the relative effectiveness and risk of laser treatment. Examples of the type of eligible clinical scenarios include DME in the presence of stable visual acuity for >3 months; DME associated with leaking microaneurysms, risk of producing symptomatic perifoveal scotomata, choroidal neovascularization or expansion of scarring leading to foveolar atrophy, or borderline reactivation after prior laser treatment. The relatively low number of eyes that received photocoagulation before or after enrollment suggests that eyes in which photocoagulation was not considered a good option were common in this trial. The trial protocol did not include any attempt to guide investigators as to the exact use of laser therapy because it was believed that no satisfactory standard exists for this purpose and that the responsibility had to remain exclusively with the investigator. Approximately 20% of eyes had received prior laser treatment; however, a subgroup analysis revealed that this treatment had no impact on the BCVA outcome. Our results should be interpreted with these factors in mind and may not be applicable to more advanced disease. However, we believe that they are relevant to patients in the intermediate stages of the development of DME.
It is proposed that ranibizumab as an adjunct to laser treatment may be more effective than either therapy alone; in addition, the combination may lead to fewer ranibizumab treatments. The recent Diabetic Retinopathy Clinical Research Network (DRCR.net) study showed that ranibizumab combined with prompt/deferred laser photocoagulation provided superior benefits compared with laser treatment alone in DME (25). However, results from the earlier READ-2 study showed that ranibizumab monotherapy led to superior improvements in BCVA compared with the combination or laser photocoagulation alone (14). The RESTORE study, which assesses the efficacy and safety of 0.5 mg ranibizumab alone or as an adjunct to laser treatment compared with laser treatment, will provide further knowledge of the efficacy and safety of ranibizumab either as monotherapy or as an adjunct to laser therapy.
The RESOLVE study included a possibility of dose-doubling that was eventually undertaken in the majority of patients receiving ranibizumab. Most patients (86%) received a dose between 0.5 and 1.0 mg inclusive during the study period. Dose doubling was included to allow for best efficacy outcomes, however, the study was not designed for precise estimates of the effect of dose doubling. Upon analysis of the actual doses used in each treatment arm over the study period, the average dose received was 0.47 mg in the 0.3–0.6 mg group and 0.76 mg in the 0.5–1.0 mg group. Because these variable dose changes resulted in a heterogeneous group within treatment arms, with overlapping between treatment arms, the study results were mainly discussed based on the pooled ranibizumab group and are considered to be representative for treatment with 0.5-mg injections.
Given the nature of diabetes and variability in patients with DME with regard to disease progression and vision loss, there is a need for an individualized treatment regimen. The RESOLVE study allowed for such a dosing regimen, because retreatment (after three monthly injections) was based on predefined visual acuity/CRT and safety criteria; this concept partly mimics clinical practice. However, unlike clinical practice, the visual acuity/CRT criteria adopted in the study were stringent to increase the likelihood of patient benefit.
Results from the RESOLVE study indicate that DME responds well to treatment with intravitreal ranibizumab over 1 year. In light of the sustained improvements in BCVA and CRT over the 12-month study period combined with a good safety profile, ranibizumab appears to be a promising pharmacological agent for the management of visual impairment due to DME. These results provide a strong basis for continuing development of ranibizumab in phase III trials in DME, and this study is a stepping stone toward increasing the treatment options for these patients.
Supplementary Material
Acknowledgments
This study was supported by Novartis Pharma.
