First-degree relatives of people with type 2 diabetes are themselves at risk of developing the disease. While the transmission of genetic information from parents to offspring contributes to diabetes risk, there are other nongenetic risk factors that are shared by family members that can be amenable to intervention. These factors are likely to include sedentary behaviors such as television viewing and computer use. The extent to which family history influences the effects of sedentary behaviors on cellular energy metabolism and other markers of diabetes risk is poorly understood. An article by Højbjerre et al. (1) in this issue of Diabetes examines the impact of 10-days' bed rest on young, nondiseased adults with or without a first-degree family history of diabetes. This commentary summarizes the findings from that study, places them in context with the findings of earlier studies, describes mechanisms of action, and identifies key areas where future research is required.
Ecological comparisons (2), cohort studies (3), clinical trials (4), and government guidelines (5) tell us that a healthy lifestyle should include regular, moderately intense physical activity. Physically active people are at lower risk of type 2 diabetes (6), heart disease (7), specific cancers (8), and early death (9). Conversely, sedentary behaviors during leisure time and at work have been associated with cardiovascular morbidity (6,10) and mortality (11). While these epidemiological studies have received considerable media attention, many are prone to confounding and reverse causality. Intervention studies where participants are confined to bed (12,13) or in other ways discouraged from exercising (14) provide some of the strongest mechanistic evidence that sedentary behaviors are harmful to health. A study by Højbjerre et al. (1) in this issue of Diabetes is one such example.
Højbjerre et al. (1) examined the effects of 10 days' bed rest on energy metabolism and mRNA expression of lipases in subcutaneous abdominal (SCAAT) and femoral (SCFAT) adipose tissue from healthy young adults with (FHx+) or without (FHx−) a family history of type 2 diabetes. Prior to bed rest, total and abdominal adipose mass and basal and insulin-stimulated glucose uptake in SCAAT were greater in FHx+ compared with FHx− individuals, suggesting that FHx+ individuals preferentially store glucose in adipose tissue during hyperinsulinemia. FHx+ participants also had lower VO2max, whole-body insulin sensitivity, and insulin-stimulated SCFAT blood flow; corresponding elevations in fasting plasma glucose and insulin-stimulated free fatty acid concentrations were also noted. Moreover, FHx+ participants had higher fasting triacylglycerol and insulin concentrations and lower hormone-sensitive lipase and lipoprotein lipase (LPL) expression in SCAAT before and after bed rest.
These characteristics are indicative of metabolic inflexibility, an acquired or inherited incapacity to switch between fat and carbohydrate oxidation as substrate availability changes (15). Metabolic inflexibility is primarily a consequence of impaired cellular glucose uptake, particularly within skeletal muscle, which is a defect that is contributed to by cellular insulin resistance and intracellular triacylglycerol accumulation. Importantly, diminished metabolic flexibility also impacts the ability to inhibit ceramide and diacylglycerol formation (16), processes that are integral to the development of cellular insulin resistance primarily because intramyocellular accumulation of these lipids interferes with insulin signaling (17,18).
In the study by Højbjerre et al., bed rest also impacted metabolic homeostasis with effects differing by FHx. Fasting plasma glucose concentrations and basal lipolysis in SCFAT decreased with bed rest, irrespective of FHx. Insulin sensitivity also declined with bed rest in both groups. Plasma lactate concentrations did not differ between groups at baseline but were lower in FHx+ participants after bed rest during insulin stimulation; bed rest increased basal LPL expression only in FHx− participants, whereas insulin-stimulated LPL expression increased markedly with bed rest only in FHx+ participants.
Insulin sensitivity improves following exercise training partly because of enhanced muscle triacylglycerol utilization (19). The metabolic changes reported by Højbjerre et al. are indicative of a diminished capacity to inhibit lipolysis, which, through the mechanisms outlined above, can impair insulin sensitivity and muscle glucose uptake. Based on exercise studies, one would predict that muscle triacylglycerol utilization would also diminish with bed rest, which could further augment cellular insulin resistance.
