Abstract
OBJECTIVE
The induction of obesity, dyslipidemia, and insulin resistance by high-fat diet in rodents can be prevented by n-3 long-chain polyunsaturated fatty acids (LC-PUFAs). We tested a hypothesis whether AMP-activated protein kinase (AMPK) has a role in the beneficial effects of n-3 LC-PUFAs.
RESEARCH DESIGN AND METHODS
Mice with a whole-body deletion of the α2 catalytic subunit of AMPK (AMPKα2−/−) and their wild-type littermates were fed on either a low-fat chow, or a corn oil-based high-fat diet (cHF), or a cHF diet with 15% lipids replaced by n-3 LC-PUFA concentrate (cHF+F).
RESULTS
Feeding a cHF diet induced obesity, dyslipidemia, hepatic steatosis, and whole-body insulin resistance in mice of both genotypes. Although cHF+F feeding increased hepatic AMPKα2 activity, the body weight gain, dyslipidemia, and the accumulation of hepatic triglycerides were prevented by the cHF+F diet to a similar degree in both AMPKα2−/− and wild-type mice in ad libitum-fed state. However, preservation of hepatic insulin sensitivity by n-3 LC-PUFAs required functional AMPKα2 and correlated with the induction of adiponectin and reduction in liver diacylglycerol content. Under hyperinsulinemic-euglycemic conditions, AMPKα2 was essential for preserving low levels of both hepatic and plasma triglycerides, as well as plasma free fatty acids, in response to the n-3 LC-PUFA treatment.
CONCLUSIONS
Our results show that n-3 LC-PUFAs prevent hepatic insulin resistance in an AMPKα2-dependent manner and support the role of adiponectin and hepatic diacylglycerols in the regulation of insulin sensitivity. AMPKα2 is also essential for hypolipidemic and antisteatotic effects of n-3 LC-PUFA under insulin-stimulated conditions.
Naturally occurring n-3 long-chain polyunsaturated fatty acids (LC-PUFAs)—namely, eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)—which are abundant in sea fish, act as hypolipidemics, reduce cardiac events, and decrease the progression of atherosclerosis [reviewed in refs (1,2).]. Studies of obese humans have also demonstrated a reduction in adiposity after n-3 LC-PUFA supplementation (3,4). In rodents fed a high-fat diet, n-3 LC-PUFAs efficiently prevented the development of obesity, hepatic steatosis, and dyslipidemia (5–8), as well as impaired glucose tolerance (8–10). However, in diabetic patients, n-3 LC-PUFAs appear to have little effect on glycemic control (3,11,12).
The hypolipidemic and antiobesity effects of n-3 LC-PUFAs depend on both the suppression of lipogenesis and the increase in fatty acid oxidation in several tissues, including the liver (13,14), adipose tissue (6), and intestine (15). This metabolic switch may reduce the accumulation of toxic fatty acid derivatives, while protecting insulin signaling in the liver and muscle (9,10,16). Our previous work has documented that the preservation of whole-body insulin sensitivity by n-3 LC-PUFAs in mice fed a high-fat diet mainly reflects improved hepatic insulin sensitivity (8). The effects of n-3 LC-PUFAs and their active metabolites (17,18) are mediated by peroxisome proliferator-activated receptors (PPAR), with PPAR-α and PPAR-δ (-β) being the main targets (14,16), although PPAR-γ, liver X receptor-α, hepatic nuclear factor-4, sterol regulatory element binding protein-1c (SREBP-1c) and carbohydrate-responsive element-binding protein are also involved (16,19–21).
It has been demonstrated that n-3 LC-PUFAs enhanced AMP-activated protein kinase (AMPK) activity in the liver (22), intestine (23), and adipose tissue (18,24). AMPK is a heterotrimeric protein consisting of a catalytic α-subunit and regulatory β- and γ-subunits, with multiple isoforms identified for each subunit [α1, α2, β1, β2, γ1, γ2, and γ3; reviewed in ref (25)]. Experiments using whole-body AMPKα2 null [AMPKα2−/−; ref (26)] mice showed the importance of the AMPKα2 subunit for whole-body insulin action, while liver-specific AMPKα2 knockout mice (27) as well as adenovirus-mediated activation of AMPKα2 in the liver (28) implicated the hepatic AMPKα2 isoform in the suppression of hepatic glucose production and maintenance of fasting blood glucose levels. Furthermore, AMPK controls metabolic fluxes in response to changing cellular energy levels, namely, the partitioning between lipid oxidation and lipogenesis (29,30).
We hypothesized that the effects of n-3 LC-PUFA on insulin sensitivity and lipid metabolism in mice fed an obesogenic high-fat diet require a functional AMPKα2 isoform. To test this hypothesis in vivo, AMPKα2−/− and wild-type mice were fed either a low-fat chow diet (Chow), a corn oil-based high-fat (cHF) diet, or cHF diet in which 15% of the lipids were replaced by n-3 LC-PUFA concentrate (cHF+F). Our results demonstrate an AMPKα2-dependent action of n-3 LC-PUFAs, in 1) the preservation of hepatic and muscle insulin sensitivity; 2) the changes in hepatic diacylglycerol content and composition; and 3) the antisteatotic effect in the liver and hypolipidemic effect under insulin-stimulated conditions, such as during hyperinsulinemic-euglycemic clamp, but not when the organism depends on lipids as substrates.
RESEARCH DESIGN AND METHODS
Four-month-old whole-body AMPKα2−/− mice (29) backcrossed to C57BL/6J mice for nine generations, and wild-type littermate controls were fed on either Chow, cHF, or cHF+F diet for nine weeks. Body weight and food consumption were recorded, and EDTA-plasma and tissues were collected for various analyses as described in the online appendix, available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1716/DC1. Male mice were used for all the experiments, except for the measurements of hepatic AMPK activity, which were performed on female mice. The experiments were conducted under the guidelines for the use and care of laboratory animals of the Institute of Physiology and followed the “Principles of laboratory animal care” (National Institutes of Health publication no. 85-23, revised 1985).
Blood and plasma parameters.
Blood glucose was measured using calibrated glucometers (LifeScan, Milpitas, CA). Nonesterified fatty acids (NEFAs), triglycerides, and total cholesterol were determined in plasma using the following enzymatic photometric tests: NEFA-C (Wako Chemicals, Neuss, Germany), triacylglycerols liquid, and cholesterol liquid (Pliva-Lachema Diagnostika, Brno, Czech Republic), respectively. Plasma insulin was measured using the Sensitive Rat Insulin RIA Kit (LINCO Research, St. Charles, MO). Total adiponectin levels and adiponectin multimeric complexes were determined using Western blotting (31).
Lipid content and gene expression in the liver.
The tissue content of triglycerides was estimated in ethanolic KOH tissue lysates as described before (8). The content and fatty acid composition of the phospholipid, diacylglycerol, triglyceride, and ceramide fractions were assessed in tissue lipid extracts; gene expression was evaluated using real-time RT-PCR (see online appendix).
Activity of α1 and α2 AMPK isoforms.
Livers were collected by freeze-clamping, AMPK was immunoprecipitated from tissue extracts, and the activity was assayed using a peptide substrate (32); see the online appendix.
