Abstract
The objective of this research is to gain a greater understanding of the cause of fasting and postprandial hyperglycemia in people with type 2 diabetes. Endogenous glucose production is excessive before eating and fails to appropriately suppress after eating in people with type 2 diabetes. This is due in part to impaired insulin-induced suppression of endogenous glucose production, which is observed early in the evolution of type 2 diabetes. Increased rates of gluconeogenesis and perhaps glycogenolysis contribute to hepatic insulin resistance. Insulin-induced stimulation of hepatic glucose uptake and hepatic glycogen synthesis are reduced in people with type 2 diabetes primarily due to decreased uptake of extracellular glucose presumably because of inadequate activation of hepatic glucokinase. Delayed insulin secretion results in higher peak glucose concentrations particularly when suppression of glucagon is impaired, whereas insulin resistance prolongs the duration of hyperglycemia, which can be marked when both hepatic and extra-hepatic insulin resistance are present.
The premise of these studies, as well as those performed by many other investigators, is that an understanding of the pathogenesis of type 2 diabetes will enable the development of targeted therapies that are directed toward correcting specific metabolic defects in a given individual. I, as well as many other investigators, believe that such therapies are likely to be more effective and to have a lower risk than would occur if everyone were treated the same regardless of the underlying cause of their hyperglycemia. While we do not yet have sufficient knowledge to truly individualize therapy, in my opinion this approach will be the norm in the not too distant future.
The discovery of insulin by Dr. Frederick Banting and Charles Best forever changed the lives of people with diabetes. When properly used, insulin prevents death from ketoacidosis in people with type 1 diabetes and reduces the symptoms from severe hyperglycemia in people with type 2 diabetes. Unfortunately, as it is currently used, insulin does not completely prevent the chronic microvascular or macrovascular complications of diabetes. This likely is because insulin, as well as all other available therapies, does not fully normalize glucose, or for that matter, lipid or protein metabolism. The premise of the research that my collaborators and I have conducted over the years is that the only way to maximize the benefit and minimize the risk is to tailor therapy to each individual's needs, and the only way to do so is to understand the cause of hyperglycemia in that particular person. While much remains to be learned, I think we have moved a long way toward this goal. The data that I will review in this article primarily focuses on the cause of fasting and postprandial hyperglycemia in type 2 diabetes. However, in general, the principles also pertain to people with type 1 diabetes.
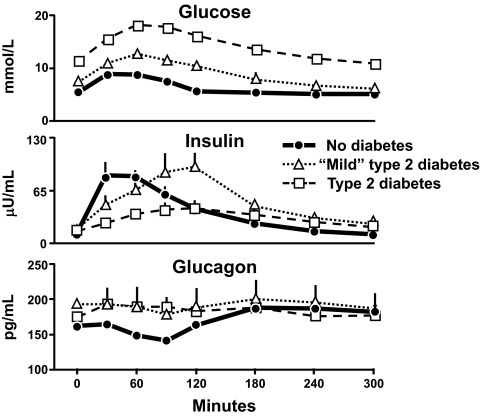
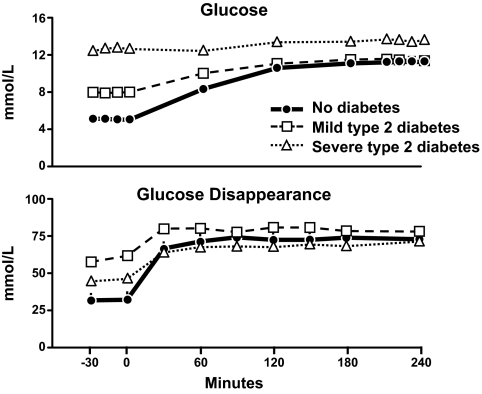
Figure 1 shows the results from a series of experiments conducted by Dr. Peter Butler in which the pattern of change in glucose, insulin, and glucagon concentrations in people with type 2 diabetes were compared with those observed in age-, sex-, and weight-matched nondiabetic subjects (1). Fifty grams of glucose was ingested at time zero. Glucose concentrations in the nondiabetic subjects averaged ∼5 mmol/l before glucose ingestion, increased to only ∼8 mmol/l after glucose ingestion, and returned to preprandial concentrations within 2 h. The reason why this occurred was because insulin concentrations rapidly increased as glucose rose reaching a peak within 30 min of glucose ingestion. Furthermore, glucagon concentrations fell as glucose rose and rose as glucose fell back to preprandial concentrations.
FIG. 1.
Glucose (upper panel), insulin (middle panel), and glucagon (lower panel) concentrations observed in people who did not have diabetes, had mild type 2 diabetes or had severe type 2 diabetes. Fifty grams of glucose was ingested at time zero (1).
The situation is quite different in people with diabetes. People with so-called mild type 2 diabetes have both fasting and postprandial hyperglycemia. In these studies, fasting glucose averaged ∼7.2 mmol/l before glucose ingestion and increased to ∼11 mmol/l after glucose ingestion. Despite higher glucose concentrations, peak insulin concentrations did not differ from those observed in the nondiabetic subjects. Furthermore, they were not achieved until 2 h after glucose ingestion. People with so-called severe type 2 diabetes have still higher fasting glucose concentrations, in this instance ∼10 mmol/l, and experience marked and prolonged hyperglycemia after eating. Once again, insulin secretion was delayed and peak insulin concentrations did not occur until 2 h after glucose ingestion. In addition, insulin concentrations during the 2 h after eating were lower than those observed in either people with mild diabetes or in people who did not have diabetes. Glucagon concentrations tended to be higher in people with either mild or severe diabetes before glucose ingestion and did not suppress and, if anything, paradoxically increased after glucose ingestion.
The data from this study (1), as well as those from studies conducted by many other investigators (2–8), established that insulin and glucagon secretion are abnormal in people with type 2 diabetes following carbohydrate ingestion. Insulin secretion is decreased and delayed, and glucagon does not suppress. The question then arises as to whether these abnormalities, either alone or in combination with insulin resistance, cause hyperglycemia. If so, to what extent do alterations in hepatic glucose metabolism contribute to fasting and postprandial hyperglycemia?
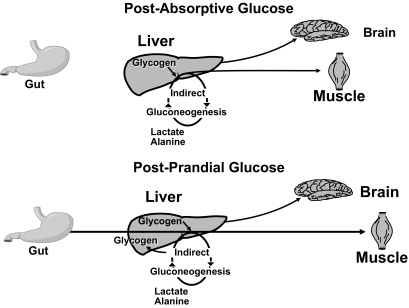
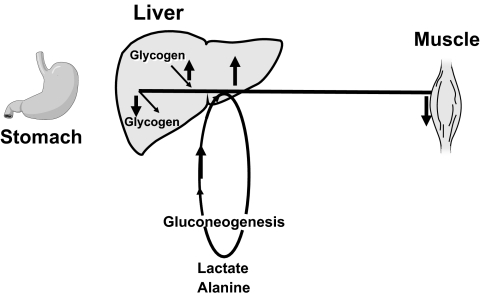
Figure 2 shows a simplified portrayal of glucose metabolism in the postabsorptive state (9). Following an overnight fast, glucose is released by the liver into the blood stream and is taken up by insulin-sensitive tissues such as muscle and tissues with limited response to insulin such as the brain. Glucose released from the liver is derived from the breakdown of glycogen and the synthesis of new glucose via the gluconeogenic pathway. The difference between the rate of glucose entering and the rate of glucose leaving the blood stream determines whether glucose rises, falls, or remains the same. Things become more complicated after eating. Glucose derived from a meal enters the portal vein after absorption from the gut. It then can pass through the liver and be released into systemic circulation, incorporated into glycogen via the direct pathway, or first degraded to three carbon precursors, such as lactate and alanine, then resynthesized via the indirect gluconeogenic pathway back to glucose-6-phospate, which in turn can then be either incorporated into glycogen or dephosphorylated and released into the systemic circulation as glucose.