It has long been acknowledged that type 2 diabetes FHx raises type 2 diabetes risk. In the Framingham Offspring Study (20), a positive parental history of diabetes almost doubled the risk of type 2 diabetes in the offspring. In Danish twins (21), 26 and 41% of the twin resemblance in diabetes was attributed to additive genetic (a2) and shared environmental (c2) factors, respectively, with the remaining variance attributed to unshared environmental factors and error (e2). The subsequent discovery of multiple type 2 diabetes associated-loci (22) has helped define the genomic regions within which causal variants reside.
The Botnia Family Study from western Finland was one of the first to investigate the metabolic consequences of diabetes FHx. This study illustrated that FHx+ offspring are predisposed to central obesity and insulin resistance, lower rates of basal (23) and maximal (24) energy expenditure, and develop type 2 diabetes more rapidly (24,25) than FHx− offspring. These observations may be related to disturbances in skeletal muscle oxidative energy metabolism; studies in insulin resistant, but otherwise healthy, FHx+ young adults (26) show that intramyocellular lipid accumulation, rates of mitochondrial phosphorylation, and the ratio of inorganic phosphate to phosphocreatine in skeletal muscle are 80, 30, and 20% lower, respectively, than in insulin-sensitive individuals with or without a positive FHx. The study by Højbjerre et al. (1) extends our understanding by defining adipose-centric mechanisms by which FHx impacts type 2 diabetes risk. The fact that this study included normal weight individuals is also of considerable interest, largely because it emphasizes the relevance of obesity-independent mechanisms linking FHx and type 2 diabetes risk.
As with the studies outlined above, the baseline characteristics of the FHx+ participants in the study by Højbjerre et al. are compatible with what one would expect to observe in FHx− individuals leading sedentary lifestyles (27). This raises an interesting unanswered question, which is whether the dysmetabolic phenotypes observed in FHx+ individuals are primarily due to a genetic predisposition that persists even in the presence of healthful lifestyles, or whether these traits segregate in certain families because of shared diabetogenic behaviors. This distinction is important because the latter may be amenable to intervention.
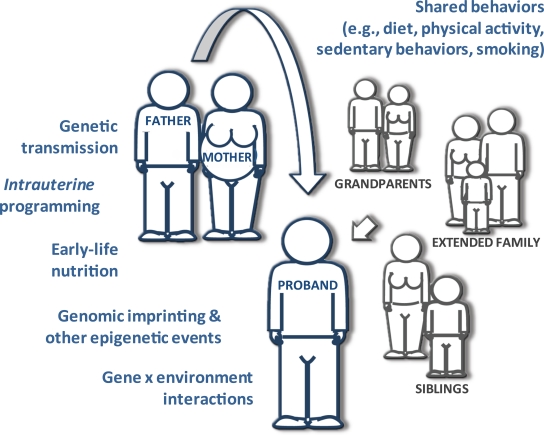
Interestingly, the correlation between the confirmed type 2 diabetes loci and FHx of the disease is fairly weak (22). This might be because type 2 diabetes FHx is often misclassified and/or because the genetic architecture of the disease is poorly understood, thus limiting the statistical power of studies that seek to test associations between these factors. An additional explanation (alluded to previously) is that diabetes FHx involves much more than simply sharing genotypes (Fig. 1). Indeed, the twin study described previously (21), studies of obesity risk within biologically unrelated social networks (where having a spouse, friend, or neighbor who later became obese raised the risk of obesity in the proband) (28), and studies of intrauterine programming (where individuals exposed to diabetes in utero were themselves at elevated risk of diabetes independently of genetic transmission) (29), indicate that nongenetic diabetogenic factors can be inherited.
FIG. 1.
“Diabetes family history” and “genetic risk” are not synonymous. The relationship between family history and risk of diabetes in the proband involves the complex interplay between genes, shared environment, shared behaviors, and epigenetic effects. Defining the details of these relationships will be necessary for the optimal prevention of type 2 diabetes. (A high-quality color representation of this figure is available in the online issue.)
Unfortunately, heightened awareness among people with an FHx+ of diabetes of the need to maintain healthful lifestyles rarely translates into action. In one study of African Americans, FHx+ individuals were more likely to report being physically inactive and consuming energy-dense foods than FHx− individuals despite being aware that such behaviors substantially raised their risk of diabetes (30). In another program for individuals from the U.K. with a positive parental history of diabetes, intensive behavior modification proved ineffective despite the participants' awareness of their heightened risk and the potential benefits of lifestyle change (31). These observations support the view that the segregation of type 2 diabetes within families involves the complex interplay between genetic and nongenetic factors and that the strong association between FHx and proband risk (20) is attributable to both shared behaviors and an underlying molecular predisposition to the disease.
