Abstract
OBJECTIVE
Skeletal muscle protein metabolism is resistant to the anabolic action of insulin in healthy, nondiabetic older adults. This defect is associated with impaired insulin-induced vasodilation and mTORC1 signaling. We hypothesized that, in older subjects, pharmacological restoration of insulin-induced capillary recruitment would improve the response of muscle protein synthesis and anabolism to insulin.
RESEARCH DESIGN AND METHODS
Twelve healthy, nondiabetic older subjects (71 ± 2 years) were randomized to two groups. Subjects were studied at baseline and during local infusion in one leg of insulin alone (Control) or insulin plus sodium nitroprusside (SNP) at variable rate to double leg blood flow. We measured leg blood flow by dye dilution; muscle microvascular perfusion with contrast enhanced ultrasound; Akt/mTORC1 signaling by Western blotting; and muscle protein synthesis, amino acid, and glucose kinetics using stable isotope methodologies.
RESULTS
There were no baseline differences between groups. Blood flow, muscle perfusion, phenylalanine delivery to the leg, and intracellular availability of phenylalanine increased significantly (P < 0.05) in SNP only. Akt phosphorylation increased in both groups but increased more in SNP (P < 0.05). Muscle protein synthesis and net balance (nmol · min−1 · 100 ml · leg−1) increased significantly (P < 0.05) in SNP (synthesis, 43 ± 6 to 129 ± 25; net balance, −16 ± 3 to 26 ± 12) but not in Control (synthesis, 41 ± 10 to 53 ± 8; net balance, −17 ± 3 to −2 ± 3).
CONCLUSIONS
Pharmacological enhancement of muscle perfusion and amino acid availability during hyperinsulinemia improves the muscle protein anabolic effect of insulin in older adults.
Recent studies have highlighted that insulin resistance is one of the potential mechanisms underlying the involuntary loss of skeletal muscle mass, strength, and function with aging (sarcopenia) (1–3). Specifically, it has been shown that, while physiological hyperinsulinemia stimulates skeletal muscle protein synthesis and overall anabolism in young individuals (4–8), in older adults this response is blunted (3,9) and can be elicited only by unphysiologically high insulin concentrations (9), or after an acute bout of aerobic exercise preceding hyperinsulinemia (1). Interestingly, this age-related insulin resistance of skeletal muscle proteins occurs in nondiabetic older individuals with normal glucose tolerance and muscle glucose metabolism. The only other abnormality apparently associated with this age-related protein anabolic resistance to insulin is endothelial dysfunction. It has been shown that the physiological insulin-stimulated increase in endothelial-dependent vasodilation is blunted in healthy older adults (3,10), and we recently found that this defect is highly correlated with the impaired response of muscle proteins to the anabolic effect of insulin (1,3,9).
Insulin increases muscle perfusion by inducing a nitric oxide-dependent vasodilation of the precapillary arterioles in skeletal muscle (11,12). Insulin-induced vasodilation may or may not be followed by increases in total blood flow but is accompanied by capillary recruitment resulting in an increased and more homogenous tissue perfusion (13–15). This mechanism allows for a greater and more diffuse capillary exchange of substrates and hormones during hyperinsulinemia in skeletal muscle. Moreover, we recently found that insulin-stimulated changes in protein synthesis are dependent on changes in blood flow and amino acid delivery to the muscle but not to changes in arterial amino acid concentration or insulin concentration (3,7,16). Thus, we hypothesize that the absence of change in muscle protein synthesis during hyperinsulinemia in older adults may be a consequence of unresponsiveness of the vascular bed to insulin. This, in turn, could hamper the physiological increase in muscle perfusion and nutrient flow to the muscle cells and blunt the insulin-stimulated increase in mammalian target of rapamycin (mTOR) signaling and muscle protein synthesis.
In this study, the purpose was to determine whether a restoration of nitric oxide dependent vasodilation during hyperinsulinemia would increase skeletal muscle protein synthesis and net balance in older adults. To test this hypothesis, we compared in older subjects the response of leg muscle protein synthesis and net balance to local insulin infusion alone or with the concomitant infusion of the nitric oxide donor sodium nitroprusside at variable rates, to mimic the physiological insulin-induced increase in blood flow across the leg observed in younger adults (3,7,16).
RESEARCH DESIGN AND METHODS
Subject characteristics.
The study was approved by the Institutional Review Board of the University of Texas Medical Branch (UTMB) and the Food and Drug Administration. All subjects gave written, informed consent before participation in the experiments. Twelve healthy, independent, but sedentary older volunteers were recruited through the UTMB Claude D. Pepper Older Americans Independence Center Volunteer Registry (Table 1). Eligibility of the volunteers was determined by clinical history, a physical examination, and several laboratory tests including a 2-h, 75-g oral glucose tolerance test. Only subjects with screening results within normal limits and a stable body weight for at least three months were included. Subjects were randomly assigned to a control group receiving insulin alone (Control) or an experimental group receiving insulin with sodium nitroprusside (SNP). To verify that the older subjects enrolled in this study were insulin resistant with regards to muscle perfusion and protein synthesis, data from a group of healthy younger subjects (n = 7, age 32 ± 2 years) undergoing the same insulin infusion protocol as the Control group are also reported in Fig. 3. The complete dataset for these younger subjects has been recently published elsewhere (16).
TABLE 1.
Subject characteristics (n = 12)
| Control | SNP | |
|---|---|---|
| n | 6 (5 M, 1 F) | 6 (4 M, 2 F) |
| Age (years) | 74 ± 3 | 69 ± 1 |
| Weight (kg) | 74.85 ± 5.18 | 73.53 ± 6.88 |
| Height (m) | 1.68 ± 0.05 | 1.71 ± 0.05 |
| BMI (kg/m2) | 26.5 ± 2.0 | 24.8 ± 1.6 |
| Leg volume (ml) | 9,489 ± 801 | 9,122 ± 727 |
Data are mean ± SE.