Hyperinsulinemic-euglycemic clamp.
Five days before the experiment, an indwelling catheter was placed into the left femoral vein under anesthesia (33). Mice were allowed to recover for 5–7 days, followed by a 6-h fast (8:00 a.m.–2:00 p.m.) prior to the experiment. The whole-body glucose turnover was determined under basal (nonstimulated) and insulin-stimulated conditions (hyperinsulinemic-euglycemic clamp), using separate groups of mice. Insulin (Actrapid, Novo Nordisk Pharma, Denmark) was infused at a constant rate of 4.8 mU/kg·min for 3 h, while d-[3-3H]glucose (Perkin Elmer, Boston, MA) was infused at a rate of 15.9 kBq/min. Throughout the infusion, glucose concentration and d-[3-3H]glucose specific activity (during the last hour of infusion) were determined in tail blood. Euglycemia (∼5.55 mmol/l) was maintained by periodically adjusting a variable infusion of 33% glucose (33). At the end of a 3-h infusion period, mice were first anesthetized by diethylether, exsanguinated through the cervical incision, and then killed by cervical dislocation, and tissues (liver and quadriceps muscle) and EDTA-plasma were collected for biochemical analyses (see supplementary Table 1 and Research Design and Methods of the online appendix for details on basic clamp parameters and methodology).
Primary cultures of hepatocytes.
Hepatocytes were isolated from livers of fed mice by a modification of the collagenase method (34) and seeded at a density of 0.5 × 106 cells per each 35-mm Petri dish. Rates of basal and insulin-stimulated de novo lipogenesis and AICAR-stimulated fatty acid oxidation were measured using [1-14C] acetate and [1-14C] palmitate, respectively (see online appendix).
Statistics.
All values are presented as means ± SE. Data were analyzed by two-way ANOVA. Comparisons were judged to be significant at P ≤ 0.05.
RESULTS
Enhancement of hepatic AMPKα2 activity by n-3 LC-PUFAs.
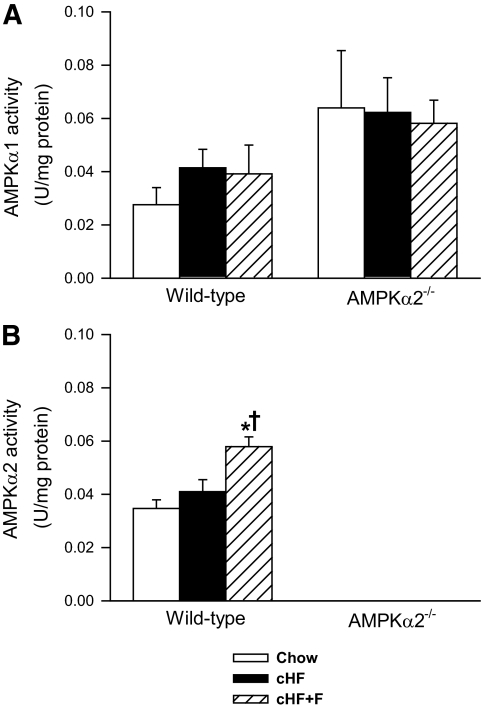
Specific activities of AMPKα1 and AMPKα2 were evaluated in the liver of ad libitum-fed mice after nine weeks of the differential dietary treatment (Fig. 1). No significant effect of either diet (Chow, cHF, and cHF+F) or genotype (wild-type versus AMPKα2−/−) on AMPKα1-specific activity was observed, although the AMPKα1 activity tended to be higher in the AMPKα2−/− mice (Fig. 1A). In contrast, AMPKα2 activity was stimulated by n-3 LC-PUFAs (cHF+F diet; Fig. 1B). AMPKα2 activity was not detected in the AMPKα2−/− mice (Fig. 1B). No changes were detected in the activity of AMPKα1 and AMPKα2 in the quadriceps muscle in response to n-3 LC-PUFAs (not shown).
FIG. 1.
Liver AMPKα1 (A) and AMPKα2 (B) activity in wild-type and AMPKα2−/− mice fed either a Chow diet, cHF, or cHF+F for 9 weeks. The data are the means ± SE (n = 5–8). In the AMPKα2−/− mice, AMPKα2 activity was below the detection limit. *P < 0.05 versus genotype Chow; †P < 0.05 versus genotype cHF.
AMPKα2 is not required for antiobesity and hypolipidemic effects of n-3 LC-PUFAs in ad libitum-fed mice.
At four months of age, at the beginning of dietary treatments, wild-type and AMPKα2−/− mice fed the Chow diet exhibited similar body weights (Table 1). In mice of both genotypes, cHF-feeding for nine weeks resulted in greater body weight gain compared with the Chow-fed mice. However, this effect was less pronounced in AMPKα2−/− mice (Table 1). In both wild-type and AMPKα2−/− mice, the cHF+F diet induced smaller body weight gain than the cHF diet (Table 1 and supplementary Figure 1). None of the differences in body weight gain could be explained by caloric intake, which was similar in all experimental groups (Table 1). The weight of fat depots increased in response to cHF feeding, while the cHF+F diet partially prevented this increase (Table 1). Triglycerides and NEFA levels in plasma of ad libitum-fed mice were similar in the Chow- and cHF-fed mice, while cholesterol levels were markedly and significantly elevated by the cHF diet. n-3 LC-PUFAs lowered plasma lipid levels independently of AMPKα2. Triglycerides and NEFA levels were strongly reduced, even below the levels observed in the Chow-fed mice (Table 1).
TABLE 1.