Hence, the effects of FHx on energy metabolism reported by Højbjerre et al. are likely to be heterogeneous in origin and involve risk factors that may, in theory at least, be amenable to preventive intervention. Future studies that build on these findings, where preintervention differences in lifestyle behaviors between FHx+/− participants are adequately controlled for, may help determine the extent to which lifestyle factors underlie differences in energy metabolism and cardiovascular risk associated with FHx and sedentary behaviors, and also outline which behaviors should be the target of preventive interventions in FHx+ individuals.
ACKNOWLEDGMENTS
The author is solely responsible for the content of this article. The author was supported in part by grants from Novo Nordisk, the Swedish Research Council, and the Swedish Diabetes Association.
No potential conflicts of interest relevant to this article were reported.
The author thanks Dr. O. Hansson (Lund University Diabetes Center, Malmö, Sweden) and Dr. F. Renström (Department of Public Health and Clinical Medicine, Genetic Epidemiology and Clinical Research Group, Umeå University, Sweden) for their helpful comments on this article.
Footnotes
REFERENCES
- 1.Højbjerre L, Sonne MP, Alibegovic AC, Dela F, Vaag A, Bruun JM, Christensen KB, Stallknecht B. Impact of physical inactivity on subcutaneous adipose tissue metabolism in healthy young male offspring of patients with type 2 diabetes. Diabetes 2010;59:2790–2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schulz LO, Bennett PH, Ravussin E, Kidd JR, Kidd KK, Esparza J, Valencia ME. Effects of traditional and western environments on prevalence of type 2 diabetes in Pima Indians in Mexico and the U.S. Diabetes Care 2006;29:1866–1871 [DOI] [PubMed] [Google Scholar]
- 3.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345:790–797 [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DMDiabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Department of Health and Human Services Physical activity and health: a report of the Surgeon General. Atlanta, Georgia, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, 1996 [Google Scholar]
- 6.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 2001;161:1542–1548 [DOI] [PubMed] [Google Scholar]
- 7.Manson JE, Hu FB, Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, Speizer FE, Hennekens CH. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med 1999;341:650–658 [DOI] [PubMed] [Google Scholar]
- 8.Thune I, Brenn T, Lund E, Gaard M. Physical activity and the risk of breast cancer. N Engl J Med 1997;336:1269–1275 [DOI] [PubMed] [Google Scholar]
- 9.Manini TM, Everhart JE, Patel KV, Schoeller DA, Colbert LH, Visser M, Tylavsky F, Bauer DC, Goodpaster BH, Harris TB. Daily activity energy expenditure and mortality among older adults. JAMA 2006;296:171–179 [DOI] [PubMed] [Google Scholar]
- 10.Jakes RW, Day NE, Khaw KT, Luben R, Oakes S, Welch A, Bingham S, Wareham NJ. Television viewing and low participation in vigorous recreation are independently associated with obesity and markers of cardiovascular disease risk: EPIC-Norfolk population-based study. Eur J Clin Nutr 2003;57:1089–1096 [DOI] [PubMed] [Google Scholar]
- 11.Dunstan DW, Barr EL, Healy GN, Salmon J, Shaw JE, Balkau B, Magliano DJ, Cameron AJ, Zimmet PZ, Owen N. Television viewing time and mortality: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Circulation 2010;121:384–391 [DOI] [PubMed] [Google Scholar]
- 12.Bergouignan A, Trudel G, Simon C, Chopard A, Schoeller DA, Momken I, Votruba SB, Desage M, Burdge GC, Gauquelin-Koch G, Normand S, Blanc S. Physical inactivity differentially alters dietary oleate and palmitate trafficking. Diabetes 2009;58:367–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamburg NM, McMackin CJ, Huang AL, Shenouda SM, Widlansky ME, Schulz E, Gokce N, Ruderman NB, Keaney JF, Jr, Vita JA. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol 2007;27:2650–2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen RH, Krogh-Madsen R, Thomsen C, Booth FW, Pedersen BK. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA 2008;299:1261–1263 [DOI] [PubMed] [Google Scholar]
