Abstract
OBJECTIVE
The present study characterizes mannose-binding lectin (MBL), an activator of the complement system and thereby important for inflammatory activation, in patients with diabetes and myocardial infarction.
RESEARCH DESIGN AND METHODS
Serum (S)-MBL was determined at hospital admission in 387 patients with type 2 diabetes (median age 70 years; 68% male) with myocardial infarction, and genotyping was performed in 287 patients. Cardiovascular events (cardiovascular mortality and nonfatal myocardial infarction or stroke) were recorded during 2.5 years.
RESULTS
Median S-MBL was 1,212 μg/l (interquartile range [IQR] 346–2,681 μg/l). Of the subjects, 54% in the geno- and phenotype subgroup had a high-coding MBL genotype (median S-MBL = 2,658 μg/l [IQR 1,715–3,829]) and 46% a low-coding MBL genotype (373 μg/l [100–765]). S-MBL did not correlate with age, BMI, creatinine clearance, glucose, or A1C. Cardiovascular events occurred in 136 (35%) patients. S-MBL did not predict events in univariable analyses (hazard ratio 0.93 [95% CI 0.85–1.01]; P = 0.09). In unadjusted analyses, the risk of events was lower in patients with a high genotype and S-MBL above the median for their genotype (0.49 [0.26–0.92]; P = 0.026) than for patients with a low genotype and S-MBL below the median for their genotype. The prediction capacity of the geno- and phenotype model was of borderline significance in adjusted Cox regression.
CONCLUSIONS
Patients with type 2 diabetes and myocardial infarction have MBL genotypes that are similar to those known in the general population. The combination of a low-coding MBL genotype with a low S-MBL appears to be prognostically unfavorable, but the association is blunted by traditional risk markers.
Macrovascular complications, not the least is myocardial infarction, are leading causes of death in patients with type 2 diabetes. An improved understanding of pathophysiological mechanisms is a prerequisite for the development of new treatment targets. Chronic, low-grade inflammation is a component in the development of atherosclerosis and coronary artery disease (1). There is evidence of increased inflammatory activation and downstream markers of inflammation, e.g., C-reactive protein, predict an increased risk of cardiovascular mortality and morbidity (1). To enable a successful modulation of disease progression, it is, however, likely that the earlier stages of the inflammatory process should be targeted.
Mannose-binding lectin (MBL) is a pattern recognition molecule of the innate immune system that has attracted interest in the context of cardiovascular disease (2). MBL activates the lectin pathway, one of three (classic, lectin, or alternative) arms, which initiates the complement cascade (3). Although MBL acts as an acute-phase reactant, circulating levels remain stable over time, only increasing two- to threefold during stress (3). However, genetic regulation accounts for a considerable variation in MBL concentration among individuals. MBL deficiency is associated with autoimmune, inflammatory, and vascular diseases (4). Information on a potential link between MBL and cardiovascular complications is contradictory. MBL levels were higher among patients with acute coronary syndromes than among control subjects in a recent study (5) and in another study comprising 890 patients with ST-segment myocardial infarction undergoing percutaneous coronary intervention, functional deficiency of complement MBL, defined as ≤100 ng/ml, was associated with reduced mortality but not with a decreased risk of the combined end points of death and shock or death, shock, and congestive heart failure (6). In contrast, low MBL levels and MBL2 genotypes have been linked to atherosclerosis (7,8), and individuals with MBL deficiency have a higher risk of coronary artery disease (9,10).
The aim of the present report was to characterize MBL geno- and phenotypes in patients with type 2 diabetes and acute myocardial infarction (AMI). A secondary aim was to test the hypothesis that this information may be useful as a prognostic marker in such patients.
RESEARCH DESIGN AND METHODS
The Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI 2) trial compared three different management strategies in patients with type 2 diabetes and suspected AMI. An extensive description of the study design has been presented elsewhere (11). In short, 1,253 patients were randomly assigned to one of three study arms receiving 1) a 24-h insulin-glucose infusion, followed by subcutaneous insulin-based long-term glucose control (group 1, n = 474); 2) the same initial treatment, followed by glucose-lowering treatment according to local practice (group 2, n = 473); or 3) glucose-lowering treatment according to local practice (group 3, n = 306). There was no significant difference in total mortality or nonfatal myocardial infarction and stroke during the median follow-up period of 2.1 years. This allowed the possibility of combining all DIGAMI 2 patients into one cohort suitable for post hoc epidemiological analyses disregarding randomized group belonging. The present study consists of 387 participants in a biochemistry substudy, in which serum (S) MBL was determined at hospital admission. In 287 of them, samples were available for both S-MBL and MBL2 genotyping.