Metabolic and plasma parameters in wild-type and AMPKα2−/− mice
| Metabolic parameters | Wild-type |
AMPKα2−/− |
||||
|---|---|---|---|---|---|---|
| Chow | cHF | cHF+F | Chow | cHF | cHF+F | |
| Food consumption (kJ/g · day) | 2.0 ± 0.1 | 1.8 ± 0.1 | 1.9 ± 0.0 | 2.0 ± 0.1 | 1.8 ± 0.1 | 2.0 ± 0.1 |
| Initial body weight (g) | 27.8 ± 0.5 | 27.9 ± 0.5 | 27.7 ± 0.5 | 27.1 ± 0.3 | 27.7 ± 0.4 | 27.3 ± 0.4 |
| Body weight gain (g) | 1.4 ± 0.2 | 7.0 ± 1.1* | 2.8 ± 0.7† | 1.8 ± 0.3 | 4.6 ± 0.7*‡ | 1.2 ± 0.4† |
| Adiposity | ||||||
| Epididymal AT (g) | 0.43 ± 0.02 | 1.52 ± 0.19* | 1.12 ± 0.14*† | 0.37 ± 0.03 | 1.19 ± 0.16*‡ | 0.64 ± 0.07*†‡ |
| Subcutaneous AT (g) | 0.20 ± 0.01 | 0.54 ± 0.05* | 0.42 ± 0.04*† | 0.17 ± 0.01 | 0.34 ± 0.03*‡ | 0.23 ± 0.01†‡ |
| Epididymal adipocytes size (μm2) | ND | 15,971 ± 1,784 | 10,232 ± 185† | ND | 13,298 ± 1,632 | 8 593 ± 896† |
| Subcutaneous adipocytes size (μm2) | 2,916 ± 610 | 6,775 ± 1,718* | 7,311 ± 1,308* | 3,395 ± 139 | 6,625 ± 926* | 5,432 ± 648* |
| Plasma metabolites | ||||||
| Triglycerides (mmol/l) | 1.17 ± 0.08 | 1.23 ± 0.11 | 0.62 ± 0.07*† | 1.04 ± 0.06 | 1.22 ± 0.08 | 0.73 ± 0.06*† |
| NEFAs (mmol/l) | 0.90 ± 0.05 | 0.94 ± 0.05 | 0.59 ± 0.04*† | 0.88 ± 0.04 | 0.99 ± 0.06 | 0.68 ± 0.04*† |
| Cholesterol (mmol/l) | 2.25 ± 0.08 | 4.12 ± 0.25* | 3.10 ± 0.20*† | 2.10 ± 0.06 | 3.94 ± 0.18* | 2.75 ± 0.14*† |
| Glucose (mmol/l) | 9.9 ± 0.4 | 10.7 ± 0.5 | 10.4 ± 0.3 | 9.7 ± 0.4 | 10.0 ± 0.4 | 9.4 ± 0.4 |
| Plasma hormones | ||||||
| Insulin fed (ng/ml) | 0.66 ± 0.11 | 1.73 ± 0.29* | 1.47 ± 0.28* | 0.60 ± 0.07 | 1.34 ± 0.20* | 0.95 ± 0.12* |
| Insulin fasted (ng/ml) | 0.13 ± 0.01 | 0.39 ± 0.06* | 0.19 ± 0.05† | 0.16 ± 0.02 | 0.22 ± 0.03‡ | 0.15 ± 0.01 |
| Total adiponectin (A.U.) | 1.15 ± 0.09 | 0.97 ± 0.10 | 1.33 ± 0.09† | 0.82 ± 0.09‡ | 0.80 ± 0.07 | 0.97 ± 0.08‡ |
| HMW: total adiponectin | 0.38 ± 0.02 | 0.36 ± 0.02 | 0.44 ± 0.02† | 0.35 ± 0.02 | 0.34 ± 0.02 | 0.39 ± 0.02 |
Data are means ± SE of 27–30 mice for metabolic parameters and 13–15 mice for other measures. AMPKα2−/− and wild-type mice were fed either a Chow diet, cHF, or cHF+F for nine weeks. Food consumption was measured weekly for nine weeks. Body weight gain (see supplementary Fig. 1) and plasma parameters were assessed in ad libitum-fed mice after nine weeks. Plasma insulin levels were also assessed in fasted mice after eight weeks. AT, adipose tissue; A.U., arbitary units; HMW, total adiponectin, ratio of high molecular weight to total adiponectin (for levels of all molecular weight forms of adiponectin; ND, no data; see supplementary Fig. 2);
*P < 0.05 vs. genotype Chow;
†P < 0.05 vs. genotype cHF;
‡P < 0.05 vs. wild-type on respective diet.
AMPKα2 is essential for the preservation of insulin sensitivity in response to n-3 LC-PUFAs.
After nine weeks of dietary treatment, no change was observed in blood glucose, but elevations were observed in plasma insulin levels in response to the cHF diet in ad libitum-fed mice of both genotypes. However, the increase in plasma insulin levels was less pronounced in AMPKα2−/− mice, closely reflecting the genotype-dependent differences in body weight gain (Table 1). A similar pattern of changes in insulin levels was also observed in fasted mice, in which n-3 LC-PUFAs significantly reduced insulin levels only in wild-type animals (Table 1). As expected, plasma levels of total as well as high molecular weight form of adiponectin, an adipokine associated with increased insulin sensitivity (35), were increased ∼1.4- and ∼1.2-fold, respectively, in wild-type mice in response to n-3 LC-PUFA supplementation (Table 1 and supplementary Fig. 2, available in an online appendix); however, no significant increase of plasma adiponectin by n-3 LC-PUFAs was observed in AMPKα2−/− mice.
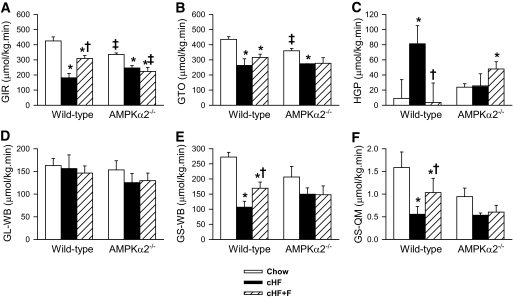
In further experiments, hyperinsulinemic-euglycemic clamps were performed to evaluate whole-body insulin sensitivity. Under basal conditions, glucose turnover rate (GTO; i.e., glucose uptake in peripheral tissues) was similar in all groups of mice (supplementary Table 1). Under insulin-stimulated conditions (Fig. 2A–F), the amount of exogenous glucose required to maintain euglycemia during the clamp, i.e., the glucose infusion rate (GIR), was ∼1.3-fold lower in AMPKα2−/− than in wild-type mice fed the Chow diet (Fig. 2A). On the other hand, GIR was decreased by the cHF diet to a similar level in mice of both genotypes, manifesting diet-induced insulin resistance. This was attributed to a decreased GTO and, in particular, to an impaired suppression of hepatic glucose production (HGP) by insulin, with HGP being ∼8.5-fold higher in the cHF-fed compared with the Chow-fed wild-type mice (Fig. 2C). In wild-type mice, cHF+F diet feeding increased GIR and GTO (∼1.9- and ∼1.2-fold increase, respectively) as compared with cHF-fed mice, while HGP was lowered to a similar level as in the Chow-fed mice. These results document the protective effects of n-3 LC-PUFAs from high-fat diet-induced insulin resistance in wild-type mice, namely, at the level of HGP. In contrast, none of these beneficial effects of n-3 LC-PUFAs was observed in AMPKα2−/− mice, in which neither the GIR (Fig. 2A) nor the GTO (Fig. 2B) differed between the cHF+F-fed and the cHF-fed mice, whereas the rate of HGP was even higher in the cHF+F-fed than in the cHF-fed AMPKα2−/− mice (Fig. 2C). Although whole-body glycolysis was similar in all the groups (Fig. 2D), the rate of whole-body glycogen synthesis, which reflects insulin sensitivity of muscle glucose metabolism, was dependent on both diet and genotype (Fig. 2E). In the Chow-fed mice, the rate of whole-body glycogen synthesis tended to be higher in wild-type mice than in AMPKα2−/− mice. Only in the former mice was it significantly affected by dietary treatment. Thus, in wild-type mice, glycogen synthesis was decreased ∼2.4-fold in response to the cHF diet, while n-3 LC-PUFAs provided a partial protection from this decrease (Fig. 2E). A similar pattern of changes in the glycogen synthesis rate in response to n-3 LC-PUFAs was observed when measured directly in the skeletal muscle (Fig. 2F). Thus, in accordance with the previous study (26), the results of clamp studies suggested impairment of insulin sensitivity in response to whole-body ablation of AMPKα2 in Chow-fed mice. However, AMPKα2−/− mice seemed to be partially protected against cHF-induced insulin resistance, while AMPKα2 was required for preservation of insulin sensitivity in the skeletal muscle and especially in the liver in response to n-3 LC-PUFA feeding.