FIG. 2.
Simplified portrayal of glucose metabolism in the postabsorptive (upper panel) and postprandial (lower panel) states (9).
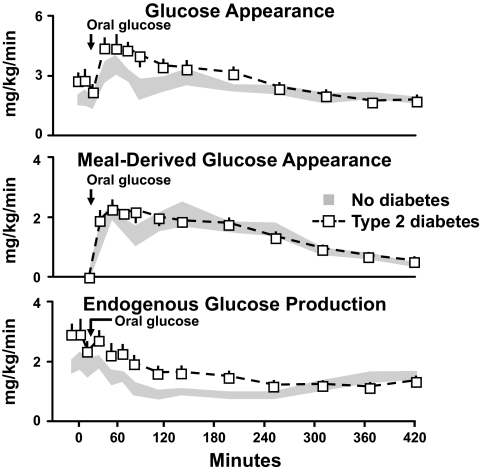
In order to determine why glucose concentrations are too high in people with type 2 diabetes both before and after eating, Dr. Dick Firth used a multiple tracer approach to simultaneously measure the total rate of glucose appearance as well as its components, which are the rate of appearance of the ingested glucose and the rate of endogenous glucose production (10). The upper panel in Fig. 3 shows the total rate of glucose appearance. As is evident, the rate of glucose appearance was higher in the people with type 2 diabetes before and after glucose ingestion. Thus, too much glucose entered the systemic circulation both before and after eating. The systemic rate of appearance of the ingested glucose, measured by tracing the rate of appearance of a tracer contained in the meal, is shown in the middle panel of Fig. 3. Somewhat to our surprise, the rate of appearance of the ingested glucose did not differ between groups. Therefore, the excessive rise in postprandial glucose concentrations was not due to too much glucose entering from the gut. However, since the glucose concentrations were markedly elevated in the people with diabetes and since hyperglycemia stimulates hepatic glucose uptake (11–14), these data should not be interpreted as indicating postprandial hepatic glucose uptake is normal in type 2 diabetes.
FIG. 3.
Rates of total body glucose appearance (upper panel), meal-derived glucose appearance (middle panel), and endogenous glucose production (lower panel) observed in people with or without type 2 diabetes. Fifty grams of glucose was ingested at time zero (10).
What then was the cause of the excessive rate of glucose appearance? The rates of endogenous glucose production are shown in the lower panel of Fig. 3. Endogenous glucose production was higher in the diabetic subjects than in the nondiabetic subjects before eating. In addition, whereas endogenous glucose production rapidly decreased in the nondiabetic subjects after glucose ingestion, suppression of endogenous glucose production was slower in the people with diabetes and required approximately 6 h for a nadir to be reached. The excess amount of glucose released due to slower suppression of endogenous glucose production entirely accounted for the higher postprandial rates of glucose appearance (10).
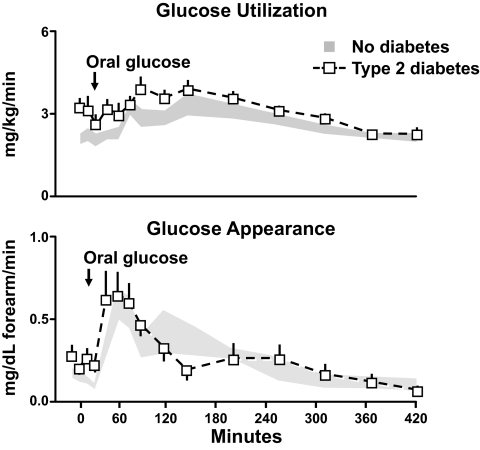
What about glucose disappearance? Was hyperglycemia due to lower rates in the diabetic subjects? As is evident from this side, the answer is no. As is evident from the upper panel of Fig. 4, if anything the rates of glucose disappearance were higher in the diabetic subjects than in the nondiabetic subjects both before and after glucose ingestion. Urinary glucose loss and muscle glucose uptake both contribute to glucose disappearance. Urinary glucose loss can be substantial when glucose concentrations exceed the renal glucose threshold as commonly happens after eating. To gain insight regarding muscle glucose uptake, Dr. Firth used the forearm catheterization method to measure forearm glucose uptake. As is evident from the lower panel of Fig. 4 in contrast to the higher rates of total body glucose disappearance, forearm glucose uptake did not differ in the diabetic and nondiabetic subjects (10). Taken together, these data indicate that people with type 2 diabetes have excessive rates of endogenous glucose production both before and after eating contributing to both fasting and postprandial hyperglycemia. In addition, while meal appearance and muscle glucose uptake did not differ in the diabetic and nondiabetic subjects, they were not appropriate for the prevailing glucose concentration.
FIG. 4.
Rates of total body glucose disappearance and forearm glucose uptake observed in people with or without type 2 diabetes. Fifty grams of glucose was ingested at time zero (10).
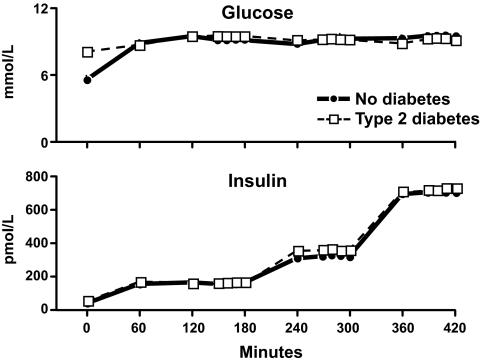
The obvious next question was why was endogenous glucose production increased in people with type 2 diabetes? Is this due to hepatic insulin resistance? As shown in Fig. 5, Dr. Rita Basu addressed this question by clamping glucose at ∼8 mmol/l, a concentration commonly observed after eating, which is sufficiently high to stimulate hepatic glucose uptake but not so high as to fully suppress endogenous glucose production (15). Glucose concentrations were maintained at this level by an infusion of glucose in people with type 2 diabetes or in people who did not have diabetes. Insulin was infused at rates that resulted in insulin concentrations that spanned the physiologic range. Somatostatin also was infused in order to inhibit hormone secretion along with replacement amounts of glucagon to ensure that portal insulin and glucagon concentrations were constant and equal throughout the experiment in the diabetic and nondiabetic subjects.
FIG. 5.
Plasma glucose (upper panel) and insulin (lower panel) concentrations observed in people with or without type 2 diabetes. Infusions of glucose, insulin, somatostatin, glucagon, and growth hormone started at time zero (15).