Investigations
Samples for assessments of the following variables were obtained as soon as possible after hospital admission: electrolytes, serum creatinine, blood lipids, glucose (millimoles per liter in whole blood), A1C, and S-MBL. Samples for genetic analyses were obtained at the time of hospital discharge. A1C was analyzed by high-performance liquid chromatography with an upper normal limit of 5.3%. Creatinine clearance (milliliters per min) was calculated using the Cockroft-Gault method. S-MBL levels were measured using an in-house, time-resolved immunofluorometric assay (12). The lower detection level was 10 μg/l, and the intra-assay and inter-assay coefficients of variation were <10%.
A full description of the genotyping of the patients is found in the supplementary data (available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc10-0903/DC1). In short, DNA was extracted from whole blood and analyzed using real-time PCR with TaqMan single nucleotide genotyping assays (Applied Biosystems, Foster City, CA) (13–15).
The presence of one of the three structural mutations in the MBL2 gene, located at 10q21.1, the D, B, and C variants (designated as “O”), significantly reduces circulating MBL. Of the three known promoter polymorphisms, only the X/Y polymorphism influences S-MBL, causing reduced MBL levels (5). In the present report, genotypes were categorized according to polymorphisms in the MBL2 gene as AA (wild-type), AO (heterozygote), or OO (homozygote). After the addition of information on the promoter region, genotypes were classified as high (including all AA with the exclusion of homozygotes for the X promotor) or low, as determined previously by Hansen et al. (16).
A composite of cardiovascular death, reinfarction, or stroke, adjudicated by an independent committee composed of experienced cardiologists, served as the primary end point.
Statistical methods
Continuous variables are presented as median and interquartile range (IQR), unless otherwise stated. The Wilcoxon Mann-Whitney test was used to study differences between groups of patients stratified by dichotomous variables, and the association between continuous variables was assessed by means of Spearman rank correlation. The Jonckheere-Terpstra test was used to compare differences between genotypes and combinations of geno- and phenotypes. The relationships between S-MBL and age, sex, BMI, previous myocardial infarction, heart failure, hypertension, creatinine clearance, B-glucose, and A1C at admission were studied by regression analyses.
The relationship between S-MBL and the primary end point was assessed using Cox proportional hazards regression and presented as hazard ratios (HR) and 95% CIs. Because of skewed distribution, S-MBL was log-transformed before analysis. No model building was performed in the principal analysis. For the outcome analysis, S-MBL was dichotomized below or above a level of 1,000 μg/l according to previous findings, demonstrating that this cutoff level has high sensitivity and specificity for risk prediction (10,17). Patients were also classified according to their genotypes in relation to the median S-MBL concentration for the respective genotype: high/above indicates high genotype with S-MBL above the median for this genotype; high/below indicates high genotype with S-MBL below the median for this genotype; low/above indicates low genotype with S-MBL above the median for this genotype; and low/below indicates low genotype with S-MBL below the median for this genotype.
To illustrate time trends for cardiovascular outcome, Kaplan-Meier curves were drawn using S-MBL less than or greater than 1,000 μg/l and the combination of geno- and phenotypes (high/above, high/below, low/above, and low/below) as strata. A log-rank test for trend was applied to assess differences in event patterns.
Cox proportional hazards regression was used to test the prediction capacity of dichotomized S-MBL (less than or greater than 1,000 μg/l), as well as of the combination of geno- and phenotypes. Cox proportional hazards regression models were adjusted with the same covariates as those used in the regression analyses.
Two-tailed statistical tests were used at a 5% significance level. SAS (version 9.2) was used for all statistical analyses.
Ethical considerations
The study followed the recommendations of the Declaration of Helsinki, and local ethics review boards approved the protocol. Written informed consent was obtained from all patients before enrollment.
RESULTS
The baseline characteristics of patients with S-MBL measured at admission are presented in Table 1. S-MBL varied between 10 and 6,382 μg/l (median 1,212 μg/l; mean 1,666 μg/l; IQR 346–2,681 μg/l). Patients (n = 287) with both an S-MBL and MBL2 genotype had baseline characteristics and S-MBL levels similar to those of the total cohort of patients with S-MBL available (n = 387). There were no significant differences in baseline characteristics between patients with high (n = 156) or low genotypes (n = 131) (Table 1). The median S-MBL in patients with a high-coding genotype was 2,658 μg/l (IQR 1,715–3,829) compared with 373 μg/l (100–765) in those with a low-coding genotype.