FIG. 2.
Insulin sensitivity assessed by hyperinsulinemic-euglycemic clamp. GIR (A), GTO (B), HGP (C), whole-body glycolysis (GL-WB; D), whole-body glycogen synthesis (GS-WB; E); and glycogen synthesis in quadriceps muscle (GS-QM; F) were measured in wild-type and AMPKα2−/− mice fed either a Chow diet, cHF, or cHF+F for 9 weeks. The data are the means ± SE (n = 5–8). *P < 0.05 versus genotype Chow; †P < 0.05 versus genotype cHF; ‡P < 0.05 versus wild-type on respective diet.
Unmasking the role of AMPKα2 in the lipid-lowering effect of n-3 LC-PUFAs under hyperinsulinemic-euglycemic conditions.
In addition to the ad libitum-fed mice (Table 1), plasma lipid levels were also measured in fasted mice, as well as in mice subjected to hyperinsulinemic-euglycemic clamp (supplementary Table 2). In contrast to the ad libitum-fed state, cHF+F diet did not affect either triglyceride or NEFA levels under fasting conditions. Under the hyperinsulinemic-euglycemic conditions, both triglyceride and NEFA levels were lower in the cHF+F-fed than in the cHF-fed wild-type mice (∼1.6-fold and ∼1.4-fold difference, respectively), but no such difference between the diets was observed in AMPKα2−/− mice. Cholesterol levels were consistently decreased by n-3 LC-PUFAs independently of both the metabolic state and genotype (supplementary Table 2).
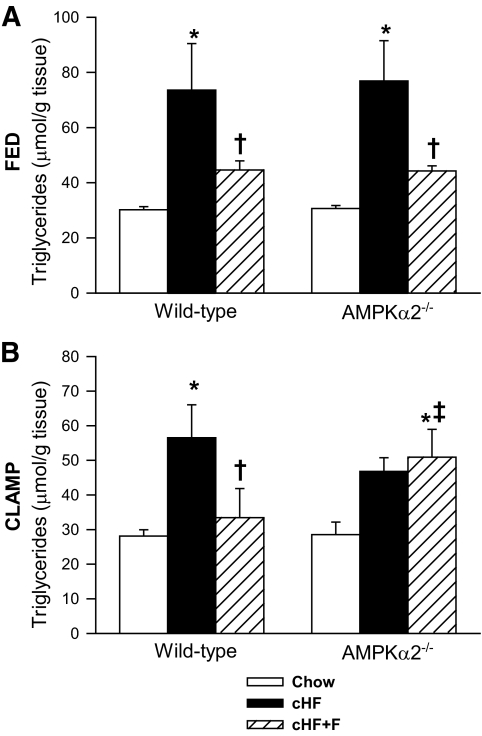
In ad libitum-fed mice of both genotypes, the hepatic triglyceride content was increased ∼twofold by cHF compared with the Chow diet, while triglyceride accumulation was increased only ∼1.3-fold by cHF+F diet in both genotypes, documenting a protection against hepatic triglyceride accumulation by n-3 LC-PUFAs (Fig. 3A). Under hyperinsulinemic-euglycemic conditions, n-3 LC-PUFAs also protected livers of wild-type mice against the cHF-induced accumulation of triglycerides. However, this effect was absent in AMPKα2−/− mice (Fig. 3B). Moreover, a strong correlation was found between plasma NEFA levels and hepatic triglyceride content assessed under the clamp conditions in the cHF+F-fed AMPKα2−/− mice (R2 = 0.43, P < 0.05) but not in wild-type mice (R2 = 0.08, P = 0.40).
FIG. 3.
Triglyceride concentration in the livers of ad libitum-fed mice (A) and mice killed at the end of a 3-h infusion period of the hyperinsulinemic-euglycemic clamp (B). Wild-type and AMPKα2−/− mice were fed either a Chow diet, cHF, or cHF+F for 9 weeks. The data are the means ± SE (A, n = 13–15; B, n = 8–14). *P < 0.05 versus genotype Chow; †P < 0.05 versus genotype cHF; ‡P < 0.05 versus wild-type on respective diet. For the detailed fatty acid composition of triglyceride fractions in the livers of ad libitum-fed mice, see supplementary Table 4.
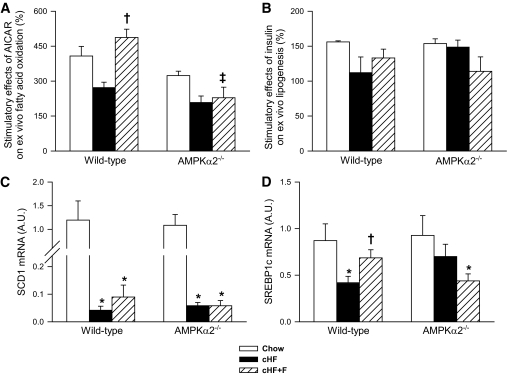
Dietary n-3 LC-PUFAs increase 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside–stimulated fatty acid oxidation and insulin-stimulated lipogenesis in cultured hepatocytes from wild-type but not from AMPKα2−/− mice.
We sought to determine whether the differential effect of n-3 LC-PUFAs on accumulation of liver triglycerides in wild-type and AMPKα2−/− mice under the clamp conditions could be explained by hepatic lipid metabolism. In cultured hepatocytes isolated from mice following the different dietary treatments, activities of both fatty acid oxidation and de novo fatty acid synthesis were evaluated. The stimulatory effects of 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), an AMPK activator, and insulin on fatty acid oxidation and synthesis are shown in Fig. 4A and B, respectively (for corresponding basal metabolic activities, see supplementary Table 3, available in an online appendix). In hepatocytes from both Chow and cHF diet-fed mice, the absence of AMPKα2 was associated with a trend for lower AICAR-stimulated fatty acid oxidation. Although hepatocytes from cHF-fed mice showed reduced stimulatory effect of AICAR irrespective of the genotype, cHF+F feeding normalized this defect in wild-type but not in AMPKα2−/− hepatocytes (Fig. 5A), suggesting AMPK-dependent induction of capacity for fatty acid oxidation by n-3 LC-PUFAs in the liver. The stimulatory effect of insulin on de novo fatty acid synthesis was reduced in hepatocytes from cHF-fed wild-type mice, whereas it was retained in the hepatocytes from cHF-fed AMPKα2−/− mice (Fig. 4B). cHF+F feeding tended to restore the stimulatory effect of insulin only in wild-type hepatocytes (Fig. 4B).
FIG. 4.
The effect of differential dietary treatment on the regulation of metabolic fluxes in the liver. AICAR-stimulated fatty acid oxidation (A) and insulin-stimulated de novo fatty acid synthesis (B) in cultured hepatocytes isolated from wild-type and AMPKα2−/− mice fed for 9 weeks either a Chow diet, cHF, or cHF+F. For basal nonstimulated rates of lipid metabolism, see supplementary Table 3. The expression of SCD-1 (C) and SREBP-1c (D) genes was quantified in total RNA isolated from the livers of mice subjected to hyperinsulinemic-euglycemic clamp following the differential dietary treatment for 9 weeks. The data are means ± SE (isolated hepatocytes, n = 3 in triplets; hepatic gene expression, n = 5–8). *P < 0.05 versus genotype Chow; †P < 0.05 versus genotype cHF; ‡P < 0.05 versus wild-type on respective diet. A.U., arbitrary units.