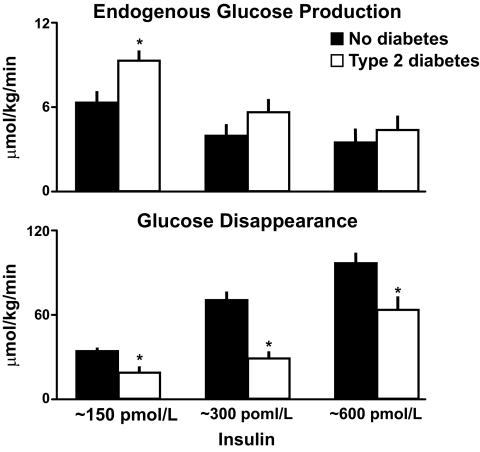
Rates of endogenous glucose production present during the final 30 min of each insulin infusion are shown in the upper panel of Fig. 6. Endogenous glucose production was suppressed in the people who did not have diabetes when insulin was increased from 150 to 300 pmol with no further suppression being observed when insulin was subsequently increased to 600 pmol. In contrast, despite matched glucose and insulin concentrations, endogenous glucose production was higher in the diabetic subjects than in the nondiabetic subjects at insulin concentrations of 150 pmol/l indicating hepatic insulin resistance. Endogenous glucose production progressively decreased when insulin concentrations were increased to 300 and then to 600 pmol/l. Of note, while the rates of endogenous glucose production were slightly higher in the people with diabetes, the differences were no longer significant at insulin concentrations above 150 pmol/l. Thus, people with type 2 diabetes have hepatic insulin resistance. These data also explain why hepatic insulin resistance will be missed when insulin action only is assessed at high insulin concentrations. A different pattern is observed for glucose disappearance. As shown in the lower panel of Fig. 6, glucose disappearance progressively increased in both groups when insulin concentrations were increased from 150 to 300 to 600 pmol/l. However, glucose disappearance was lower in the people with diabetes at all three insulin concentrations.
FIG. 6.
Rates of endogenous glucose production (upper panel) and total body glucose disappearance (lower panel) observed during the final 30 min of a hyperinsulinemic clamp when glucose concentrations were clamped at ∼8 mmol/l in people with or without type 2 diabetes (15). *P < 0.05 vs. no diabetes.
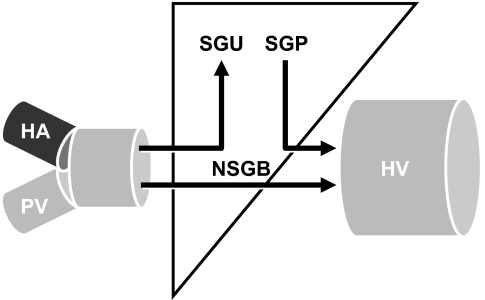
Why was insulin-induced stimulation of glucose disappearance lower in the people with diabetes? Is insulin-induced stimulation of hepatic glucose uptake, muscle glucose uptake, or a combination of both decreased in people with type 2 diabetes? In order to address this question, Dr. Ananda Basu used the splanchnic catheterization method, whose development was pioneered by Dr. John Wahren (16). With this method, blood samples are simultaneously obtained from an artery and from the hepatic vein. Splanchnic blood flow is measured by infusing indocyanine green. Since both flow and glucose concentration are known, net splanchnic glucose balance (NSGB) (Fig. 7) can be calculated by subtracting the amount of glucose leaving the splanchnic bed from the amount of glucose entering. Splanchnic glucose uptake (SGU) also can be calculated if a glucose tracer is infused. For example, if 10 labeled molecules of glucose enter the splanchnic bed and 9 leave, then SGU must have been one. Since the ratio of labeled to unlabeled glucose in the artery is known, total SGU can be determined. Splanchnic glucose production (SGP) then is calculated as the algebraic sum of NSGB and SGU.
FIG. 7.
A schematic portrayal of the splanchnic catheterization method. HA, hepatic artery; HV, hepatic vein; PV, hepatic vein (16).
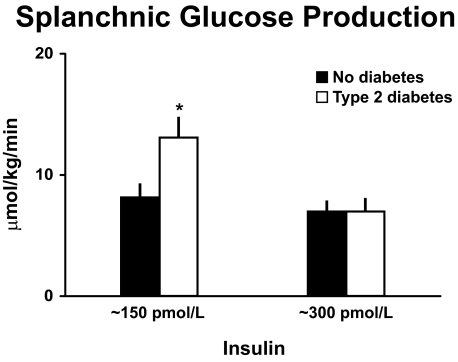
Figure 8 shows SGP measured in the presence of comparably elevated glucose and insulin concentrations in the diabetic and nondiabetic subjects. The pattern is virtually identical to that observed with endogenous glucose production in that the rate of SGP was increased in the people with type 2 diabetes at low insulin concentrations but no longer differed from people who did not have diabetes at higher insulin concentrations (16). These data indicate that insulin-induced suppression of SGP is impaired in people with type 2 diabetes. Since the liver is presumed to be the primary source of SGP, these data strongly imply that people with type 2 diabetes have increased rates of hepatic glucose release.
FIG. 8.
SGP. Rates of SGP observed during the final 30 min of a hyperinsulinemic clamp when glucose concentrations were clamped at ∼8 mmol/l in people with or without type 2 diabetes (16). *P < 0.05 vs. no diabetes.
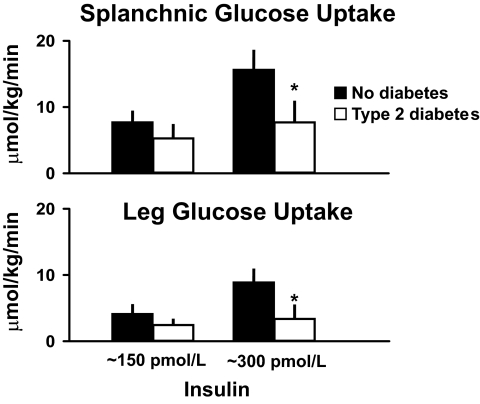
Is insulin-stimulated hepatic glucose uptake also impaired in people with type 2 diabetes? Fig. 9 shows that it is. SGU, shown in the upper panel, was slightly lower in the people with type 2 diabetes at low insulin concentrations and significantly lower at higher insulin concentrations. The same pattern was observed for leg glucose uptake, shown in the lower panel, with leg glucose uptake being slightly lower at low insulin concentrations and significantly lower at higher insulin concentrations. When total muscle mass was estimated using measurements obtained with dual-energy X-ray absorptiometry scanning under these experimental conditions, muscle accounted for approximately two-thirds and liver accounted for one-third of the decrease in total body glucose disappearance that was observed in the people with type 2 diabetes (16).
FIG. 9.
Rates of SGU (upper panel) and leg glucose uptake (lower panel) observed during the final 30 min of a hyperinsulinemic clamp when glucose concentrations were clamped at ∼8 mmol/l in people with or without type 2 diabetes (16). *P < 0.05 vs. no diabetes.