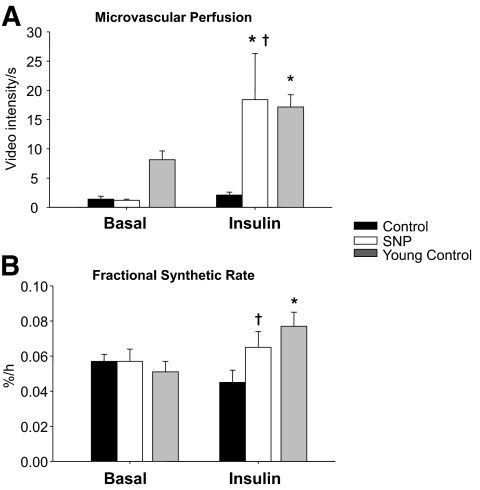
FIG. 3.
Microvascular blood perfusion (A) measured by CEU and fractional synthetic rate (B) in the basal state and during local insulin infusion with (SNP) or without (Control) concomitant infusion with the vasodilator, sodium nitroprusside. To provide normal reference values, previously published data (16) from a group of younger individuals (n = 7, age = 32 ± 2) undergoing the same insulin infusion protocol as the Control group are also shown. Data are the mean ± SE. *P < 0.05 versus baseline, †P < 0.05 versus Control.
Study design.
All subjects were instructed to eat their normal diets and refrain from strenuous physical activity for the week before participating in the study. The evening before the study, the subjects were admitted to the UTMB Clinical Research Center. They were given a standardized dinner (10 kcal/kg of body wt; 60% carbohydrate, 20% fat, and 20% protein) and a snack at 11:00 P.M., after which they were allowed only water until the end of the experiment the next day. The next morning, a polyethylene catheter was inserted into a forearm vein for stable isotope tracer (Isotec Inc., Sigma-Aldrich, Miamisburg, OH) and dextrose infusion. A retrograde catheter was placed in the contralateral hand and kept at ∼60°C to sample arterialized blood so as to measure systemic insulin and indocyanine green (ICG) concentrations. Using aseptic procedure and local anesthesia, catheters were placed retrogradely into the common femoral artery and vein of one leg for blood sampling. The arterial catheter was also used for the infusion of ICG, insulin, and sodium nitroprusside (SNP group only). After collecting a background blood sample, a primed-continuous infusion of l-[ring-13C6]phenylalanine (priming dose 2 μmol/kg, infusion rate 0.05 μmol · kg−1 · min−1) and d-[6,6-2H2]glucose (priming dose 19 μmol/kg, infusion rate 0.22 μmol · kg−1 · min−1) were started to measure phenylalanine and glucose kinetics (Fig. 1). After 4 h, an insulin infusion (0.15 mU · min−1 · 100 ml of leg−1) was started directly into the femoral artery to increase leg insulin concentrations to postprandial values while avoiding excessive systemic hyperinsulinemia and consequent hypoaminoacidemia (6,7,9). During insulin infusion, arterialized blood glucose concentrations were measured frequently (every 5–10 min), and 20% dextrose containing 2% d-[6,6-2H2]glucose was infused in the forearm catheter at a variable rate to maintain basal values of arterial blood glucose. To reproduce the insulin-induced vasodilation observed in young healthy adults (3,7,16), subjects in the SNP group also received an infusion of sodium nitroprusside administered at variable rate, starting at 0.01 μg · min−1 · 100 ml of leg−1 (average dose, 0.06 ± 0.01 μg · min−1 · 100 ml of leg−1), to double leg blood flow as determined by frequent Doppler ultrasound measurements of the superficial femoral artery (HDI-5000 ultrasound system, Philips ATL Ultrasound, Andover, MA). Systemic blood pressure was also frequently monitored during SNP infusion using an arm cuff. At 2.5–3 h (basal) and 5.5–6 h (insulin infusion), ICG dye (0.5 mg/ml) was infused into the common femoral artery and four blood samples taken from the common femoral vein and the hand vein to measure blood flow in the entire leg. After ICG, Perflutren Lipid Microsphere (Definity, Lantheus Medical Imaging, N. Billerica, MA) was infused in the wrist vein to measure muscle perfusion of the vastus lateralis, after which four additional blood samples were obtained from the femoral artery and vein to measure the concentrations of amino acids, glucose, insulin, and the free phenylalanine and glucose enrichments. Biopsies of the lateral aspect of the vastus lateralis muscle of the leg bearing the femoral catheters were taken at 2, 4, 5.5, and 7 h after starting the tracer infusion using aseptic procedure, local anesthesia, and a 5 mm Bergström needle. The first two biopsies were taken from the same incision at a different angle to sample areas at least 5 cm apart. The third and fourth biopsies were taken from a new incision about 10 cm apart from the first, using the same technique used for the first two biopsies. The tissue was immediately rinsed with ice-cold saline, blotted, frozen in liquid nitrogen, and stored at −80°C until analyzed. After the fourth biopsy, all infusions were stopped, the catheters were removed, and the subjects were fed and discharged after a 2-h observation period.
FIG. 1.
Study design. Blood and muscle samples are indicated by arrows. ICG indicates indocyanine green infusion for blood flow measurement. CEU indicates contrast enhanced ultrasound measurement of muscle perfusion using Definity microspheres.
Analytical methods.
Plasma glucose concentrations were measured using an automated glucose analyzer (Yellow Springs Instrument Co., Yellow Springs, OH). Insulin concentrations were measured by sandwich enzyme-linked immunosorbent assay (Linco, St. Charles, MS) using a microplate reader (Bio-Rad, Hercules, CA). Serum ICG concentrations in the femoral vein and hand vein were determined spectrophotometrically (Beckman Coulter, Fullerton, CA) at λ = 805, allowing for the calculation of blood flow as previously described (17,18).