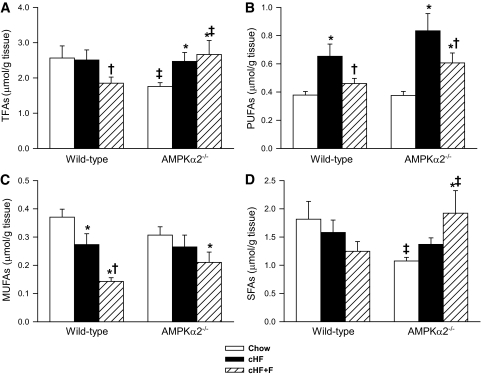
FIG. 5.
The composition of fatty acids in hepatic diacylglycerol fraction in ad libitum-fed wild-type and AMPKα2−/− mice: total fatty acids (TFAs; A), PUFAs (B), monounsaturated fatty acids (MUFAs; C), and saturated fatty acids (SFAs; D). Animals were fed either a Chow diet, cHF, or cHF+F for 9 weeks. The data are the means ± SE (n = 13–15). *P < 0.05 versus genotype Chow; †P < 0.05 versus genotype cHF; ‡P < 0.05 versus wild-type on respective diet. For the detailed fatty acid composition of diacylglycerol fractions in the livers of ad libitum fed mice, see supplementary Table 4.
To further characterize hepatic effects of differential dietary treatment, the expression of selected genes was quantified in total RNA isolated from the livers of mice subjected to hyperinsulinemic-euglycemic clamp (Fig. 4C and D). Feeding cHF diet suppressed expression of lipogenic genes stearoyl-CoA desaturase (SCD-1) and SREBP-1c in all groups (except for SREBP-1c in AMPKα2−/− mice). This suppression was partially counteracted by cHF+F diet in wild-type but not AMPKα2−/− mice (Fig. 4C and D). Together with the de novo fatty acid synthesis data, these results further support the AMPKα2-dependent improvement of liver insulin sensitivity by n-3 LC-PUFAs.
Changes in hepatic diacylglycerol levels are associated with insulin-sensitizing effects of n-3 LC-PUFAs.
To identify factors predisposing animals to insulin resistance in an AMPKα2-dependent manner, a detailed analysis of hepatic lipids in ad libitum-fed mice was performed. No major genotype-dependent differences in the contents of either ceramides or phospholipids were observed (supplementary Table 4). In contrast, hepatic content of diacylglycerols was affected in a genotype- and diet-dependent manner (Fig. 5A). Wild-type mice fed the cHF+F diet had lower diacylglycerol content than genotype-matched cHF diet-fed mice, while this effect of the cHF+F diet was not observed in AMPKα2−/− mice. Moreover, the analysis of fatty acid composition of the diacylglycerol fraction in the liver revealed that wild-type as well as AMPKα2−/− mice fed cHF diet were characterized by marked increase in the level of PUFA but not monounsaturated or saturated fatty acids (Fig. 5B, C, and D and supplementary Table 4). The increase in the PUFA content tended to be smaller in the wild-type compared with AMPKα2−/− mice (∼1.7-fold and ∼2.2-fold, respectively). Administration of n-3 LC-PUFAs completely prevented accumulation of hepatic polyunsaturated diacylglycerols in wild-type mice, whereas their level in the AMPKα2−/− animals, although decreased, was still significantly higher compared with genotype-matched Chow-fed mice (Fig. 5B). Regarding polyunsaturated diacylglycerols, α-linolenic acid (18:3n-3) appeared to be by far the most differentially regulated PUFA in the diacylglycerol fraction in the two genotypes (supplementary Table 4). In addition, cHF+F diet markedly reduced hepatic content of monounsaturated diacylglycerols in wild-type but not in knockout animals (Fig. 5C). Hepatic diacylglycerol levels and their fatty acid composition were also analyzed in mice subjected to hyperinsulinemic-euglycemic clamp (supplementary Table 5 and supplementary Fig. 3). No significant differences among the groups were observed in total diacylglycerols content or in their saturated or monounsaturated fatty acid fractions (supplementary Fig. 3).
DISCUSSION
Previous animal studies demonstrated that n-3 LC-PUFAs could counteract the development of both hepatic steatosis (8,18,36,37) and hepatic insulin resistance (8,9,16), while suppressing lipogenesis and augmenting lipid catabolism in the liver (8,13,19,21). Using mice with a whole-body deletion of AMPKα2 and high-fat feeding, we show for the first time that AMPKα2 is required for the effect of n-3 LC-PUFAs to preserve whole-body, muscle, and especially hepatic insulin sensitivity, as well as to suppress hepatic and plasma triglycerides as well as NEFA levels under hyperinsulinemic-euglycemic clamp conditions. In contrast, AMPKα2 was not required for protection by n-3 LC-PUFAs from hepatic lipid accumulation and dyslipidemia in ad libitum-fed mice.
In addition to AMPKα2, PPARα was previously identified as an important determinant of n-3 LC-PUFA's effect on lipid metabolism, especially short-term modulation of hepatic gene expression (14) and insulin sensitivity (16). Thus, the reduction in hepatic triglyceride concentrations by fish oil feeding did not rescue insulin action in PPARα-null mice, while hepatic diacylglycerol concentrations were decreased by fish oil in a PPARα-dependent manner and were associated with a preserved hepatic insulin sensitivity (16). It is generally accepted that 1) diacylglycerols rather than triglycerides or ceramides mediate hepatic insulin resistance in mice fed a high-fat diet (19,38,39), 2) diacylglycerol-induced insulin resistance depends on activation of protein kinase C, and 3) that polyunsaturated diacylglycerols in particular are better protein kinase C activators than saturated diacylglycerol species [reviewed in refs (38,39)]. Also our results showed that cHF diet-induced insulin resistance was associated primarily with the accumulation of PUFA in hepatic diacylglycerols and that n-3 LC-PUFA completely prevented cHF diet-induced increase in PUFA diacylglycerols in wild-type mice, whereas in AMPKα2−/− animals, the content of these lipids was still significantly higher compared with the control. Moreover, it was only in ad libitum-fed mice but not in mice subjected to hyperinsulinemic-euglycemic clamps that the levels of hepatic diacylglycerols and their fatty acid compositions were associated with hepatic insulin sensitivity. It is possible that under clamp conditions AMPKα2-dependent effects of n-3 LC-PUFAs on liver diacylglycerols were masked by metabolic changes occurring during a 3-h infusion of insulin and glucose.