P.Ma. has served on advisory boards for Novartis and its competitors and has received honoraria and travel and accommodation payments from them. F.B. has served on advisory boards for Novartis and has received honoraria and travel and accommodation payments from them as well as from Allergan, Pfizer, and Solvay. L.L.H. presented interim data of the study (Macula Update, Leipzig 2008) and has received honoraria and travel and accommodation payments from Novartis. S.P.H. has served on advisory boards for Novartis and its competitors and has received honoraria and travel and accommodation payments from them. M.L., on behalf of his employer, has performed contract research and consultancy services for Novartis and its competitors, including serving on advisory boards. P.Mi. has received a consultancy fee from Novartis Pharma, Pfizer, Solvay, and Allergan and lecture fees/honoraria and travel and accommodation payments from Novartis Pharma, Pfizer, Solvay, and Allergan. D.S. has served on advisory boards for Novartis and has received honoraria and travel and accommodation payments from them. U.E.K.W.-S., on behalf of her employer, has performed contracted research for Novartis and its competitors. S.W. has received a consultancy fee from Novartis Pharma and lecture fees/honoraria from Novartis Pharma, Pfizer, and Allergan and is an Advisory Board Member for studies sponsored by Novartis as well as several other companies. No other potential conflicts of interest relevant to this article were reported.
P.Ma. assisted with the design of the study, participated in the study as principal investigator, contributed to the collection of research data, contributed to discussion, and reviewed/edited the manuscript. F.B. contributed to the collection of research data and manuscript preparation, and reviewed/edited the manuscript. J.G.G. was principal investigator at the Bern study center, contributed to the collection of research data and manuscript preparation, and reviewed/edited the manuscript. L.L.H. was the principal investigator of the institution with the highest rate of recruitment and reviewed/edited the manuscript. S.P.H. contributed to manuscript preparation and reviewed/edited the manuscript. M.L., P.Mi., D.S. participated as principal investigator, contributed to the collection of research data and manuscript preparation and reviewed/edited the manuscript. U.E.K.W.-S. contributed to data collection and evaluation of imaging data at the Bern Photographic Reading Center and manuscript preparation and reviewed/edited the manuscript. M.G. participated in the study as clinical trial head, contributed to manuscript preparation, and reviewed/edited the manuscript. A.W. was the study statistician, researched data, and contributed to manuscript preparation. S.W. contributed to development of the protocol, supervised reading center activities and data analysis, contributed to manuscript preparation, and reviewed/edited the manuscript.
Parts of this study were presented in abstract form at the annual meeting of the American Academy of Ophthalmology, Atlanta, Georgia, 8–11 November 2008; at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, 3–9 May 2009; at the 45 annual meeting of the European Association for the Study of Diabetes, Vienna, Austria, 29 September–2 October 2009; and at the 27 annual meeting of the American Society of Retinal Specialists, New York, 30 September–4 October 2009.
We thank the following Novartis employees who assisted with the analysis and/or interpretation of these data: Y. Hashad, G. Burian, O. Gerstner, Ch. Ortmann, and S. Osborne. We also thank third parties that assisted in the study conduct: Global Central Labs PPD (Belgium), Bern Reading Center (Switzerland), MEDIDATA (Germany), and VA certifiers B. Bhogal, A. Lea, and K. Burke. Medical writing and editorial support was provided by Aditi Gandhe and Shivani Mittra of Novartis Healthcare, India.
Footnotes
Clinical trial reg. no. NCT00284050, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying editorial, p. 2484.