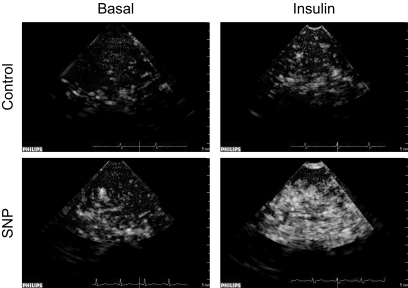
Muscle perfusion was measured at basal and during hyperinsulinemia using contrast-enhanced ultrasound (CEU) as described by others (19–22). Briefly, ultrasound imaging of the vastus lateralis muscle was performed in a transaxial plane ∼15–20 cm above the patella over the midportion of the vastus lateralis muscle using a P4-2 phased array transducer. A suspension of perflutren lipid microspheres (Definity) was infused intravenously (3.5 ml/min for 8 min) using a mechanical index of 1.3 and a compression of 80%. Once the systemic microbubble concentrations reached steady state (∼2 min), background images were obtained at a frame rate of 1/s. Intermittent imaging was then performed using an internal timer at pulsing intervals ranging from 1 to 25 s, allowing for progressively greater replenishment of the ultrasound beam elevation between destructive pulses. Depth, focus, and gain were optimized at the beginning of each experiment and held constant throughout. Data were recorded on a magneto optical disk and digitized for analysis using an offline system. A minimum of three images were acquired at each pulsing interval. The background-subtracted video intensity at each pulsing interval was measured from a region of interest within the vastus lateralis muscle. Pulsing interval versus video intensity data were curve fitted to the function: y = A(1 – e−βt) where y is the video intensity at pulsing interval t, A is the plateau video intensity (an index of microvascular blood volume), and β is the rate of microvascular refilling (an indicator of microvascular flow velocity) (19). Representative CEU images are shown in Fig. 2.
FIG. 2.
Representative contrast enhanced ultrasound images of Definity microsphere infusion into the vastus lateralis of one control and one SNP subject during the basal period and during insulin infusion with or without concomitant SNP infusion. Each image is captured during the 10-s pulsing interval.
Free phenylalanine enrichment and 13C6 concentration in blood and tissue fluid were measured by gas chromatography-mass spectrometry (GCMS, 6,890 Plus GC, 5973N MSD, 7,683 autosampler, Agilent Technologies, Palo Alto, CA) after addition of an internal standard (L-15N-phenylalanine), extraction, purification, and tert-butyldimethylsilyl derivatization (23). 2H2-glucose enrichment was measured by GCMS after extraction by ion-exchange chromatography and pentaacetate derivatization (23). Muscle tissue samples were mechanically homogenized, and intracellular free amino acids and muscle proteins were extracted as described previously (23). The incorporation of labeled phenylalanine into the mixed muscle proteins was measured by GCMS, after protein hydrolysis and amino acid extraction, using the external standard curve approach (24).
Muscle tissue samples were also used to measure phosphorylated and total protein expression of mTOR, Akt/PKB, 4E-BP1, S6K1, ERK1/2, and rpS6. The primary phospho-antibodies (Cell Signaling, Beverly, MA) used were phospho-mTOR (Ser 2,448, 1:1,000 in 5% nonfat dairy milk), phospho-Akt (Ser 473, 1:1,000), phospho-4E-BP1 (Thr 37/46, 1:1,000), phospho-ERK1/2 (Thr202/Tyr204, 1:1,000), phospho-p70 S6K1 (Thr 389, 1:500), phospho-eEF2 (Thr 56, 1:2000), and phospho-rpS6 (Ser 235/236, 1:500). A dilution of 1:1,000 was used for total expression of each protein. Fifty micrograms of total protein homogenate was loaded in duplicate into each lane, and the samples were separated on a 7.5% or 15% polyacrylamide gel (150 V, 1 h) (Criterion, Bio-Rad, Hercules, CA), depending on the size of the target protein. Specific details of our immunoblotting technique have been reported elsewhere (25). All samples were normalized to a rodent internal loading control, and final data were reported as phosphorylated protein relative to total protein.
Calculations.
The kinetics of intracellular free phenylalanine in response to exogenous hyperinsulinemia and pharmacological enhancement of muscle perfusion were calculated using two-pool, three-pool, and precursor-product models. With the two-pool model, phenylalanine enrichments and concentrations in the femoral artery and vein were measured to estimate muscle protein synthesis, breakdown, and net balance without any consideration of the intracellular recycling of amino acids from breakdown to synthesis (26). The three-pool model also included intracellular amino acid kinetics. A limitation of this methodology is that the kinetic parameters thus derived are blood flow dependent. For this reason, we also measured the fractional synthetic rate of mixed muscle proteins using the precursor-product model (27).
Leg glucose utilization was calculated as the net glucose uptake and fractional extraction across the leg (23); basal whole-body endogenous glucose production and utilization were calculated using the single-pool model (23). During clamp, endogenous glucose production was calculated by subtracting the exogenous glucose infusion rate from basal whole-body endogenous glucose production and utilization. Insulin delivery to the leg was calculated as the product of femoral insulin concentration by blood flow (16).
Details on the calculations, including formulas, are reported in the online appendix available at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0415/DC1.
Statistical analyses.