The failure of n-3 LC-PUFAs to decrease hepatic lipids in AMPKα2−/− mice under clamp conditions could be due to primary alterations in metabolic fluxes in the liver, reflecting 1) increased de novo fatty acid synthesis, 2) decreased secretion of VLDL triglycerides, or 3) reduced fatty acid oxidation. De novo fatty acid synthesis was not the responsible factor, because hepatocytes of the n-3 LC-PUFA-fed AMPKα2−/− mice showed decreased insulin-stimulated de novo fatty acid synthesis and reduced expression of SREBP-1c and SCD-1, as compared with hepatocytes isolated from n-3 LC-PUFA-fed wild-type mice, reflecting probably low insulin sensitivity of the liver in AMPKα2−/− mice. Moreover, AICAR-stimulated fatty acid oxidation in hepatocytes from n-3 LC-PUFA-fed AMPKα2−/− mice was markedly reduced as compared with those from wild-type mice, suggesting decreased hepatic capacity for fatty acid oxidation in the absence of AMPKα2, which could contribute to enhanced lipid accumulation. Therefore, these experiments supported a major role of hepatic AMPKα2 in the regulation of both insulin sensitivity and lipid metabolism by n-3 LC-PUFAs. However, the differential modulation of lipid accumulation by n-3 LC-PUFAs in the livers of wild-type and AMPKα2−/− mice under clamp conditions could also be secondary to the AMPKα2-dependent effects of n-3 LC-PUFAs in other tissues, resulting in a relatively high hepatic uptake of circulating NEFA in AMPKα2−/− mice. This is supported by persistently elevated plasma levels of NEFA in AMPKα2−/− mice, as well as by a significant correlation between plasma NEFA levels and hepatic triglyceride content observed under clamp conditions in AMPKα2−/− but not wild-type mice fed n-3 LC-PUFA-containing diet. Moreover, it has been shown in humans with nonalcoholic fatty liver disease that most of hepatic triglycerides arise from circulating NEFA (40). That plasma NEFA levels under clamp conditions were reduced only in wild-type but not in AMPKα2−/− mice fed n-3 LC-PUFAs may reflect a role of AMPKα2 in muscle lipid uptake mediated by lipoprotein lipase (41), as well as the antilipolytic effect of AMPK in adipose tissue, documented for AMPKα1 (42). In any case, decreased fatty acid oxidation in situ in the liver and, possibly even more importantly, abundant supply of circulating NEFA could be responsible for the lack of the antisteatotic effect of n-3 LC-PUFAs in AMPKα2−/− mice under clamp conditions (Fig. 6).
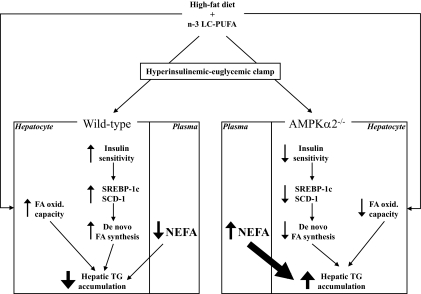
FIG. 6.
Putative involvement of AMPKα2 in antisteatotic action of n-3 LC-PUFAs in the liver. Dietary intake of n-3 LC-PUFAs increases the capacity of hepatocytes to oxidize fatty acids in wild-type (left panels) but not in AMPKα2−/− mice (right panels). When insulin and glucose levels are high, such as during hyperinsulinemic-euglycemic clamp, wild-type mice fed n-3 LC-PUFAs exhibit improved hepatic insulin sensitivity and decreased plasma levels of NEFAs as compared with high-fat diet-fed controls. This is associated with increased expression of lipogenic genes such as SREBP-1c and SCD-1 and increased drive for de novo fatty acid synthesis. Despite the elevated lipogenic drive under clamp conditions, the livers of wild-type mice fed n-3 LC-PUFAs show reduced accumulation of triglycerides. However, in AMPKα2−/− mice fed n-3 LC-PUFAs, hepatic triglyceride content is markedly elevated despite reduced rates of de novo fatty acid synthesis. This effect could be secondary to persisting elevated NEFA levels in circulation and thus better substrate availability in AMPKα2−/− mice under clamp conditions. FA, fatty acids; TG, triglycerides.
Previous studies reported contradictory results, showing either 1) activation of AMPK in rat liver (22) and murine adipose tissue (24) or 2) no changes in AMPK activity in the liver, skeletal muscle, and heart of mice (43) in response to dietary n-3 LC-PUFAs. These discrepancies could be related to differences in dietary n-3 LC-PUFA intake, nutritional state of animals, and other parameters. In accordance with the involvement of AMPKα2 in various effects of n-3 LC-PUFAs, our results document activation of AMPKα2 (but not AMPKα1) in the liver of mice by long-term n-3 LC-PUFA treatment, in the absence of significant changes in either the AMP to ATP ratio assessed in whole liver extracts [not shown and ref (44)] or the phosphorylation status of LKB1, an upstream kinase for AMPK [not shown and ref (45)]. In addition, no effect on AMPK activity in either cultured hepatocytes (21) or embryonic kidney cells (not shown) of n-3 LC-PUFAs added to the cell culture medium could be detected. Therefore, the activation of AMPKα2 by n-3 LC-PUFAs probably does not depend on a direct interaction between n-3 LC-PUFAs and AMPK. On the other hand, induction of adiponectin by n-3 LC-PUFAs [results of this study and refs (46,47)] could be involved, because adiponectin activates AMPK in both the liver and skeletal muscle (35). Adiponectin is also required for the activation of AMPK upon administration of PPARγ agonists thiazolidinediones, whereas mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to these compounds (48). Thus, absence of AMPKα2 may blunt adiponectin-mediated effects of n-3 LC-PUFAs. In accordance with the previous study (49), plasma adiponectin levels tended to be reduced in AMPKα2−/− mice. Moreover, the induction of adiponectin by n-3 LC-PUFAs in AMPKα2−/− mice was compromised (Table 1 and supplementary Figure 2).
AMPKα1 and AMPKα2 contribute equally to total AMPK activity in the liver (50). In mice with liver-specific ablation of AMPKα2 (27), hepatic AMPKα2 was essential for suppressing hepatic glucose production and maintaining fasting blood glucose levels; however, the absence of AMPKα2 did not affect inhibitory action of insulin on hepatic glucose production. In our study, although fasting blood glucose levels were unaltered by whole-body ablation of AMPKα2, the beneficial effect of dietary n-3 LC-PUFAs on hepatic insulin sensitivity was clearly AMPKα2-dependent. Differential regulation of glucose homeostasis in the above transgenic models likely reflects the complexity of whole-body (26) versus liver-specific (27) deletion of AMPKα2. In contrast to the previous report, showing induction of adiposity and adipocyte hypertrophy in AMPKα2−/− mice fed a lard-based high-fat diet (49), our study documented a relatively low weight gain, low adiposity, and smaller fat cells in AMPKα2−/− mice fed a corn-oil based high-fat diet. This discrepancy could be related to the differences in the composition of experimental high-fat diets; however, our results are consistent with the elevated sympathetic tonus of AMPKα2−/− mice (26), which may stimulate energy dissipation in these animals. In any case, lower body weight of cHF-fed AMPKα2−/− mice as compared with their wild-type counterparts could be related to better insulin sensitivity of the former mice, as suggested by the differences in insulinemia, results of hyperinsulinemic-euglycemic clamp, stimulatory effect of insulin on lipogenesis, and expression of lipogenic genes in the liver.