Table 1.
Baseline characteristics of all patients with S-MBL, of patients with both S-MBL and genotype, and of patients grouped according to genotype (encoding high or low S-MBL concentration)
| Variable | All patients | Geno- and phenotyped patients | High genotype | Low genotype |
|---|---|---|---|---|
| n | 387 | 287 | 156 | 131 |
| Age (years) | 70.0 (61.1–77.1) | 69.9 (60.7–76.7) | 67.9 (60.4–75.7) | 70.7 (61.1–77.1) |
| Male sex | 264 (68) | 194 (68) | 107 (69) | 87 (66) |
| BMI (kg/m2) | 28 (26–31) | 28 (26–31) | 28 (26–31) | 28 (25–31) |
| Diabetes duration (years) | 6.0 (2.0–14.0) | 6.0 (1.0–14.0) | 5.5 (0–13.0) | 8.0 (2.0–14.5) |
| Blood pressure (mmHg) | ||||
| Systolic | 130 (120–150) | 130 (120–150) | 135 (120–150) | 130 (115–145) |
| Diastolic | 74 (65–85) | 74 (63–85) | 75 (64–85) | 70 (60–80) |
| Previous medical history | ||||
| Myocardial infarction | 149 (39) | 109 (38) | 59 (38) | 50 (38) |
| Angina pectoris | 202 (52) | 145 (51) | 79 (51) | 66 (50) |
| Heart failure | 77 (20) | 53 (19) | 25 (16) | 28 (21) |
| Hypertension | 214 (55) | 160 (56) | 92 (59) | 68 (52) |
| Hyperlipidemia | 134 (35) | 101 (35) | 51 (33) | 50 (38) |
| Current smoker | 84 (22) | 61 (21) | 32 (21) | 29 (22) |
| Medication before admission | ||||
| Insulin | 141 (36) | 104 (36) | 51 (33) | 53 (40) |
| Metformin | 102 (26) | 73 (25) | 35 (22) | 38 (29) |
| Sulfonylurea | 150 (39) | 108 (38) | 50 (32) | 58 (44) |
| β-Blocker | 176 (46) | 123 (43) | 69 (45) | 54 (41) |
| Aspirin | 204 (53) | 148 (52) | 87 (56) | 61 (47) |
| ACE inhibitor | 122 (32) | 94 (33) | 53 (34) | 41 (31) |
| Lipid-lowering | 119 (31) | 90 (31) | 46 (30) | 44 (34) |
| Biochemical analysis at admission | ||||
| Blood glucose (mmol/l) | 11.8 (9.2–14.9) | 11.9 (9.5–14.9) | 12.0 (9.3–15.1) | 11.9 (9.6–14.9) |
| A1C (%) | 7.1 (6.2–8.5) | 7.1 (6.3–8.6) | 7.0 (6.3–8.7) | 7.3 (6.3–8.5) |
| Serum creatinine (μmol/l) | 91 (78–112) | 89 (75–107) | 88 (75–108) | 90 (76–106) |
| Creatinine clearance (ml/min) | 72 (49–92) | 74 (51–96) | 78 (52–97) | 70 (49–95) |
| Serum cholesterol (mmol/l) | 5.0 (4.2–5.8) | 5.1 (4.4–5.9) | 5.1 (4.6–5.9) | 4.9 (4.2–6.0) |
| Serum triglycerides (mmol/l) | 1.7 (1.2–2.6) | 1.8 (1.2–2.7) | 1.9 (1.2–2.7) | 1.7 (1.1–2.7) |
Data are n (%) or median (quartile 1–quartile 3). All variables refer to the time of hospital admission, unless otherwise stated.
The genetic analyses revealed that 173 patients belonged to genotype AA (60.3%), 104 to AO (36.2%), and 10 to OO (3.5%). S-MBL differed significantly among patients with different genotypes (Jonckheere-Terpstra test; P < 0.001). The respective medians and IQRs for the three groups were AA 2,538 μg/l (IQR 1,498–3,772), AO 384 μg/l (130–702), and OO 12 μg/l (10–65).