Statistical analyses were performed using the statistical software SigmaStat version 3.5 (Systat Software Inc., San Jose, CA). The primary end points were measures of blood flow, muscle perfusion, muscle protein synthesis, and net muscle phenylalanine balance. Secondary end points were all remaining measures of muscle amino acid turnover, glucose kinetics, and phosphorylation of intracellular signals. Subjects' characteristics were analyzed using one-way ANOVA with the exception of sex, a categorical variable, which was analyzed using the Fisher exact test. To determine the effects of vasodilation on the response of muscle protein turnover to insulin in aging, comparisons were carried out using ANOVA for repeated measures, with the factors being subject, group (Control, SNP), and time (basal, insulin). Post hoc comparisons were carried out using the Tukey-Kramer test. For data that did not pass the normality test, the values were transformed using natural log (ln) or the reciprocal of the value. All values are listed as means ± SEM. Differences were considered significant at P < 0.05.
RESULTS
Blood flow, blood pressure, and microvascular perfusion.
Basal leg blood flow, systemic blood pressure (Table 2), and microvascular perfusion (Fig. 3) did not differ between groups. During insulin infusion, blood flow and microvascular perfusion increased significantly only in the SNP group (P < 0.05), with a significant difference between groups (P < 0.05). Conversely, systemic blood pressure did not change significantly in either group.
TABLE 2.
Leg blood flow, systemic blood pressure, leg and systemic insulin and glucose concentrations, and kinetics in two groups of healthy older subjects in the basal state and during local insulin infusion in one leg with (SNP) or without (Control) concomitant infusion of sodium nitroprusside
| Control |
SNP |
|||
|---|---|---|---|---|
| Basal | Insulin | Basal | Insulin | |
| Leg blood flow (ml · min−1 · 100 ml leg−1) | 2.6 ± 0.5 | 3.4 ± 0.6 | 2.4 ± 0.5 | 5.6 ± 0.7*,† |
| Systemic blood pressure | ||||
| Systolic (mmHg) | 132 ± 4 | 126 ± 5 | 128 ± 5 | 126 ± 6 |
| Diastolic (mmHg) | 67 ± 3 | 67 ± 3 | 71 ± 5 | 69 ± 6 |
| Insulin | ||||
| Systemic concentration (pmol/l) | 32 ± 6 | 87 ± 19* | 39 ± 7 | 101 ± 34* |
| Femoral vein concentration (pmol/l) | 39 ± 8 | 394 ± 124* | 36 ± 7 | 290 ± 55* |
| Delivery to the leg (μU · min−1 · 100 ml leg−1) | 15 ± 2 | 173 ± 20* | 15 ± 4 | 270 ± 50*,† |
| Glucose | ||||
| Arterial concentration (mmol/l) | 5.2 ± 0.2 | 5.3 ± 0.2 | 5.2 ± 0.1 | 5.3 ± 0.2 |
| Leg uptake (μmol · min−1 · 100 ml leg−1) | 0.4 ± 0.2 | 3.3 ± 1.5* | 0.5 ± 0.1 | 1.9 ± 0.4* |
| Leg fractional extraction (%) | 3.0 ± 2.5○ | 14.4 ± 7.1*,○ | 3.5 ± 1.1 | 6.5 ± 1.3* |
| Endogenous production (μmol · kg−1 · min−1) | 8.6 ± 0.4 | 6.7 ± 0.4* | 9.1 ± 0.2 | 6.5 ± 0.2* |
| Whole-body uptake (μmol · kg−1 · min−1) | 8.6 ± 0.4 | 13.5 ± 0.7* | 9.1 ± 0.2 | 13.7 ± 0.9* |
Date are mean ± SE.
*P < 0.05 vs. basal,
† indicates group × time effect (P < 0.05). ○ values excluding a single outlier: basal 3.7 ± 2.9; insulin 7.4 ± 1.3.
Insulin and glucose kinetics.
Insulin and glucose concentrations and kinetics are presented in Table 2. There were no basal differences between the two groups. During hyperinsulinemic-euglycemic clamp, systemic and femoral insulin concentrations increased significantly in both groups (P < 0.05), with no significant differences between groups. Insulin delivery to the leg increased with insulin infusion in both groups (P < 0.05) but was significantly higher in the SNP group (P < 0.05). Arterial glucose concentration was successfully maintained at basal levels in both groups. Leg and whole-body glucose uptake increased significantly (P < 0.05) and endogenous glucose production decreased (P < 0.05) from basal levels in both groups, with no differences between groups. Leg glucose fractional extraction increased in both groups with hyperinsulinemia (P < 0.05) and was somewhat higher in the Control group without reaching statistical significance. This was due to high variability attributable to a single outlier in the Control group. Statistical analysis performed after excluding the outlier confirmed the lack of differences between groups in response to insulin.
Phenylalanine concentrations, enrichments, and kinetics.