In conclusion, the preservation of hepatic insulin sensitivity by n-3 LC-PUFAs in mice fed a high-fat diet depends on AMPKα2. The accumulation of diacylglycerols, which is regulated in an AMPKα2-dependent manner, could contribute to the modulation of hepatic insulin sensitivity in response to dietary n-3 LC-PUFAs. On the other hand, the AMPKα2-dependent acute changes in lipid metabolism and hepatic triglyceride accumulation, which are unmasked under insulin-stimulated conditions such as during hyperinsulinemic-euglycemic clamp, largely reflect the extrahepatic action of n-3 LC-PUFAs. Our results are relevant for the development of novel strategies for prevention and treatment of the metabolic syndrome.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the Czech Science Foundation (301/10/1420) and the Ministry of Education, Youth and Sports (1M6837805002, COST BMB0602-OC08007) of the Czech Republic. Further support included grants from the European Commission (LSHM-CT-2004-005272, EXGENESIS) and from EPAX AS (Norway), as well as the research project AV0Z50110509. No other potential conflicts of interest relevant to this article were reported.
T.J. and M.R. performed experiments and wrote the manuscript. O.K., Z.M.J., D.M., V.K., M.H., and P.J. performed experiments. I.M. quantified tissue nucleotides. M.B. performed analysis of hepatic lipids and contributed to discussion. J.G. performed analysis of hepatic lipids. S.H. contributed to experiments on hepatocytes. T.E.J. contributed to analysis of muscle metabolism. P.F. introduced AMPKα2−/− mouse model to the Prague laboratory and contributed to discussion. S.H. performed experiments on kidney cells. B.V. developed AMPKα2−/− mice and contributed to discussion. J.K. wrote the manuscript.
The authors thank D. Grahame Hardie for the sheep AMPKα1 and AMPKα2 antibodies, Z. Szulc for the gift of N-palmitoyl-D-erythro-sphingosine (C17 base), and J. Jones (NMR Research Unit, Department of Biochemistry and Center for Neurosciences and Cell Biology, University of Coimbra, Coimbra, Portugal) for critical reading of the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Flachs P, Rossmeisl M, Bryhn M, Kopecky J. Cellular and molecular effects of n-3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin Sci 2009;116:1–16 [DOI] [PubMed] [Google Scholar]
- 2.Nettleton JA, Katz R. n-3 long-chain polyunsaturated fatty acids in type 2 diabetes: a review. J Am Diet Assoc 2005;105:428–440 [DOI] [PubMed] [Google Scholar]
- 3.Mori TA, Bao DQ, Burke V, Puddey IB, Watts GF, Beilin LJ. Dietary fish as a major component of a weight-loss diet: effect on serum lipids, glucose, and insulin metabolism in overweight hypertensive subjects. Am J Clin Nutr 1999;70:817–825 [DOI] [PubMed] [Google Scholar]
- 4.Couet C, Delarue J, Ritz P, Antoine JM, Lamisse F. Effect of dietary fish oil on body fat mass and basal fat oxidation in healthy adults. Int J Obes 1997;21:637–643 [DOI] [PubMed] [Google Scholar]
- 5.Ruzickova J, Rossmeisl M, Prazak T, Flachs P, Sponarova J, Veck M, Tvrzicka E, Bryhn M, Kopecky J. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids 2004;39:1177–1185 [DOI] [PubMed] [Google Scholar]
- 6.Flachs P, Horakova O, Brauner P, Rossmeisl M, Pecina P, Franssen-van Hal N, Ruzickova J, Sponarova J, Drahota Z, Vlcek C, Keijer J, Houstek J, Kopecky J. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce beta-oxidation in white fat. Diabetologia 2005;48:2365–2375 [DOI] [PubMed] [Google Scholar]
- 7.Ikemoto S, Takahashi M, Tsunoda N, Maruyama K, Itakura H, Ezaki O. High-fat diet-induced hyperglycemia and obesity in mice: differential effects of dietary oils. Metabolism 1996;45:1539–1546 [DOI] [PubMed] [Google Scholar]
- 8.Kuda O, Jelenik T, Jilkova Z, Flachs P, Rossmeisl M, Hensler M, Kazdova L, Ogston N, Baranowski M, Gorski J, Janovska P, Kus V, Polak J, Mohamed-Ali V, Burcelin R, Cinti S, Bryhn M, Kopecky J. n-3 fatty acids and rosiglitazone improve insulin sensitivity through additive stimulatory effects on muscle glycogen synthesis in mice fed a high-fat diet. Diabetologia 2009;52:941–951 [DOI] [PubMed] [Google Scholar]
- 9.Storlien LH, Kraegen EW, Chisholm DJ, Ford GL, Bruce DG, Pascoe WS. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science 1987;237:885–888 [DOI] [PubMed] [Google Scholar]
- 10.Jucker BM, Cline GW, Barucci N, Shulman GI. Differential effects of safflower oil versus fish oil feeding on insulin-stimulated glycogen synthesis, glycolysis, and pyruvate dehydrogenase flux in skeletal muscle: a 13C nuclear magnetic resonance study. Diabetes 1999;48:134–140 [DOI] [PubMed] [Google Scholar]
- 11.Fasching P, Ratheiser K, Waldhäusl W, Rohac M, Osterrode W, Nowotny P, Vierhapper H. Metabolic effects of fish-oil supplementation in patients with impaired glucose tolerance. Diabetes 1991;40:583–589 [DOI] [PubMed] [Google Scholar]
- 12.Pelikánová T, Kohout M, Válek J, Kazdová L, Base J. Metabolic effects of omega-3 fatty acids in type 2 (non-insulin-dependent) diabetic patients. Ann N Y Acad Sci 1993;683:272–278 [DOI] [PubMed] [Google Scholar]
- 13.Teran-Garcia M, Adamson AW, Yu G, Rufo C, Suchankova G, Dreesen TD, Tekle M, Clarke SD, Gettys TW. Polyunsaturated fatty acid suppression of fatty acid synthase (FASN): evidence for dietary modulation of NF-Y binding to the Fasn promoter by SREBP-1c. Biochem J 2007;402:591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanderson LM, de Groot PJ, Hooiveld GJ, Koppen A, Kalkhoven E, Müller M, Kersten S. Effect of synthetic dietary triglycerides: a novel research paradigm for nutrigenomics. PLoS One 2008;3:e1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Schothorst EM, Flachs P, Franssen-van Hal NL, Kuda O, Bunschoten A, Molthoff J, Vink C, Hooiveld GJ, Kopecky J, Keijer J. Induction of lipid oxidation by polyunsaturated fatty acids of marine origin in small intestine of mice fed a high-fat diet. BMC Genomics 2009;10:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neschen S, Morino K, Dong J, Wang-Fischer Y, Cline GW, Romanelli AJ, Rossbacher JC, Moore IK, Regittnig W, Munoz DS, Kim JH, Shulman GI. n-3 Fatty acids preserve insulin sensitivity in vivo in a peroxisome proliferator-activated receptor-alpha-dependent manner. Diabetes 2007;56:1034–1041 [DOI] [PubMed] [Google Scholar]
- 17.Serhan CN. Novel omega-3-derived local mediators in anti-inflammation and resolution. Pharmacol Ther 2005;105:7–21 [DOI] [PubMed] [Google Scholar]