There were no significant differences in S-MBL between men and women or between patients with or without a history of myocardial infarction, heart failure, or hypertension (Table 2). Moreover, there were no significant correlations between S-MBL and age, BMI, creatinine clearance, glucose, and A1C at admission (Spearman correlation).
Table 2.
Differences between groups of patients stratified by dichotomous variables (Wilcoxon Mann-Whitney test) and associations between continuous variables (Spearman rank correlation) in all patients with S-MBL and in patients with both S-MBL and genotype available
| Variable | All patients | P value | Geno- and phenotyped patients | P value |
|---|---|---|---|---|
| n | 387 | 287 | ||
| Wilcoxon Mann-Whitney test | S-MBL (μg/1) | S-MBL (μg/1) | ||
| Sex | ||||
| Male | 1,357 (337–2,835) | 0.44 | 1,555 (301–3,287) | 0.19 |
| Female | 1,046 (347–2,394) | 1,071 (371–2,336) | ||
| Previous myocardial infarction | ||||
| Yes | 1,354 (346–2,568) | 0.71 | 1,475 (303–2,878) | 0.96 |
| No | 1,158 (347–2,836) | 1,297 (346–2,930) | ||
| Previous heart failure | ||||
| Yes | 943 (241–2,100) | 0.13 | 943 (239–2,392) | 0.23 |
| No | 1,270 (373–2,860) | 1,446 (386–2,930) | ||
| Previous hypertension | ||||
| Yes | 1,337 (386–2,836) | 0.18 | 1,542 (407–2,941) | 0.09 |
| No | 1,041 (234–2,605) | 1,026 (n177–2,778) | ||
| Spearman rank correlation | Correlation coefficient | Correlation coefficient | ||
| Age | −0.08 | 0.13 | −0.08 | 0.21 |
| BMI | −0.03 | 0.52 | −0.03 | 0.65 |
| Biochemical analysis at admission | ||||
| Creatinine clearance | 0.08 | 0.15 | 0.04 | 0.49 |
| Glucose | 0.06 | 0.22 | 0.05 | 0.43 |
| A1C | −0.01 | 0.84 | −0.04 | 0.48 |
Data are median (quartile 1–quartile 3) or correlation coefficient.
A univariable regression analysis of S-MBL and age, sex, BMI, creatinine clearance, glucose, A1C, a history of myocardial infarction, heart failure, and hypertension was performed separately in the dataset with all patients (n = 387) and in the geno- and phenotyped patients (n = 287). Only hypertension showed a weak relationship (unadjusted P = 0.05) in patients with both geno- and phenotype available (data not shown).
Mortality and morbidity
During the follow-up period of 2.5 (range 1.04–3.00) years, 35% of the total cohort (n = 387) had a cardiovascular event (Table 3). The corresponding number in the subgroup with both S-MBL and genotype available (n = 287) was 30.0%.
Table 3.
Number and proportion of events and the predictive value of S-MBL (Cox proportional hazards regression models) for respective events in all patients with S-MBL and in patients with both S-MBL and genotype available
| All patients |
Geno- and phenotyped patients |
|||||
|---|---|---|---|---|---|---|
| n (%) | HR (95% CI) | P value | n (%) | HR (95% CI) | P value | |
| n | 387 | 287 | ||||
| Death | 95 (24.6) | 0.93 (0.84–1.04) | 0.20 | 56 (19.5) | 0.97 (0.85–1.12) | 0.71 |
| CV death | 77 (19.9) | 0.95 (0.85–1.08) | 0.43 | 43 (15.0) | 0.99 (0.84–1.16) | 0.89 |
| Reinfarction | 58 (15.0) | 0.95 (0.83–1.09) | 0.45 | 41 (14.3) | 0.93 (0.79–1.09) | 0.35 |
| Stroke | 25 (6.5) | 0.87 (0.72–1.06) | 0.17 | 20 (7.0) | 0.89 (0.71–1.10) | 0.27 |
| CV event | 136 (35.1) | 0.93 (0.85–1.01) | 0.09 | 86 (30.0) | 0.92 (0.82–1.02) | 0.12 |
CV, cardiovascular.
The predictive value of S-MBL for the composite end point, its separate components, and total mortality are shown in Table 3. No model building was performed, because S-MBL did not predict the primary end point or any components in the univariable analysis.