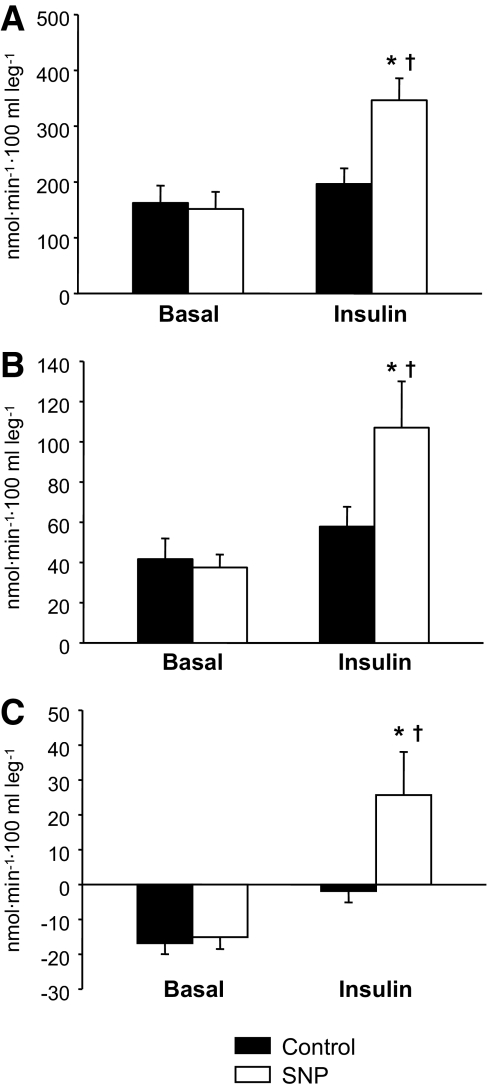
There were no basal differences between the groups for any concentration, enrichment, and kinetic parameter (Table 3, Fig. 4). During insulin infusion, phenylalanine arterial concentrations did not change in either group. Venous and muscle phenylalanine concentrations decreased in both Control and SNP, with no group differences. Phenylalanine enrichment in the artery, vein, and muscle increased slightly, yet significantly, in both Control and SNP groups (P < 0.05) with no group differences. Phenylalanine delivery to the leg, output from the leg, net balance, leg rate of disappearance, and utilization for protein synthesis increased significantly in the SNP group only (P < 0.05) and were significantly higher than in the Control group during hyperinsulinemia (P < 0.05). Phenylalanine transport into and out of the muscle increased significantly from the basal level only in the SNP group (P < 0.05). Muscle protein fractional synthetic rate was not different between groups at baseline but was significantly higher in the SNP group during insulin infusion (Fig. 3B). The leg phenylalanine rate of appearance did not significantly change in either group. However, phenylalanine release from proteolysis increased significantly in the SNP group only (P < 0.05). Similarly, insulin infusion significantly increased phenylalanine recycling from protein breakdown to synthesis (without appearing in circulation) only in the SNP group (P < 0.05). Intracellular phenylalanine availability tended to increase only in the SNP group as compared with Control (P = 0.10).
TABLE 3.
Leg free phenylalanine concentrations, enrichments, and kinetics in two groups of healthy older subjects at baseline and during local insulin infusion in one leg with (SNP) or without (Control) concomitant infusion of the vasodilator sodium nitroprusside
| Control |
SNP |
|||
|---|---|---|---|---|
| Basal | Insulin | Basal | Insulin | |
| Phenylalanine concentration (μmol/l) | ||||
| Femoral artery | 63 ± 3 | 61 ± 4 | 62 ± 3 | 63 ± 3 |
| Femoral vein | 70 ± 3 | 62 ± 4* | 68 ± 4 | 59 ± 4* |
| Muscle | 80 ± 10 | 65 ± 10* | 79 ± 4 | 67 ± 4* |
| Phenylalanine enrichment (tracer/tracee, %) | ||||
| Femoral artery | 8.9 ± 0.5 | 9.9 ± 0.2* | 9.1 ± 0.4 | 10.0 ± 0.1* |
| Femoral vein | 6.6 ± 0.4 | 7.7 ± 0.2* | 6.9 ± 0.3 | 8.4 ± 0.2* |
| Muscle | 5.6 ± 0.2 | 6.9 ± 0.5* | 5.8 ± 0.3 | 7.2 ± 0.6* |
| Phenylalanine kinetics (nmol · min−1 · 100 ml leg−1) | ||||
| Net balance | −17 ± 3 | −2 ± 3 | −15 ± 3 | 26 ± 12*,† |
| Delivery to the leg | 162 ± 31 | 196 ± 28 | 151 ± 31 | 346 ± 39*,† |
| Output from the leg | 179 ± 32 | 198 ± 27 | 166 ± 34 | 321 ± 33*,† |
| Leg rate of appearance | 53 ± 10 | 54 ± 7 | 47 ± 9 | 69 ± 13 |
| Leg rate of disappearance | 36 ± 9 | 52 ± 9 | 32 ± 6 | 94 ± 23*,† |
| Transport into the muscle | 115 ± 28 | 168 ± 37 | 103 ± 23 | 225 ± 46* |
| Transport out of the muscle | 132 ± 28 | 170 ± 35 | 118 ± 26 | 200 ± 38* |
| Release from proteolysis | 58 ± 12 | 60 ± 8 | 53 ± 9 | 81 ± 12* |
| Utilization for protein synthesis | 42 ± 10 | 58 ± 10 | 38 ± 6 | 107 ± 23*,† |
| Intracellular availability | 173 ± 36 | 227 ± 40 | 156 ± 31 | 307 ± 58* |
| Recycling | 6 ± 2 | 6 ± 5 | 6 ± 2 | 13 ± 2* |
Data are mean ± SE.
*P < 0.05 vs. basal;
† indicates group × time effect (P < 0.05).
FIG. 4.
Phenylalanine delivery to the leg (A), phenylalanine utilization for protein synthesis (B), and phenylalanine net balance (C) measured in the basal state and during local insulin infusion with (SNP) or without (Control) concomitant infusion with the vasodilator, sodium nitroprusside. Data are the mean ± SE. *P < 0.05 versus baseline, †P < 0.05 versus Control.
Cell signaling.
Phosphorylation of all measured cell signals did not differ between groups in the basal state (Fig. 5). During insulin infusion, Akt/PKB phosphorylation increased in both groups (P < 0.05), but more in SNP (P < 0.05) at the end of the infusion (biopsy 4). Phosphorylation of mTOR and S6K1 increased in both groups (P < 0.05) with no differences between groups. 4E-BP1 phosphorylation increased with insulin (P < 0.05) in both groups with no differences between groups at mid-infusion (biopsy 3), but it remained significantly elevated only in SNP (P < 0.05) at the end of the infusion (biopsy 4). Phosphorylation of ERK1/2, eEF2, and rpS6 did not change significantly during hyperinsulinemia in either group (data not shown). There were no changes in total protein expression for the measured proteins.
FIG. 5.