References
- 1.Diabetes. Fact sheet No. 312 [article online], 2009. Available from http://www.who.int/mediacentre/factsheets/fs312/en/ Accessed 20 December 2009
- 2.Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ 2004;82:844–851 [PMC free article] [PubMed] [Google Scholar]
- 3.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology 1998;105:1801–1815 [DOI] [PubMed] [Google Scholar]
- 4.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 1998;21:1414–1431 [DOI] [PubMed] [Google Scholar]
- 5.Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol 1985;103:1796–806 [PubMed] [Google Scholar]
- 6.Diabetic Retinopathy Clinical Research Network A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology 2008;115:1447–1449, 1449.e1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cekiç O, Chang S, Tseng JJ, Akar Y, Barile GR, Schiff WM. Cataract progression after intravitreal triamcinolone injection. Am J Ophthalmol 2005;139:993–998 [DOI] [PubMed] [Google Scholar]
- 8.Thomas D, Bunce C, Moorman C, Laidlaw DA. A randomised controlled feasibility trial of vitrectomy versus laser for diabetic macular oedema. Br J Ophthalmol 2005;89:81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aiello LP, Bursell SE, Clermont A, Duh E, Ishii H, Takagi C, Mori F, Ciulla TA, Ways K, Jirousek M, Smith LE, King GL. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes 1997;46:1473–1480 [DOI] [PubMed] [Google Scholar]
- 10.Poulaki V. Hypoxia in the pathogenesis of retinal disease. In Retinal Vascular Disease. Joussen AM, Gardner TW, Kirchhof B, Ryan SJ. Eds. Berlin, Germany, Springer, 2007, p. 121–138 [Google Scholar]
- 11.Simó R, Hernández C. Intravitreous anti-VEGF for diabetic retinopathy: hopes and fears for a new therapeutic strategy. Diabetologia 2008;51:1574–1580 [DOI] [PubMed] [Google Scholar]
- 12.Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 2006;26:859–870 [DOI] [PubMed] [Google Scholar]
- 13.Chun DW, Heier JS, Topping TM, Duker JS, Bankert JM. A pilot study of multiple intravitreal injections of ranibizumab in patients with center-involving clinically significant diabetic macular edema. Ophthalmology 2006;113:1706–1712 [DOI] [PubMed] [Google Scholar]
- 14.Nguyen QD, Shah SM, Heier JS, Do DV, Lim J, Boyer D, Abraham P, Campochiaro PAREAD-2 Study Group Primary end point (six months) results of the Ranibizumab for Edema of the mAcula in diabetes (READ-2) study. Ophthalmology 2009;116:2175–2181.e1 [DOI] [PubMed] [Google Scholar]
- 15.Cunningham ET, Jr, Adamis AP, Altaweel M, Aiello LP, Bressler NM, D'Amico DJ, Goldbaum M, Guyer DR, Katz B, Patel M, Schwartz SDMacugen Diabetic Retinopathy Study Group A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology 2005;112:1747–1757 [DOI] [PubMed] [Google Scholar]
- 16.Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, Sanchez JG, Wu L, Maia M, Berrocal MH, Solis-Vivanco A, Farah ME. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology 2007;114:743–750 [DOI] [PubMed] [Google Scholar]
- 17.Lam DS, Lai TY, Lee VY, Chan CK, Liu DT, Mohamed S, Li CL. Efficacy of 1.25 mg versus 2.5 mg intravitreal bevacizumab for diabetic macular edema: six-month results of a randomized controlled trial. Retina 2009;29:292–299 [DOI] [PubMed] [Google Scholar]
- 18.Soheilian M, Ramezani A, Obudi A, Bijanzadeh B, Salehipour M, Yaseri M, Ahmadieh H, Dehghan MH, Azarmina M, Moradian S, Peyman GA. Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema. Ophthalmology 2009;116:1142–1150 [DOI] [PubMed] [Google Scholar]
- 19.Diabetic Retinopathy Clinical Research Network, Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK, Elman MJ, Friedman SM, Greven CM, Maturi RK, Pieramici DJ, Shami M, Singerman LJ, Stockdale CR. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology 2007;114:1860–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider SANCHOR Study Group Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:1432–1444 [DOI] [PubMed] [Google Scholar]
- 21.Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S, Shams N. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol 2008;145:239–248 [DOI] [PubMed] [Google Scholar]
- 22.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RYMARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–1431 [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Arumi J. Safety profile of diabetic macular edema patients treated over 12 months with ranibizumab in the RESOLVE study. Poster presented at the Annual Meeting of the Association for Research in Vision and Ophthalmology, 1–5 May 2010, Fort Lauderdale, FL [Google Scholar]
- 24.Lanzetta P, Burian G, Weichselberger A, Hashad Y. Safety profiles of diabetic versus non-diabetic AMD patients in randomized controlled clinical studies with ranibizumab. Poster presented at the Annual Meeting of the Association for Research in Vision and Ophthalmology, 1–5 May 2010, Fort Lauderdale, FL [Google Scholar]
- 25.The Diabetic Retinopathy Clinical Research Network Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010;117:1064–1077.e35 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.