Patients with S-MBL >1,000 μg/l had lower event rates than those below that level in a univariable Cox regression analysis (HR 0.68 [95% CI 0.48–0.95]; P = 0.02). This pattern remained after univariable adjustments for age, admission glucose, BMI, sex, previous myocardial infarction, and hypertension, but not with adjustment for creatinine clearance, A1C, or previous heart failure. However, the dichotomized S-MBL did not predict events (P = 0.09) in a multiple Cox regression model adjusting for significant confounders using a best subset selection criterion (age, BMI, admission glucose, creatinine clearance, and myocardial infarction).
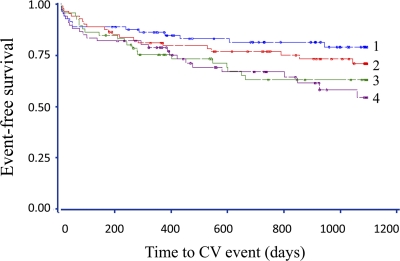
When we grouped the patients according to their genotype and S-MBL above or below the median for their genotype (high/above [n = 78], high/below [n = 78], low/above [n = 65], and low/below [n = 66]), there was a significant trend among the four groups to predict survival free from cardiovascular event (log-rank test P = 0.01) (Fig. 1). Using the low/below group as a reference in Cox regression analyses, patients with high/above were the only group with a significantly lower event rate (HR = 0.49 [95% CI 0.26–0.92]; P = 0.03). For patients in the high/below and low/above groups compared with low/below, the HRs were 0.66 ([0.37–1.17], P = 0.15) and 0.89 ([0.51–1.57], P = 0.69), respectively. These results remained after univariable adjustments for admission glucose, A1C, BMI, sex, previous myocardial infarction, and hypertension, but not for age, creatinine clearance, or previous heart failure. In a multiple Cox regression model adjusting for significant confounders using a best subset selection criterion (age, BMI, creatinine clearance, and previous myocardial infarction), the predictive capacity of the geno- and phenotype model was of borderline significance (P = 0.07).
Figure 1.
Kaplan-Meier curves for cardiovascular (CV) events in patients grouped according to genotype (encoding high or low S-MBL) and S-MBL level (above or below median S-MBL concentration for respective genotype) (log-rank test for trend P = 0.01). 1, high/above; 2, high/below; 3, low/above; 4, low/below. ●, censored event.
CONCLUSIONS
This study is the first to examine the distribution of MBL geno- and phenotypes and the impact of MBL on prognosis in patients with type 2 diabetes and myocardial infarction. The patients had a MBL2 genotype distribution similar to that of the general population and S-MBL did not contribute much information on cardiovascular prognosis beyond traditional risk markers. The results suggest that the combination of a low geno- and phenotype is associated with a poorer outcome, but they does not explain whether a low or a high MBL level is harmful in this patient group.
In the present cohort, the distribution of MBL genotypes was similar to that in a general European population (18,19), with the majority of patients, 60%, belonging to the wild-type genotype (AA), whereas the remainder had one or two alleles with mutations (AO or OO). As expected, the phenotypes were distributed with the highest S-MBL levels in patients belonging to the AA group and in those classified as having a high genotype, i.e., also including information on polymorphisms in the promoter region (19).
The present S-MBL median of 1,212 μg/l, with no sex difference, was higher than that detected in outpatients with type 2 diabetes and healthy control subjects (median 666 and 728 μg/l, respectively) (17). One explanation for the higher S-MBL in this cohort is that MBL was measured at admission for AMI and was thereby possibly influenced by stress. In a study of patients with acute coronary syndrome and matched control subjects, patients had higher MBL than control subjects (median 855 and 441 μg/l; P < 0.0001) (5).
The present study does not resolve the issues of whether a low or a high S-MBL and which genotypes are harmful in patients with type 2 diabetes and myocardial infarction. In the unadjusted analysis, S-MBL <1,000 μg/l and the combination of a low genotype with S-MBL below the median for this genotype was associated with a poorer outcome, but this finding did not remain after adjustments.
One limitation of the present report is that patients with both geno- and phenotypes available had a lower event rate than that for the total cohort (Table 3). One possible explanation is that genotypes were measured at the time of hospital discharge, thereby eliminating patients who died in the hospital from the genotyped subgroup. The results of the correlation and regression analyses for continuous S-MBL were, however, similar in the two groups, suggesting that findings in the genotyped group are representative. Only a larger study will be able to elucidate the prognostic significance of determining MBL geno- and phenotypes.