- 15.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 2000;49:677–683 [DOI] [PubMed] [Google Scholar]
- 16.Moro C, Bajpeyi S, Smith SR. Determinants of intramyocellular triglyceride turnover: implications for insulin sensitivity. Am J Physiol Endocrinol Metab 2008;294:E203–E213 [DOI] [PubMed] [Google Scholar]
- 17.Chibalin AV, Leng Y, Vieira E, Krook A, Björnholm M, Long YC, Kotova O, Zhong Z, Sakane F, Steiler T, Nylén C, Wang J, Laakso M, Topham MK, Gilbert M, Wallberg-Henriksson H, Zierath JR. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell 2008;132:375–386 [DOI] [PubMed] [Google Scholar]
- 18.Zierath JR. The path to insulin resistance: paved with ceramides? Cell Metab 2007;5:161–163 [DOI] [PubMed] [Google Scholar]
- 19.Goodpaster BH, Kelley DE. Skeletal muscle triglyceride: marker or mediator of obesity-induced insulin resistance in type 2 diabetes mellitus? Curr Diab Rep 2002;2:216–222 [DOI] [PubMed] [Google Scholar]
- 20.Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D'Agostino RB., Sr Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med 2007;167:1068–1074 [DOI] [PubMed] [Google Scholar]
- 21.Poulsen P, Kyvik KO, Vaag A, Beck-Nielsen H. Heritability of type II (non-insulin-dependent) diabetes mellitus and abnormal glucose tolerance: a population-based twin study. Diabetologia 1999;42:139–145 [DOI] [PubMed] [Google Scholar]
- 22.Frayling TM. Genome-wide association studies provide new insights into type 2 diabetes aetiology. Nat Rev Genet 2007;8:657–662 [DOI] [PubMed] [Google Scholar]
- 23.Groop L, Forsblom C, Lehtovirta M, Tuomi T, Karanko S, Nissén M, Ehrnström BO, Forsén B, Isomaa B, Snickars B, Taskinen MR. Metabolic consequences of a family history of NIDDM (the Botnia study): evidence for sex-specific parental effects. Diabetes 1996;45:1585–1593 [DOI] [PubMed] [Google Scholar]
- 24.Isomaa B, Forsén B, Lahti K, Holmström N, Wadén J, Matintupa O, Almgren P, Eriksson JG, Lyssenko V, Taskinen MR, Tuomi T, Groop LC. A family history of diabetes is associated with reduced physical fitness in the Prevalence, Prediction and Prevention of Diabetes (PPP)-Botnia study. Diabetologia 2010;53:1709–1713 [DOI] [PubMed] [Google Scholar]
- 25.Lyssenko V, Almgren P, Anevski D, Perfekt R, Lahti K, Nissén M, Isomaa B, Forsen B, Homström N, Saloranta C, Taskinen MR, Groop L, Tuomi TBotnia study group Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes 2005;54:166–174 [DOI] [PubMed] [Google Scholar]
- 26.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 2004;350:664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franks PW, Ekelund U, Brage S, Wong MY, Wareham NJ. Does the association of habitual physical activity with the metabolic syndrome differ by level of cardiorespiratory fitness? Diabetes Care 2004;27:1187–1193 [DOI] [PubMed] [Google Scholar]
- 28.Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med 2007;357:370–379 [DOI] [PubMed] [Google Scholar]
- 29.Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 2000;49:2208–2211 [DOI] [PubMed] [Google Scholar]
- 30.Baptiste-Roberts K, Gary TL, Beckles GL, Gregg EW, Owens M, Porterfield D, Engelgau MM. Family history of diabetes, awareness of risk factors, and health behaviors among African Americans. Am J Public Health 2007;97:907–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinmonth AL, Wareham NJ, Hardeman W, Sutton S, Prevost AT, Fanshawe T, Williams KM, Ekelund U, Spiegelhalter D, Griffin SJ. Efficacy of a theory-based behavioural intervention to increase physical activity in an at-risk group in primary care (ProActive UK): a randomised trial. Lancet 2008;371:41–48 [DOI] [PubMed] [Google Scholar]