Phosphorylation of Akt (A), mTOR (B), S6K1 (C), and 4E-BP1 (D) in the skeletal muscle of two groups of healthy elderly subjects in the basal state and during local insulin infusion (biopsy 3, Bx 3; and biopsy 4, Bx 4) in one leg with (SNP) or without (Control) concomitant infusion of the vasodilator sodium nitroprusside. Representative phosphorylated (e.g., P-Protein) and total protein blots for each panel are from a single control and SNP subject. The basal, Bx3, and Bx4 samples were run on the same gel for each subject. Data are the mean ± SE. *P < 0.05 versus baseline, †P < 0.05 versus Control.
DISCUSSION
This study shows for the first time that pharmacologically increasing blood flow and muscle perfusion during hyperinsulinemia in older adults enhances muscle protein synthesis and overcomes age-related insulin resistance of muscle protein anabolism.
Specifically, local infusion of sodium nitroprusside during hyperinsulinemia in our older subjects more than doubled total leg blood flow, as planned, and induced a ninefold increase in muscle microvascular perfusion, thus increasing amino acid delivery to the muscle and stimulating muscle protein synthesis to levels comparable to those seen during insulin infusion alone in young healthy subjects (Fig. 3). Conversely, when insulin was infused alone in a control group, it induced neither vasodilation nor an increase in amino acid delivery to the leg. Additionally, muscle protein synthesis did not increase, confirming previous reports by our group and others that the physiological vasodilatory and muscle protein anabolic effect of insulin observed in young healthy subjects is lost with aging (3,10). These data provide strong evidence that insulin's ability to stimulate blood flow and amino acid delivery are necessary for it to induce a muscle protein synthetic response. This interpretation is further corroborated by our recent finding that pharmacologic blockade of insulin-induced vasodilation eliminates the anabolic response of muscle to insulin infusion in young subjects (16).
Interestingly, phenylalanine release from muscle protein breakdown, but not the leg rate of appearance, increased in the group undergoing pharmacological vasodilation, although to a lesser extent than protein synthesis. As a result, phenylalanine net balance across the leg switched from negative to positive, indicating net muscle protein accretion in the SNP group during hyperinsulinemia. This significant positive effect of vasodilation on net balance indicates that the increase in muscle protein breakdown was not due to a modeling artifact or washout effect induced by enhanced blood flow, because in those instances net balance would remain negative. Instead, the significant change in muscle protein breakdown appears to be due to acceleration in intracellular protein turnover, as demonstrated by the significant increase in phenylalanine intracellular recycling from breakdown to synthesis. An increase in intracellular amino acid recycling also occurs in younger subjects during isolated physiological hyperinsulinemia, as long as amino acid availability does not decrease (7,28). This may partially explain the conflicting results in the literature of the effects of insulin on protein synthesis and breakdown. For instance, in contrast to data from our laboratory (1,3,7,9,16) and others (4,6,29–31), hyperinsulinemia has been reported to have no effect on muscle protein synthesis but to inhibit muscle protein breakdown (32–36). We believe this discrepancy may be due to differences in amino acid availability. In studies reporting an insulin-induced increase in muscle protein synthesis (1,3,4,6,7,9,16,29,30), amino acid delivery increased due to either exogenous infusion or increased blood flow with no change in amino acid concentrations, as in the present study. Conversely, studies showing no increase in synthesis with decreased breakdown also reported decreased or unchanged amino acid availability (32,35,37,38), mainly due to significant reductions in blood amino acid concentrations induced by systemic hyperinsulinemia.
Blood flow—and, more importantly, muscle perfusion—emerge as important contributors to the anabolic effect of insulin on muscle proteins and the age-related insulin resistance of muscle protein metabolism. Enhanced microvascular perfusion increased insulin delivery to the muscle and the amount of tissue exposed to insulin. Insulin has been reported to promote its own transport across the endothelial barrier to reach its receptor on the end target tissue, such as skeletal muscle, and this process may be saturable (39). During this process, insulin also increases the microvascular surface available for uptake via endothelial-dependent capillary recruitment (13,40), thus facilitating its own delivery to the skeletal muscle. This mechanism may conceivably be lost in older subjects due to endothelial dysfunction and inability to recruit capillaries (3,41). Thus, restoring vasodilation and capillary recruitment during hyperinsulinemia in our older subjects might have led to the observed improvement in the age-related insulin resistance of muscle protein metabolism by augmenting the amount of muscle tissue exposed to insulin. This explanation is supported by increased Akt/PKB phosphorylation in the SNP group compared with the Control group during hyperinsulinemia. Enhanced Akt/PKB phosphorylation, however, did not result in a further increase in glucose uptake in the SNP group, which was somewhat lower, although not significantly, than in the Control group due to an outlier. The relatively modest improvement in Akt/PKB phosphorylation may have been insufficient to further activate downstream signals in the glucose uptake pathway, which were not measured in this study, or glucose uptake may have already been elevated in these two groups of healthy, nondiabetic subjects. This would not be surprising, as we have already shown that glucose metabolism is more insulin-sensitive than protein metabolism in healthy nondiabetic subjects (7), as well as that age-related insulin resistance in muscle protein metabolism does not correlate with the insulin sensitivity of glucose metabolism (7). However, this explanation is unlikely to apply under our experimental conditions, because we used a physiological insulin dose insufficient to maximally stimulate glucose uptake. A recent theory (42) suggests, in fact, that increased perfusion enhances only maximal glucose uptake, when large arteriovenous glucose gradients occur. Conversely, as compared with glucose, amino acids are present in the blood at much lower concentrations and with larger arteriovenous gradients. Thus, their uptake may be more sensitive to changes in blood flow. Lastly, it is also possible that we missed differences in glucose uptake due to the blood sampling schedule.