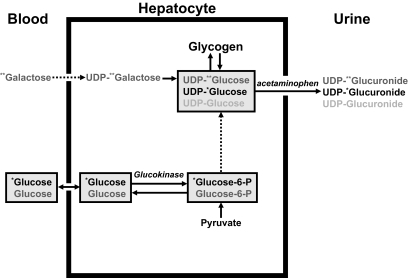
Why is SGU decreased in people with type 2 diabetes? To gain insight into this question, Dr. Andy Basu used a method pioneered by the late Dr. Bernie Landau and colleagues (17) where acetaminophen is used to sample the hepatic uridine-5-diphosphate (UDP)-glucose pool during the infusion of labeled galactose. A schematic of this method is shown in Fig. 10. When infused in trace amounts, galactose is converted to UDP-galactose within hepatocyte, which in turn equilibrates with the UDP-glucose pool. Enrichment of UDP-glucose can be determined by giving acetaminophen and measuring the enrichment of UDP-glucuronide in the urine. Since UDP-glucose is the obligate precursor of glycogen, flux through the UDP-glucose pool into glycogen can be calculated. The enrichment of the intravenously infused glucose tracer also can be measured in the same manner allowing estimation of the proportion of the hepatic UDP-glucose pool that is derived from the uptake of extracellular glucose.
FIG. 10.
Schematic portrayal of glucose metabolism in the blood, liver, and urine when labeled glucose and galactose are infused intravenously and acetaminophen is given by mouth in order to sample UDP-glucuronide in the urine (16). * and ** represent respectively labeled glucose and galactose.
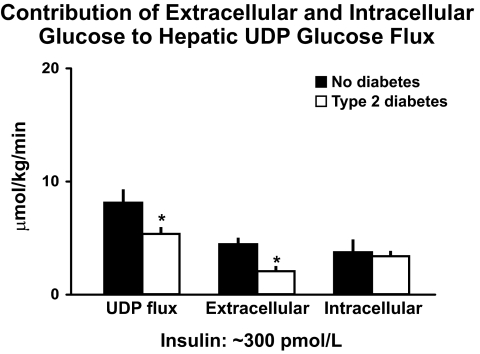
As is evident in Fig. 11, UDP flux was lower in the people with diabetes implying decreased rates of hepatic glycogen synthesis. Of particular interest, the decrease in UDP glucose flux was entirely accounted for by a decrease in the rate of uptake of extracellular glucose with no difference in the proportion derived from intracellular sources. Since phosphorylation of glucose by glucokinase is the rate limiting step in the uptake of extracellular glucose by the liver (18,19), these data strongly suggest a defect in hepatic glucokinase activity. This conclusion is supported by studies that have shown decreased activity of this enzyme in people with type 2 diabetes (20) and by the observation that hepatic glucose uptake is reduced in people with genetic defects in glucokinase activation (21). Thus, people with type 2 diabetes have excessive rates of hepatic glucose release, impaired hepatic glucose uptake, decreased hepatic glycogen synthesis, and decreased uptake of extracellular glucose.
FIG. 11.
The contribution of extracellular and intracellular glucose to the hepatic UDP-glucose pool when glucose is clamped at ∼145 mg/dl and insulin at ∼300 pmol/l in people with or without type 2 diabetes (16). *P < 0.05 vs. no diabetes.
These data established that endogenous glucose production is increased in people with type 2 diabetes. However these studies (1,10,16) and many other studies in the literature at that time (22–27) were performed in people whose fasting glucose concentrations generally were substantially above the nondiabetic range (e.g., 10–13 mmol/l). The question then arose as to whether endogenous glucose production is increased in people with mild as well as severe type 2 diabetes. If so, it would suggest that increased rates of endogenous glucose production contribute to rather than are caused by the abnormal metabolic milieu that accompanies elevated glucose concentrations.
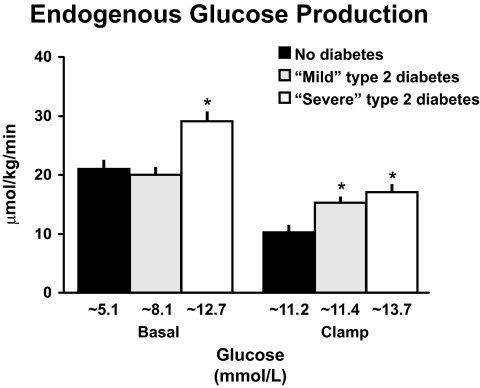
To address this question, Dr. Rita Basu (28) performed hyperglycemic, hyperinsulinemic clamps in people with so-called severe diabetes, whose fasting glucose concentrations averaged ∼12 mmol/l; in people with mild diabetes, whose fasting glucose concentrations averaged ∼8 mmol/l; and in people who did not have diabetes, whose fasting glucose concentrations averaged ∼5 mmol/l (Fig. 12 upper panel). On each occasion, Dr. Rita Basu began an infusion of somatostatin, insulin, and glucagon at time zero to be sure that portal concentrations of insulin and glucagon were constant and equal in all groups during the clamp. She also infused sufficient glucose to raise glucose concentrations to ∼11 mmol/l in the subjects with either mild diabetes or no diabetes. Glucose concentrations in the people with severe diabetes remained elevated at concentrations that averaged ∼12 mmol/l. Fasting insulin concentrations in the people with mild diabetes were higher than those present in the people who did not have diabetes implying higher hepatic insulin concentrations (Fig. 12 lower panel). In contrast, insulin concentrations were comparable in all groups during the clamp. The somatostatin infusion and glucagon infusions resulted in near complete suppression of C-peptide and constant but equal plasma glucagon concentrations in both groups (data not shown).
FIG. 12.
Plasma glucose and insulin concentrations observed in people without diabetes, people with mild type 2 diabetes and people with severe type 2 diabetes. Infusions of glucose, insulin, somatostatin, glucagon, and growth hormone started at time zero (28).
The left side of Fig. 13 shows the rates of endogenous glucose production that were present before the clamp. Consistent with previous studies (22–26) and those discussed above (1,10,16,27), endogenous glucose production was increased in people with severe diabetes but did not differ in people with mild diabetes or no diabetes. However, since people with mild diabetes had higher glucose and insulin concentrations, this implied abnormal regulation of endogenous glucose production. This became evident during the clamp. As shown on the right side of Fig. 13, when glucose and insulin concentrations were matched, endogenous glucose production was higher in both the mild and severe diabetic groups. Dr. Gerlies Bock (29) recently used a similar experimental design to establish that insulin-induced suppression of endogenous glucose production also is impaired in people with pre-diabetes (data not shown). Thus, abnormal regulation of hepatic glucose metabolism occurs early in the evolution of type 2 diabetes, strongly suggesting it is involved in the pathogenesis of the disease.
FIG. 13.
Rates of endogenous glucose production observed in people who did not have diabetes, had mild diabetes, or had severe diabetes either before (basal) or during a hyperglycemic clamp (28). *P < 0.05 vs. no diabetes.
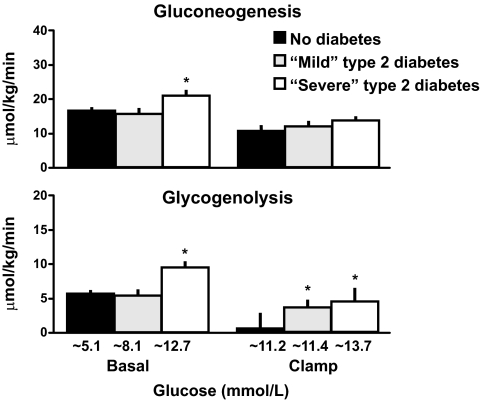
Increased rates of endogenous glucose production can be caused by increased rates of gluconeogenesis, increased rates of glycogenolysis, or a combination of both. Figure 14 (upper panel) shows the rates of gluconeogenesis measured using the deuterated water method that was pioneered by Dr. Bernie Landau (30). This method is based on the fact that following the ingestion of deuterated water, the fifth carbon of glucose is labeled with deuterium during gluconeogenesis, whereas the second carbon of glucose is labeled with deuterium during both the gluconeogenesis and glycogenolysis (30). While the assumptions of this method continue to be evaluated (31,32), it is widely accepted as providing an index of gluconeogenesis. Consistent with previous studies (23,25), the data on the left side of Fig. 14 indicate that gluconeogenesis was increased in the people with severe diabetes before the clamp. However, as shown on the right side of Fig. 14, perhaps due to the fact that glucagon was lowered to comparable levels by the somatostatin infusion, the rates of gluconeogenesis that were present during the clamp no longer differed among the groups.