- 18.González-Périz A, Horrillo R, Ferré N, Gronert K, Dong B, Morán-Salvador E, Titos E, Martínez-Clemente M, López-Parra M, Arroyo V, Clària J. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J 2009;23:1946–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jump DB. Fatty acid regulation of gene transcription. Crit Rev Clin Lab Sci 2004;41:41–78 [DOI] [PubMed] [Google Scholar]
- 20.Neschen S, Morino K, Rossbacher JC, Pongratz RL, Cline GW, Sono S, Gillum M, Shulman GI. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-gamma-dependent mechanism in mice. Diabetes 2006;55:924–928 [DOI] [PubMed] [Google Scholar]
- 21.Dentin R, Benhamed F, Pégorier JP, Foufelle F, Viollet B, Vaulont S, Girard J, Postic C. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J Clin Invest 2005;115:2843–2854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suchankova G, Tekle M, Saha AK, Ruderman NB, Clarke SD, Gettys TW. Dietary polyunsaturated fatty acids enhance hepatic AMP-activated protein kinase activity in rats. Biochem Biophys Res Commun 2005;326:851–858 [DOI] [PubMed] [Google Scholar]
- 23.Gabler NK, Radcliffe JS, Spencer JD, Webel DM, Spurlock ME. Feeding long-chain n-3 polyunsaturated fatty acids during gestation increases intestinal glucose absorption potentially via the acute activation of AMPK. J Nutr Biochem 2009;20:17–25 [DOI] [PubMed] [Google Scholar]
- 24.Kopecky J, Rossmeisl M, Flachs P, Kuda O, Brauner P, Jilkova Z, Stankova B, Tvrzicka E, Bryhn M. n-3 PUFA: bioavailability and modulation of adipose tissue function. Proc Nutr Soc 2009;68:361–369 [DOI] [PubMed] [Google Scholar]
- 25.Carling D. The AMP-activated protein kinase cascade–a unifying system for energy control. Trends Biochem Sci 2004;29:18–24 [DOI] [PubMed] [Google Scholar]
- 26.Viollet B, Andreelli F, Jørgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest 2003;111:91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andreelli F, Foretz M, Knauf C, Cani PD, Perrin C, Iglesias MA, Pillot B, Bado A, Tronche F, Mithieux G, Vaulont S, Burcelin R, Viollet B. Liver adenosine monophosphate-activated kinase-alpha2 catalytic subunit is a key target for the control of hepatic glucose production by adiponectin and leptin but not insulin. Endocrinology 2006;147:2432–2441 [DOI] [PubMed] [Google Scholar]
- 28.Foretz M, Ancellin N, Andreelli F, Saintillan Y, Grondin P, Kahn A, Thorens B, Vaulont S, Viollet B. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes 2005;54:1331–1339 [DOI] [PubMed] [Google Scholar]
- 29.Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab 2009;9:407–416 [DOI] [PubMed] [Google Scholar]
- 30.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005;1:15–25 [DOI] [PubMed] [Google Scholar]
- 31.Polak J, Kovacova Z, Jacek M, Klimcakova E, Kovacikova M, Vitkova M, Kuda O, Sebela M, Samcova E, Stich V. An increase in plasma adiponectin multimeric complexes follows hypocaloric diet-induced weight loss in obese and overweight pre-menopausal women. Clin Sci (Lond) 2007;112:557–565 [DOI] [PubMed] [Google Scholar]
- 32.Hardie DG, Salt IP, Davies SP. Analysis of the role of the AMP-activated protein kinase in the response to cellular stress. Methods Mol Biol 2000;99:63–74 [DOI] [PubMed] [Google Scholar]
- 33.Burcelin R, Crivelli V, Dacosta A, Roy-Tirelli A, Thorens B. Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. Am J Physiol Endocrinol Metab 2002;282:E834–E842 [DOI] [PubMed] [Google Scholar]
- 34.Foretz M, Guichard C, Ferré P, Foufelle F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc Natl Acad Sci U S A 1999;96:12737–12742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 2002;8:1288–1295 [DOI] [PubMed] [Google Scholar]
- 36.Kajikawa S, Harada T, Kawashima A, Imada K, Mizuguchi K. Highly purified eicosapentaenoic acid prevents the progression of hepatic steatosis by repressing monounsaturated fatty acid synthesis in high-fat/high-sucrose diet-fed mice. Prostaglandins Leukot Essent Fatty Acids 2009;80(4):229–238 [DOI] [PubMed] [Google Scholar]
- 37.Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab 2006;4:107–110 [DOI] [PubMed] [Google Scholar]
- 38.Wakelam MJO. Diacylglycerol—when is it an intracellular messenger? BBA-Mol Cell Biol Lipids 1998;1436:117–126 [DOI] [PubMed] [Google Scholar]
- 39.Schmitz-Peiffer C, Biden TJ. Protein kinase C function in muscle, liver, and beta-cells and its therapeutic implications for type 2 diabetes. Diabetes 2008;57:1774–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohira M, Miyashita Y, Murano T, Watanabe F, Shirai K. Metformin promotes induction of lipoprotein lipase in skeletal muscle through activation of adenosine monophosphate-activated protein kinase. Metabolism 2009;58:1408–1414 [DOI] [PubMed] [Google Scholar]
- 42.Daval M, Diot-Dupuy F, Bazin R, Hainault I, Viollet B, Vaulont S, Hajduch E, Ferré P, Foufelle F. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem 2005;280:25250–25257 [DOI] [PubMed] [Google Scholar]
- 43.Dobrzyn A, Dobrzyn P, Miyazaki M, Ntambi JM. Polyunsaturated fatty acids do not activate AMP-activated protein kinase in mouse tissues. Biochem Biophys Res Commun 2005;332:892–896 [DOI] [PubMed] [Google Scholar]
- 44.Salati LM, Clarke SD. Fatty acid inhibition of hormonal induction of acetyl-coenzyme A carboxylase in hepatocyte monolayers. Arch Biochem Biophys 1986;246:82–89 [DOI] [PubMed] [Google Scholar]
- 45.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol 2003;13:2004–2008 [DOI] [PubMed] [Google Scholar]
- 46.Flachs P, Mohamed-Ali V, Horakova O, Rossmeisl M, Hosseinzadeh-Attar MJ, Hensler M, Ruzickova J, Kopecky J. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia 2006;49:394–397 [DOI] [PubMed] [Google Scholar]
- 47.Itoh M, Suganami T, Satoh N, Tanimoto-Koyama K, Yuan X, Tanaka M, Kawano H, Yano T, Aoe S, Takeya M, Shimatsu A, Kuzuya H, Kamei Y, Ogawa Y. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol 2007;27:1918–1925 [DOI] [PubMed] [Google Scholar]
- 48.Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem 2006;281:2654–2660 [DOI] [PubMed] [Google Scholar]
- 49.Villena JA, Viollet B, Andreelli F, Kahn A, Vaulont S, Sul HS. Induced adiposity and adipocyte hypertrophy in mice lacking the AMP-activated protein kinase-alpha2 subunit. Diabetes 2004;53:2242–2249 [DOI] [PubMed] [Google Scholar]
- 50.Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J 2000;346:659–669 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.