Several studies have linked a low S-MBL or genotypes associated with a low S-MBL to cardiovascular disease (7–10,20). There are, however, reports of an association between macrovascular disease and high MBL (5,6,21). These discrepant findings may relate to differences in study design, the composition of patient populations, and the type of events studied. Pesonen et al. (5) noted that patients with acute coronary syndrome had high S-MBL and genotypes, but this was in comparison with healthy control subjects. Furthermore, patients with type 2 diabetes may react differently to MBL than those without type 2 diabetes. In the study by Trendelenburg et al. (6) few patients had diabetes. In studies with a high proportion of patients with diabetes, low S-MBL or low MBL genotypes were related to a poor prognosis (9,10).
The possible relationship between low MBL and an increased risk of atherosclerosis may have several explanations. In a study comprising 2,176 children, homozygosity for the MBL variant alleles was associated with a greater impairment in flow-mediated dilation during infection compared with that in control subjects without infection, suggesting that an MBL gene-environment interaction, possibly occurring early in life, may have an impact on the initiation and progression of atherosclerosis (22).
The idea underlying the use of the combination of genotype and MBL levels below or above the median for the respective genotype was to combine information on genetic susceptibility and ongoing inflammation. The inability of this combination to predict risk in the present cohort may be due to a lack of power, but it also indicates that easily available factors such as age and renal function are more useful as clinical risk markers than S-MBL.
MBL may play a different role in different settings, which limits its usefulness as an overall risk marker or a treatment target. However, before abandoning the concept of MBL and the notion of the potential importance of the innate immune system for the progression of cardiovascular diseases, it is important to look further into downstream steps of the complement cascade.
Supplementary Material
Acknowledgments
This study was supported by grants from the Swedish Heart-Lung Foundation, the Swedish Research Council (8691), and AFA Insurance and by unconditional grants from Aventis Sweden and Novo Nordisk Denmark.
No other potential conflicts of interest relevant to this article were reported.
L.G.M., L.R., and T.K.H. researched and prepared data, wrote the manuscript, and revised/edited the manuscript. A.H. and R.S. performed laboratory analyses, contributed to discussion, and revised/edited the manuscript. K.M. researched data and contributed to discussion. J.Ö. assisted in statistical analyses and reviewed/edited the manuscript.
We thank Mattias Molin, BSc, Statistical Consulting Group, Göteborg, Sweden, for valuable support with the database.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Libby P, Ridker PM, Hansson GK. Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 2009;54:2129–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bjerre M, Hansen TK, Flyvbjerg A. Complement activation and cardiovascular disease. Horm Metab Res 2008;40:626–634 [DOI] [PubMed] [Google Scholar]
- 3. Ip WK, Takahashi K, Ezekowitz RA, Stuart LM. Mannose-binding lectin and innate immunity. Immunol Rev 2009;230:9–21 [DOI] [PubMed] [Google Scholar]
- 4. Thiel S, Frederiksen PD, Jensenius JC. Clinical manifestations of mannan-binding lectin deficiency. Mol Immunol 2006;43:86–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pesonen E, Hallman M, Sarna S, Andsberg E, Haataja R, Meri S, Persson K, Puolakkainen M, Ohlin H, Truedsson L: Mannose-binding lectin as a risk factor for acute coronary syndromes. Ann Med 2009;41:591–598 [DOI] [PubMed] [Google Scholar]
- 6. Trendelenburg M, Theroux P, Stebbins A, Granger C, Armstrong P, Pfisterer M: Influence of functional deficiency of complement mannose-binding lectin on outcome of patients with acute ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Eur Heart J 2010;31:1181–1187 [DOI] [PubMed] [Google Scholar]