FIG. 14.
Rates of gluconeogenesis (upper panel) and glycogenolysis (lower panel) observed in people who did not have diabetes, had mild diabetes, or had severe diabetes either before (basal) or during a hyperglycemic clamp (28). *P < 0.05 vs. no diabetes.
Rates of glycogenolysis can be estimated by subtracting the rate of gluconeogenesis from the rate of endogenous glucose production. As shown in the lower panel of Fig. 14, the rate of glycogenolysis was increased in the people with severe diabetes before the clamp but did not differ in people with mild diabetes or no diabetes. However, hyperglycemia is known to be a potent suppressor of glycogenolysis (33). This is evident from the data shown on the right side of Fig. 14 since rates of glycogenolysis in the people who did not have diabetes were almost completely suppressed when their glucose was raised to 200 mg/dl. In contrast, minimal suppression occurred in the people with either mild or severe diabetes, indicating that the rates of glycogenolysis were not inappropriate for the prevailing glucose and insulin concentrations.
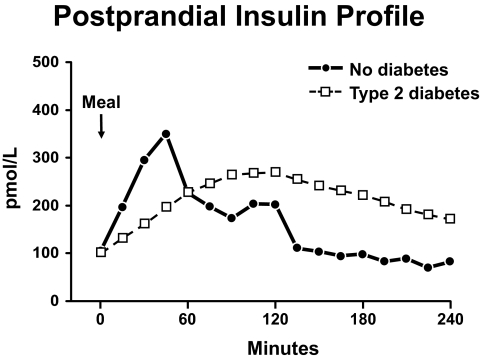
What about insulin secretion? Do alterations in insulin secretion and insulin action have the same effect on postprandial glucose concentrations? Dr. Ananda Basu attempted to answer this question by determining the metabolic effects of diabetic and nondiabetic insulin profiles in insulin-sensitive and insulin-resistant people (34). Figure 15 shows the insulin concentrations that were observed after Dr. Andy Basu fed a carbohydrate containing meal to people who had either severe type 2 diabetes or no diabetes. As anticipated, the meal resulted in a prompt increase in insulin concentrations in people who did not have diabetes with levels peaking at ∼45 min. In contrast, there was a delay in insulin release in the people with type 2 diabetes with concentrations not reaching a peak until approximately 2 h after eating.
FIG. 15.
Postprandial insulin profile. Plasma insulin concentrations observed in people with and without type 2 diabetes following ingestion of a meal at time zero (34).
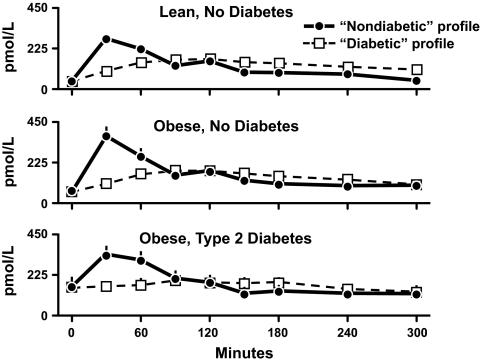
Dr. Andy Basu then studied separate groups of lean nondiabetic subjects, obese nondiabetic subjects, and obese subjects with type 2 diabetes on two occasions. On each occasion he infused somatostatin to inhibit endogenous insulin secretion and used a computer to infuse the identical amount of insulin as either a nondiabetic or diabetic profile (34). Figure 16 shows the resultant insulin concentrations. The insulin concentrations peaked at 30 min during the nondiabetic profile and at 120 min during the diabetic profile. Note that this resulted in substantially lower insulin concentrations during the first hour of the diabetic insulin profile in all three groups. Dr. Basu also infused glucose in a pattern and an amount that mimicked the meal appearance rate that normally occurs following ingestion of a meal containing 50 g of glucose. Thus, both groups received identical amounts of glucose and insulin on all occasions; only the pattern of the insulin infusion differed. Figure 17 shows the resultant glucose concentrations. The diabetic insulin profile caused a higher peak glucose concentration than the nondiabetic profile did in all three groups. However, this difference was transient with glucose concentrations falling within three hours to values that no longer differed from those observed during the nondiabetic insulin profile. These data indicate that a delay in the rate of the rise of insulin resulted in a higher peak glucose concentration but only minimally prolonged the duration of hyperglycemia.
FIG. 16.
Plasma insulin concentrations observed in lean nondiabetic subjects (upper panel), obese nondiabetic subjects (middle panel), and obese people with type 2 diabetes (lower panel) in the presence of either a nondiabetic or diabetic insulin profile. A prandial glucose infusion and infusions of somatostatin, insulin, glucagon, and growth hormone were started at time zero (34).
FIG. 17.
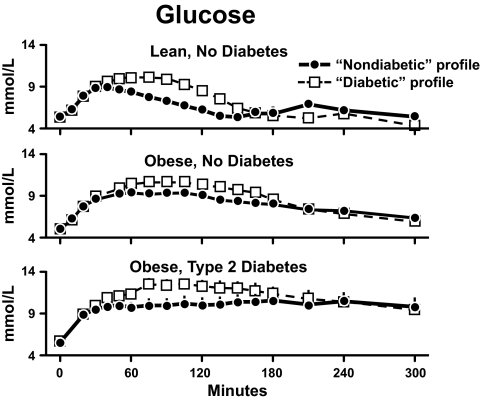
Glucose concentrations observed in lean nondiabetic subjects (upper panel), obese nondiabetic subjects (middle panel), and obese people with type 2 diabetes (lower panel) in the presence of either a nondiabetic or diabetic insulin profile. A prandial glucose infusion and infusions of somatostatin, insulin, glucagon, and growth hormone were started at time zero (34).
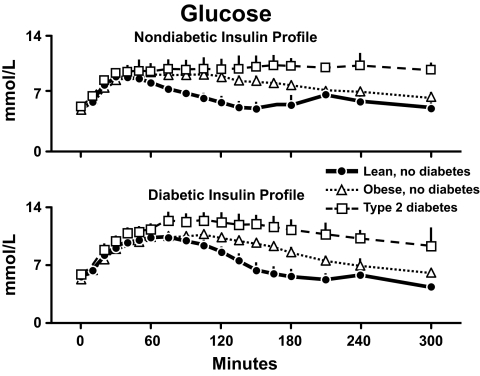
In order to evaluate the effects of the differences in insulin action, the results observed during the nondiabetic profile are shown in the upper panel of Fig. 18, and those observed during the diabetic profile are shown in the lower panel. Peak glucose concentrations did not differ in the lean and obese subjects regardless of whether insulin was infused as a nondiabetic profile or a diabetic profile. However, the duration of hyperglycemia was more prolonged in the obese nondiabetic subjects then in the lean nondiabetic subjects. The duration of hyperglycemia was even more prolonged in the people with type 2 diabetes during both the nondiabetic and the diabetic insulin profiles. Thus, insulin resistance prolonged the duration of hyperglycemia but had a minimal effect on the peak glucose concentration. Note that the glucose concentrations were substantially higher in the obese diabetic subjects than in the obese nondiabetic subjects.