The large SNP-induced increase in blood flow and microvascular perfusion elevated amino acid transport into the muscle. However, mTOR phosphorylation at Ser 2,448, which typically responds to changes in amino acid availability (43), increased in both groups to essentially the same extent as during hyperinsulinemia. It was recently reported that elevated levels of nitric oxide inhibit mTOR signaling (44). Because sodium nitroprusside is a direct nitric oxide donor, the excess nitric oxide produced by sodium nitroprusside infusion may have blunted the activation of mTOR in response to elevated amino acid delivery in the SNP group. Nonetheless, the lack of a group difference in activation of the mTOR signaling pathway clearly did not impede an increase in muscle protein synthesis in the SNP group. As mTOR, S6K1, and 4E-BP1 were equally elevated in both groups during hyperinsulinemia, the differences in protein kinetics observed between SNP and Control subjects may have been due to the already active translational machinery being exposed to more amino acids in the SNP group. This interpretation is supported by previous findings that amino acid availability and transport to the muscle appear to modulate the effect of insulin on muscle protein synthesis and breakdown (3,6,7,9,16). Finally, we cannot exclude the possibility that our muscle-sampling schedule, while adequate to detect changes in protein turnover, was not timed to capture the largest changes in intracellular signaling under these experimental conditions.
Overall, these results suggest that pharmacological restoration of nitric oxide dependent vasodilation during hyperinsulinemia can restore the physiological anabolic response of skeletal muscle proteins to insulin in older adults. This effect appears mediated by increased availability of amino acids to skeletal muscle under hyperinsulinemic conditions. Further studies, to examine the effect of isolated vasodilation on muscle protein turnover, are needed to clarify this point. The results of this study are an encouraging first step for future investigations of the potential use of pharmacological agents to treat sarcopenia in older adults, particularly those whose physical limitations prevent lifestyle interventions, such as exercise (1,45–47), known to improve muscle function and metabolism.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the National Institutes of Health R01-AG-18311, P30-AG-024832, S10-RR-16650, T32-HD-07539, M01-RR-00073, and UL1-RR-029876. Definity was provided by Lantheus Medical Imaging, N. Billerica, MA, under Definity Research Grant No. 23012.
No potential conflicts of interest relevant to this article were reported.
K.L.T. and J.L.L. researched data, contributed to discussion, and wrote the manuscript. S.F., S.D., H.C.D., and C.S.F. researched data and reviewed the manuscript. M.J.D., M.S.M., and B.B.R. contributed to discussion and reviewed the manuscript. E.V. researched data, contributed to discussion, and wrote the manuscript.
The authors thank the study participants for their time and effort; Shelley Medina, UTMB, and Ming Zheng, UTMB, for their technical support; and Dr. Sarah Toombs-Smith, UTMB, for editing this manuscript.
Footnotes
Clinical trial reg. no. NCT00690534; clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Fujita S, Rasmussen BB, Cadenas JG, Drummond MJ, Glynn EL, Sattler FR, Volpi E. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes 2007;56:1615–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, de Rekeneire N, Harris TB, Kritchevsky S, Tylavsky FA, Nevitt M, Cho YW, Newman ABHealth, Aging, and Body Composition Study Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009;32:1993–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J 2006;20:768–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennet WM, Connacher AA, Scrimgeour CM, Jung RT, Rennie MJ. Euglycemic hyperinsulinemia augments amino acid uptake by human leg tissues during hyperaminoacidemia. Am J Physiol 1990;259:E185–E194 [DOI] [PubMed] [Google Scholar]
- 5.Bennet WM, Rennie MJ. Protein anabolic actions of insulin in the human body. Diabet Med 1991;8:199–207 [DOI] [PubMed] [Google Scholar]
- 6.Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest 1995;95:811–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita S, Rasmussen BB, Cadenas JG, Grady JJ, Volpi E. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab 2006;291:E745–E754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillier T, Long W, Jahn L, Wei L, Barrett EJ. Physiological hyperinsulinemia stimulates p70(S6k) phosphorylation in human skeletal muscle. J Clin Endocrinol Metab 2000;85:4900–4904 [DOI] [PubMed] [Google Scholar]
- 9.Fujita S, Glynn EL, Timmerman KL, Rasmussen BB, Volpi E. Supraphysiological hyperinsulinaemia is necessary to stimulate skeletal muscle protein anabolism in older adults: evidence of a true age-related insulin resistance of muscle protein metabolism. Diabetologia 2009;52:1889–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meneilly GS, Elliot T, Bryer-Ash M, Floras JS. Insulin-mediated increase in blood flow is impaired in the elderly. J Clin Endocrinol Metab 1995;80:1899–1903 [DOI] [PubMed] [Google Scholar]
- 11.Scherrer U, Randin D, Vollenweider P, Vollenweider L, Nicod P. Nitric oxide release accounts for insulin's vascular effects in humans. J Clin Invest 1994;94:2511–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 1994;94:1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baron AD, Tarshoby M, Hook G, Lazaridis EN, Cronin J, Johnson A, Steinberg HO. Interaction between insulin sensitivity and muscle perfusion on glucose uptake in human skeletal muscle: evidence for capillary recruitment. Diabetes 2000;49:768–774 [DOI] [PubMed] [Google Scholar]
- 14.Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S, Barrett E. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 2001;50:2682–2690 [DOI] [PubMed] [Google Scholar]