- 7. Madsen HO, Videm V, Svejgaard A, Svennevig JL, Garred P. Association of mannose-binding-lectin deficiency with severe atherosclerosis. Lancet 1998;352:959–960 [DOI] [PubMed] [Google Scholar]
- 8. Hegele RA, Ban MR, Anderson CM, Spence JD. Infection-susceptibility alleles of mannose-binding lectin are associated with increased carotid plaque area. J Investig Med 2000;48:198–202 [PubMed] [Google Scholar]
- 9. Best LG, Davidson M, North KE, MacCluer JW, Zhang Y, Lee ET, Howard BV, DeCroo S, Ferrell RE. Prospective analysis of mannose-binding lectin genotypes and coronary artery disease in American Indians: the Strong Heart Study. Circulation 2004;109:471–475 [DOI] [PubMed] [Google Scholar]
- 10. Saevarsdottir S, Oskarsson OO, Aspelund T, Eiriksdottir G, Vikingsdottir T, Gudnason V, Valdimarsson H. Mannan binding lectin as an adjunct to risk assessment for myocardial infarction in individuals with enhanced risk. J Exp Med 2005;201:117–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malmberg K, Rydén L, Wedel H, Birkeland K, Bootsma A, Dickstein K, Efendic S, Fisher M, Hamsten A, Herlitz J, Hildebrandt P, MacLeod K, Laakso M, Torp-Pedersen C, Waldenström A. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 2005;26:650–661 [DOI] [PubMed] [Google Scholar]
- 12. Thiel S, Møller-Kristensen M, Jensen L, Jensenius JC. Assays for the functional activity of the mannan-binding lectin pathway of complement activation. Immunobiology 2002;205:446–454 [DOI] [PubMed] [Google Scholar]
- 13. Henckaerts L, Nielsen KR, Steffensen R, Van Steen K, Mathieu C, Giulietti A, Wouters PJ, Milants I, Vanhorebeek I, Langouche L, Vermeire S, Rutgeerts P, Thiel S, Wilmer A, Hansen TK, Van den Berghe G. Polymorphisms in innate immunity genes predispose to bacteremia and death in the medical intensive care unit. Crit Care Med 2009;37:192–201, e1–3 [DOI] [PubMed] [Google Scholar]
- 14. Van Hoeyveld E, Houtmeyers F, Massonet C, Moens L, Van Ranst M, Blanckaert N, Bossuyt X. Detection of single nucleotide polymorphisms in the mannose-binding lectin gene using minor groove binder-DNA probes. J Immunol Methods 2004;287:227–230 [DOI] [PubMed] [Google Scholar]
- 15. Mølle I, Steffensen R, Thiel S, Peterslund NA. Chemotherapy-related infections in patients with multiple myeloma: associations with mannan-binding lectin genotypes. Eur J Haematol 2006;77:19–26 [DOI] [PubMed] [Google Scholar]
- 16. Hansen TK, Tarnow L, Thiel S, Steffensen R, Stehouwer CD, Schalkwijk CG, Parving HH, Flyvbjerg A. Association between mannose-binding lectin and vascular complications in type 1 diabetes. Diabetes 2004;53:1570–1576 [DOI] [PubMed] [Google Scholar]
- 17. Hansen TK, Gall MA, Tarnow L, Thiel S, Stehouwer CD, Schalkwijk CG, Parving HH, Flyvbjerg A. Mannose-binding lectin and mortality in type 2 diabetes. Arch Intern Med 2006;166:2007–2013 [DOI] [PubMed] [Google Scholar]
- 18. Garred P, Larsen F, Seyfarth J, Fujita R, Madsen HO. Mannose-binding lectin and its genetic variants. Genes Immun 2006;7:85–94 [DOI] [PubMed] [Google Scholar]
- 19. Steffensen R, Thiel S, Varming K, Jersild C, Jensenius JC. Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin (MBL) gene by polymerase chain reaction with sequence-specific primers. J Immunol Methods 2000;241:33–42 [DOI] [PubMed] [Google Scholar]
- 20. Rugonfalvi-Kiss S, Endrész V, Madsen HO, Burián K, Duba J, Prohászka Z, Karádi I, Romics L, Gönczöl E, Füst G, Garred P. Association of Chlamydia pneumoniae with coronary artery disease and its progression is dependent on the modifying effect of mannose-binding lectin. Circulation 2002;106:1071–1076 [DOI] [PubMed] [Google Scholar]
- 21. Keller TT, van Leuven SI, Meuwese MC, Wareham NJ, Luben R, Stroes ES, Hack CE, Levi M, Khaw KT, Boekholdt SM. Serum levels of mannose-binding lectin and the risk of future coronary artery disease in apparently healthy men and women. Arterioscler Thromb Vasc Biol 2006;26:2345–2350 [DOI] [PubMed] [Google Scholar]
- 22. Charakida M, Donald AE, Leary S, Halcox JP, Turner MW, Johnson M, Loukogeorgakis SP, Okorie MI, Davey Smith G, Deanfield JE, Klein NJ. Endothelial response to childhood infection: the role of mannose-binding lectin (MBL). Atherosclerosis 2010;208:217–221 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.