FIG. 18.
Glucose concentrations observed in lean nondiabetic subjects, obese nondiabetic subjects, and obese people with type 2 diabetes in the presence of either a nondiabetic insulin profile (upper panel) or diabetic insulin profile (lower panel). A prandial glucose infusion and infusions of somatostatin, glucagon, and growth hormone also were started at time zero (34).
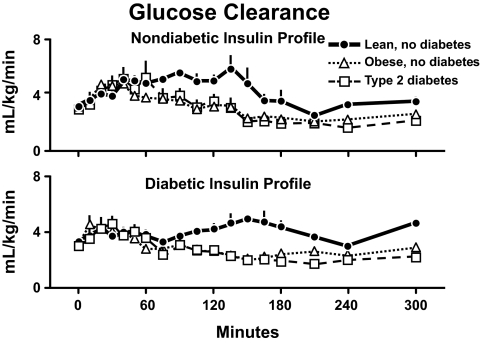
Why did this happen? Fig. 19 shows the rates of glucose clearance in the lean and obese individuals who did not have diabetes (34). Despite identical insulin concentrations and higher glucose concentrations, the rates of glucose clearance were lower in the obese individuals documenting the presence of insulin resistance. Glucose clearance also was lower in the obese subjects with type 2 diabetes. However, the rates did not differ from those observed in the obese subjects who did not have diabetes whether measured during the nondiabetic or diabetic insulin profiles. Therefore, the higher glucose concentrations in the obese diabetic were not due to lower rates of glucose clearance.
FIG. 19.
Rates of glucose clearance observed in lean nondiabetic subjects, obese nondiabetic subjects, and obese people with type 2 diabetes in the presence of either a nondiabetic insulin profile (upper panel) or diabetic insulin profile (lower panel). A prandial glucose infusion and infusions of somatostatin, glucagon, and growth hormone also were started at time zero (34).
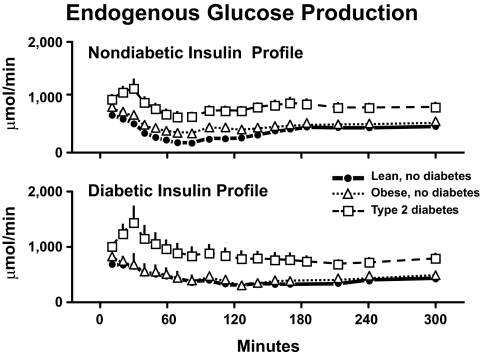
Figure 20 shows the rates of endogenous glucose production. Endogenous glucose production was comparably suppressed in the lean and obese nondiabetic subjects. On the other hand, suppression of endogenous glucose production was markedly impaired in the subjects with type 2 diabetes whether measured during the nondiabetic or the diabetic insulin profiles. Thus, a delay in insulin secretion results in higher peak glucose concentrations, and a decrease in insulin action results in prolonged hyperglycemia, which is particularly severe when decreased glucose clearance is accompanied by excessive rates of endogenous glucose production.
FIG. 20.
Rates of endogenous glucose production observed in lean nondiabetic subjects, obese nondiabetic subjects, and obese people with type 2 diabetes in the presence of either a nondiabetic insulin profile (upper panel) or diabetic insulin profile (lower panel). A prandial glucose infusion and infusions of somatostatin, insulin, glucagon, and growth hormone also were started at time zero (34).
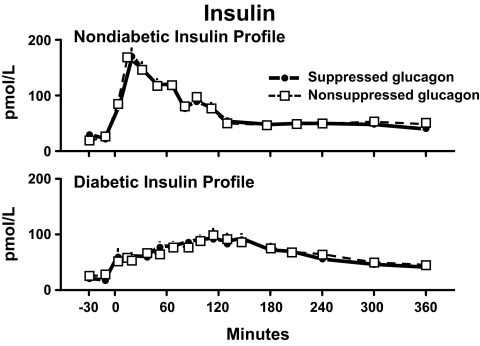
What about glucagon? Does the lack of suppression of glucagon contribute to postprandial hyperglycemia? As illustrated in the bottom panel of Fig. 1, glucose ingestion suppresses glucagon concentrations in people who do not have diabetes. In contrast, glucagon concentrations either do not decrease or paradoxically increase in people with type 2 diabetes (1,3,8,35). Can the 40–50 pg/ml difference in glucagon concentration that arises from the lack of suppression of glucagon cause postprandial hyperglycemia? In order to address this question, Dr. Pankaj Shah studied a group of healthy nondiabetic subjects on two occasions (36). He infused insulin as a nondiabetic insulin profile on both occasions. On one occasion, glucagon was suppressed; on the other occasion, it was not. Dr. Shah used the same experimental design in a separate set of subjects except he infused a diabetic insulin profile on both occasions. Figure 21 shows that the resultant insulin concentrations were virtually identical on the suppressed and the nonsuppressed study days. However, as expected, the nondiabetic profile resulted in insulin concentrations that peaked at 30 min (upper panel), whereas the diabetic profile resulted in insulin concentrations that peaked at 120 min (lower panel).
FIG. 21.
Plasma insulin concentrations observed during nondiabetic (upper panel) or diabetic (lower panel) insulin profiles when glucagon concentrations were either maintained constant by glucagon infusion started at time zero (nonsuppressed study day) or permitted to decrease by delaying the glucagon infusion until 120 min. A prandial glucose infusion and infusions of somatostatin and growth hormone also were started at time zero (36).
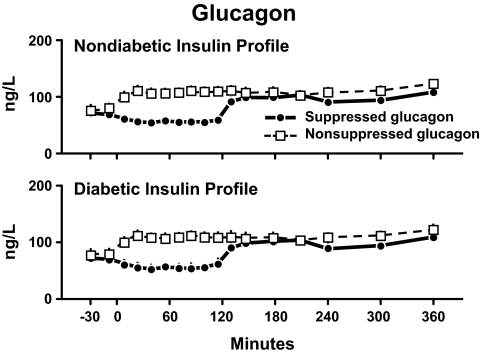
Figure 22 shows the glucagon concentrations that were achieved during the experiments. To create these patterns, Dr. Shah infused somatostatin from 0 to 360 min on both occasions in order to inhibit glucagon release. On one occasion, he infused glucagon at a rate of 0.65 ng/kg/min throughout the experiment, which resulted in a slight increase in peripheral glucagon concentrations. Since endogenous secretion decreased, this meant that portal glucagon concentrations either did not change or minimally increased during the experiment. In contrast, on the other occasion he delayed starting the glucagon infusion until 120 min. This resulted in an approximate 50 pg/ml difference in glucagon on the 2 study days that was only present during the first 2 h of the experiment (36).
FIG. 22.
Plasma glucagon concentrations observed during nondiabetic (upper panel) or diabetic (lower panel) insulin profiles when glucagon concentrations were either maintained constant by glucagon infusion started at time zero (nonsuppressed study day) or permitted to decrease by delaying the glucagon infusion until 120 min. A prandial glucose infusion and infusions of somatostatin and growth hormone also were started at time zero (36).
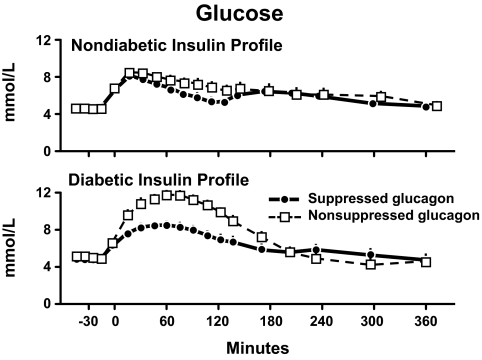
The upper panel of Fig. 23 shows the glucose concentrations that resulted when 35 g of glucose also was infused in the same prandial profile as was used in the experiments by Basu et al. (34). Of note, in the presence of the nondiabetic profile, the peak glucose concentrations were the same whether or not glucagon was suppressed. While the glucose area above basal was significantly higher on the suppressed than the nonsuppressed study days, the difference was small.
FIG. 23.
Plasma glucose concentrations observed during nondiabetic (upper panel) or diabetic (lower panel) insulin profiles when glucagon concentrations were either maintained constant by glucagon infusion started at time zero (nonsuppressed study day) or permitted to decrease by delaying the glucagon infusion until 120 min. A prandial glucose infusion and infusions of somatostatin and growth hormone also were started at time zero (36).
An entirely different outcome was observed in the presence of the diabetic insulin profile. As shown in the lower panel of Fig. 23, the lack of suppression of glucagon resulted in peak glucose concentrations that were ∼50–60 mg/dl higher than were observed when glucagon was suppressed. Also of interest, despite the delayed rise in insulin that occurred during the diabetic profile, glucose concentrations on the suppressed glucagon study day peaked at only 145 mg/dl. These data suggest that an agent that suppresses glucagon in the presence of a rapid increase in insulin concentration will have a minimal effect on postprandial glucose concentrations. On the other hand, if insulin secretion is delayed, as typically occurs in people with diabetes, then an agent that suppresses glucagon likely will substantially lower postprandial glucose concentrations.
As indicated in Fig. 24, many factors contribute to fasting and postprandial hyperglycemia. People with type 2 diabetes have excessive rates of endogenous glucose production that fail to appropriately suppress after eating (10,16,28). Rates of gluconeogenesis and perhaps glycogenolysis are increased early in the evolution of diabetes (28,37). Insulin-induced stimulation of hepatic glucose uptake is impaired in people with type 2 diabetes. This leads to lower rates of hepatic glycogen synthesis primarily due to reduced uptake of extracellular glucose presumably because of inadequate activation of hepatic glucokinase (16,38). Delayed insulin secretion results in higher peak glucose concentrations particularly when suppression of glucagon is impaired, whereas insulin resistance prolongs the duration of hyperglycemia, which can be marked when both hepatic and extra-hepatic insulin resistance are present (36).
FIG. 24.
Schematic summary of alterations in glucose metabolism observed in people with type 2 diabetes. An arrow pointing up indicates an increase and an arrow pointing down indicates a decrease in flux.
The premise of all of these studies, as well as others that my colleagues and I have performed, is that an understanding of the pathogenesis of type 2 diabetes enables the development of targeted therapies that are directed toward correcting specific metabolic defects in a given individual. I, as well as many other investigators, believe that such therapies are likely to be more effective and have a lower risk than would occur if everyone were treated the same regardless of the underlying cause of their hyperglycemia. While we do not yet have sufficient knowledge to truly individualize therapy, in my opinion this approach will be the norm in the not too distant future.
ACKNOWLEDGMENTS
R.A.R.'s research has been supported by the National Institute of Diabetes and Digestive and Kidney Diseases (DK-29953), the American Diabetes Association, the Mayo Clinic and its Center for Translational Science Activities (one UL1 RR024150), as well as the Earl and Annette R. McDonough Professorship of Medicine, Mayo Clinic College of Medicine.
No potential conflicts of interest relevant to this article were reported.
The Banting Lecture was presented at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
I would like to thank my mentors, Jack Gerich and Morey Haymond, for teaching me the art of clinical research. I would like to thank my long-time collaborators, Dr. Claudio Cobelli and Gianna Toffolo, as well as my many superb postdoctoral fellows. In particular, I would like to thank the individuals whose work has served as the basis of this paper, including Drs. Richard Firth, Peter Butler, Ananda Basu, Rita Basu, Adrian Vella, and Pankaj Shah. I am deeply grateful to my colleagues on the Mayo Endocrine Research Unit, including Drs. Cheryl Conover, Norman Eberhardt, Mike Jensen, Yogish Kudva, Jim Levine, John Miles, and Sree Nair for their friendship, creativity, and constructive criticism.
REFERENCES
- 1.Butler PC, Rizza RA. Contribution to postprandial hyperglycemia and effect on initial splanchnic glucose clearance of hepatic glucose cycling in glucose-intolerant or NIDDM patients. Diabetes 1991;40:73–81 [PubMed] [Google Scholar]
- 2.Reaven GM, Chen Y-DI, Coulston AM, Greenfield MS, Hollenbeck C, Lardinois C, Liu G, Schwartz H. Insulin secretion and action in noninsulin-dependent diabetes mellitus. Am J Med 1983;30:85–93 [DOI] [PubMed] [Google Scholar]
- 3.Müller WA, Faloona GR, Aguilar-Parada E, Unger RH. Abnormal alpha-cell function in diabetes: response to carbohydrate and protein ingestion. N Engl J Med 1970;283:109–115 [DOI] [PubMed] [Google Scholar]
- 4.Gerich JE, Lorenzi M, Karam JH, Schneider V, Forsham PH. Abnormal pancreatic glucagon secretion and postprandial hyperglycemia in diabetes mellitus. JAMA 1975;234:159–165 [PubMed] [Google Scholar]
- 5.Perley M, Kipnis DM. Plasma insulin responses to glucose and tolbutamide of normal weight and obese diabetic and nondiabetic subjects. Diabetes 1966;15:867–874 [DOI] [PubMed] [Google Scholar]
- 6.Porte D., Jr Banting lecture 1990: β-cells in type II diabetes mellitus. Diabetes 1991;40:166–180 [DOI] [PubMed] [Google Scholar]
- 7.Polonsky K, Frank B, Pugh W, Addis A, Karrison T, Meier P, Tager H, Rubenstein A. The limitations to and valid use of C-peptide as a marker of the secretion of insulin. Diabetes 1986;35:379–386 [DOI] [PubMed] [Google Scholar]
- 8.Knop FK, Vilsbøll T, Madsbad S, Holst JJ, Krarup T. Inappropriate suppression of glucagon during OGTT but not during isoglycaemic i.v. glucose infusion contributes to the reduced incretin effect in type 2 diabetes mellitus. Diabetologia 2007;50:797–805 [DOI] [PubMed] [Google Scholar]
- 9.Dinneen S, Gerich J, Rizza R. Carbohydrate metabolism in non-insulin-dependent diabetes mellitus. N Engl J Med 1992;327:707–713 [DOI] [PubMed] [Google Scholar]
- 10.Firth RG, Bell PM, Marsh HM, Hansen I, Rizza RA. Postprandial hyperglycemia in patients with noninsulin-dependent diabetes mellitus: role of hepatic and extrahepatic tissues. J Clin Invest 1986;77:1525–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergman RN, Bucolo RJ. Interaction of insulin and glucose in the control of hepatic glucose balance. Am J Physiol 1974;227:1314–1322 [DOI] [PubMed] [Google Scholar]
- 12.Cherrington AD, Williams PE, Harris MS. Relationship between the plasma glucose level and glucose uptake in the conscious dog. Metabolism 1978;27:787–791 [DOI] [PubMed] [Google Scholar]
- 13.Myers SR, Diamond MP, Adkins-Marshall BA, Williams PE, Stinsen R, Cherrington AD. Effects of small changes in glucagon on glucose production during a euglycemic, hyperinsulinemic clamp. Metabolism 1991;40:66–71 [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Ferrannini E, Hendler R, Felig P, Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes 1983;32:35–45 [DOI] [PubMed] [Google Scholar]
- 15.Basu R, Basu A, Johnson CM, Schwenk WF, Rizza RA. Insulin dose-response curves for stimulation of splanchnic glucose uptake and suppression of endogenous glucose production differ in nondiabetic humans and are abnormal in people with type 2 diabetes. Diabetes 2004;53:2042–2050 [DOI] [PubMed] [Google Scholar]
- 16.Basu A, Basu R, Shah P, Vella A, Johnson CM, Nair KS, Jensen MD, Schwenk WF, Rizza RA. Effects of type 2 diabetes on the ability of insulin and glucose to regulate splanchnic and muscle glucose metabolism: evidence for a defect in hepatic glucokinase activity. Diabetes 2000;49:272–283 [DOI] [PubMed] [Google Scholar]
- 17.Wajngot A, Chandramouli V, Schumann WC, Efendic S, Landau BR. Quantitation of glycogen/glucose-1-P cycling in liver. Metabolism 1991;40:877–881 [DOI] [PubMed] [Google Scholar]
- 18.Pilkis SJ, Weber IT, Harrison RW, Bell GI. Glucokinase: structural analysis of a protein involved in susceptibility to diabetes. J BiolChem 1994;269:21925–21928 [PubMed] [Google Scholar]
- 19.Seoane J, Barberà A, Télémaque-Potts S, Newgard CB, Guinovart JJ. Glucokinase overexpression restores glucose utilization and storage in cultured hepatocytes from male Zucker diabetic fatty rats. J Biol Chem 1999;274:31833–31838 [DOI] [PubMed] [Google Scholar]
- 20.Caro JF, Triester S, Patel VK, Tapscott EB, Frazier NL, Dohm GL. Liver glucokinase: decreased activity in patients with type II diabetes. Horm Metab Res 1995;27:19–22 [DOI] [PubMed] [Google Scholar]
- 21.Velho G, Petersen KF, Perseghin G, Hwang JH, Rothman DL, Pueyo ME, Cline GW, Froguel P, Shulman GI. Impaired hepatic glycogen synthesis in glucokinase-deficient (MODY-2) subjects. J Clin Invest 1996;98:1755–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bogardus C, Lillioja S, Howard BV, Reaven G, Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest 1984;74:1238–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boden G, Chen X, Capulong E, Mozzoli M. Effects of free fatty acids on gluconeogenesis and autoregulation of glucose production in type 2 diabetes. Diabetes 2001;50:810–816 [DOI] [PubMed] [Google Scholar]
- 24.Tappy L, Acheson K, Curchod B, Schneiter P, Normand S, Pachiaudi C, Temler E, Riou JP, Jéquier E. Overnight glucose metabolism in obese non-insulin-dependent diabetic patients and in healthy lean individuals. Clin Physiol 1994;14:251–265 [DOI] [PubMed] [Google Scholar]
- 25.Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus: a 13C nuclear magnetic resonance study. J Clin Invest 1992;90:1323–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radziuk J, Pye S. Production and metabolic clearance of glucose under basal conditions in type II (non-insulin-dependent) diabetes mellitus. Diabetologia 2001;44:983–991 [DOI] [PubMed] [Google Scholar]
- 27.Campbell PJ, Mandarino LJ, Gerich JE. Quantification of the relative impairment in actions of insulin on hepatic glucose production and peripheral glucose uptake in non-insulin-dependent diabetes mellitus. Metabolism 1988;37:15–21 [DOI] [PubMed] [Google Scholar]
- 28.Basu R, Schwenk WF, Rizza RA. Both fasting glucose production and disappearance are abnormal in people with “mild” and “severe” type 2 diabetes. Am J Physiol Endocrinol Metab 2004;287:E55–E62 [DOI] [PubMed] [Google Scholar]
- 29.Bock G, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Chandramouli V, Landau BR, Rizza RA. Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose: role of increased rates of gluconeogenesis. Diabetes 2007;56:1703–1711 [DOI] [PubMed] [Google Scholar]
- 30.Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest 1996;98:378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bock G, Schumann WC, Basu R, Burgess SC, Yan Z, Chandramouli V, Rizza RA, Landau BR. Evidence that processes other than gluconeogenesis may influence the ratio of deuterium on the fifth and third carbons of glucose: implications for the use of 2H2O to measure gluconeogenesis in humans. Diabetes 2008;57:50–55 [DOI] [PubMed] [Google Scholar]
- 32.Basu R, Chandramouli V, Schumann W, Basu A, Landau BR, Rizza RA. Additional evidence that transaldolase exchange, isotope discrimination during the triose-isomerase reaction, or both occur in humans: effects of type 2 diabetes. Diabetes 2009;58:1539–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen KF, Laurent D, Rothman DL, Cline GW, Shulman GI. Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans. J Clin Invest 1998;101:1203–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basu A, Alzaid A, Dinneen S, Caumo A, Cobelli C, Rizza RA. Effects of a change in the pattern of insulin delivery on carbohydrate tolerance in diabetic and nondiabetic humans in the presence of differing degrees of insulin resistance. J Clin Invest 1996;97:2351–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerich JE, Tsalikian E, Lorenzi M, Schneider V, Bohannon NV, Gustafson G, Karam JH. Normalization of fasting hyperglucagonemia and excessive glucagon responses to intravenous arginine in human diabetes mellitus by prolonged infusion of insulin. J Clin Endocrinol Metab 1975;41:1178–1180 [DOI] [PubMed] [Google Scholar]
- 36.Shah P, Basu A, Basu R, Rizza R. Impact of lack of suppression of glucagon on glucose tolerance in humans. Am J Physiol Endocrinol Metab 1999;277:E283–E290 [DOI] [PubMed] [Google Scholar]
- 37.Basu R, Chandramouli V, Dicke B, Landau B, Rizza R. Obesity and type 2 diabetes impair insulin-induced suppression of glycogenolysis as well as gluconeogenesis. Diabetes 2005;54:1942–1948 [DOI] [PubMed] [Google Scholar]
- 38.Basu A, Basu R, Shah P, Vella A, Johnson CM, Jensen M, Nair KS, Schwenk WF, Rizza RA. Type 2 diabetes impairs splanchnic uptake of glucose but does not alter intestinal glucose absorption during enteral glucose feeding: additional evidence for a defect in hepatic glucokinase activity. Diabetes 2001;50:1351–1362 [DOI] [PubMed] [Google Scholar]