- 15.Vincent MA, Dawson D, Clark AD, Lindner JR, Rattigan S, Clark MG, Barrett EJ. Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes 2002;51:42–48 [DOI] [PubMed] [Google Scholar]
- 16.Timmerman KL, Lee JL, Dreyer HC, Dhanani S, Glynn EL, Fry CS, Drummond MJ, Sheffield-Moore M, Rasmussen BB, Volpi E. Insulin stimulates human skeletal muscle protein synthesis via an indirect mechanism involving endothelial-dependent vasodilation and mammalian target of rapamycin complex 1 signaling. J Clin Endocrinol Metab 2010;95:3848–3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci 1971;41:459–473 [DOI] [PubMed] [Google Scholar]
- 18.Jorfeldt L, Juhlin-Dannfelt A. The influence of ethanol on splanchnic and skeletal muscle metabolism in man. Metabolism 1978;27:97–106 [DOI] [PubMed] [Google Scholar]
- 19.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 2006;55:1436–1442 [DOI] [PubMed] [Google Scholar]
- 20.Dawson D, Vincent MA, Barrett EJ, Kaul S, Clark A, Leong-Poi H, Lindner JR. Vascular recruitment in skeletal muscle during exercise and hyperinsulinemia assessed by contrast ultrasound. Am J Physiol Endocrinol Metab 2002;282:E714–E720 [DOI] [PubMed] [Google Scholar]
- 21.Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong-Poi H, Barrett EJ. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab 2006;290:E1191–E1197 [DOI] [PubMed] [Google Scholar]
- 22.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 1998;97:473–483 [DOI] [PubMed] [Google Scholar]
- 23.Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine. Principles and Practice of Kinetic Analysis. New York, Wiley-Liss, 1992 [Google Scholar]
- 24.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom 1992;6:421–424 [DOI] [PubMed] [Google Scholar]
- 25.Dreyer HC, Drummond MJ, Glynn EL, Fujita S, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases human skeletal muscle AS160/TBC1D4 phosphorylation in association with enhanced leg glucose uptake during postexercise recovery. J Appl Physiol 2008;105:1967–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research. Principles and Practice of Kinetic Analysis. New York, Wiley-Liss, 2005 [Google Scholar]
- 27.Toffolo G, Foster DM, Cobelli C. Estimation of protein fractional synthetic rate from tracer data. Am J Physiol 1993;264:E128–E135 [DOI] [PubMed] [Google Scholar]
- 28.Bell JA, Fujita S, Volpi E, Cadenas JG, Rasmussen BB. Short-term insulin and nutritional energy provision do not stimulate muscle protein synthesis if blood amino acid availability decreases. Am J Physiol Endocrinol Metab 2005;289:E999–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guillet C, Prod'homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J 2004;18:1586–1587 [DOI] [PubMed] [Google Scholar]
- 30.Hillier TA, Fryburg DA, Jahn LA, Barrett EJ. Extreme hyperinsulinemia unmasks insulin's effect to stimulate protein synthesis in the human forearm. Am J Physiol 1998;274:E1067–E1074 [DOI] [PubMed] [Google Scholar]
- 31.Wolf RF, Heslin MJ, Newman E, Pearlstone DB, Gonenne A, Brennan MF. Growth hormone and insulin combine to improve whole-body and skeletal muscle protein kinetics. Surgery 1992;112:284–292 [PubMed] [Google Scholar]
- 32.Denne SC, Liechty EA, Liu YM, Brechtel G, Baron AD. Proteolysis in skeletal muscle and whole body in response to euglycemic hyperinsulinemia in normal adults. Am J Physiol 1991;261:E809–E814 [DOI] [PubMed] [Google Scholar]
- 33.Heslin MJ, Newman E, Wolf RF, Pisters PW, Brennan MF. Effect of hyperinsulinemia on whole body and skeletal muscle leucine carbon kinetics in humans. Am J Physiol 1992;262:E911–E918 [DOI] [PubMed] [Google Scholar]
- 34.Louard RJ, Fryburg DA, Gelfand RA, Barrett EJ. Insulin sensitivity of protein and glucose metabolism in human forearm skeletal muscle. J Clin Invest 1992;90:2348–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Möller-Loswick AC, Zachrisson H, Hyltander A, Körner U, Matthews DE, Lundholm K. Insulin selectively attenuates breakdown of nonmyofibrillar proteins in peripheral tissues of normal men. Am J Physiol 1994;266:E645–E652 [DOI] [PubMed] [Google Scholar]
- 36.Wilkes EA, Selby AL, Atherton PJ, Patel R, Rankin D, Smith K, Rennie MJ. Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age-related sarcopenia. Am J Clin Nutr 2009;90:1343–1350 [DOI] [PubMed] [Google Scholar]
- 37.Nygren J, Nair KS. Differential regulation of protein dynamics in splanchnic and skeletal muscle beds by insulin and amino acids in healthy human subjects. Diabetes 2003;52:1377–1385 [DOI] [PubMed] [Google Scholar]
- 38.Tessari P, Inchiostro S, Biolo G, Vincenti E, Sabadin L. Effects of acute systemic hyperinsulinemia on forearm muscle proteolysis in healthy man. J Clin Invest 1991;88:27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eggleston EM, Jahn LA, Barrett EJ. Hyperinsulinemia rapidly increases human muscle microvascular perfusion but fails to increase muscle insulin clearance: evidence that a saturable process mediates muscle insulin uptake. Diabetes 2007;56:2958–2963 [DOI] [PubMed] [Google Scholar]
- 40.Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 2004;53:1418–1423 [DOI] [PubMed] [Google Scholar]
- 41.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol 2009;297:H425–H432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clerk LH, Vincent MA, Lindner JR, Clark MG, Rattigan S, Barrett EJ. The vasodilatory actions of insulin on resistance and terminal arterioles and their impact on muscle glucose uptake. Diabetes Metab Res Rev 2004;20:3–12 [DOI] [PubMed] [Google Scholar]
- 43.Du M, Shen QW, Zhu MJ, Ford SP. Leucine stimulates mammalian target of rapamycin signaling in C2C12 myoblasts in part through inhibition of adenosine monophosphate-activated protein kinase. J Anim Sci 2007;85:919–927 [DOI] [PubMed] [Google Scholar]
- 44.Frost RA, Nystrom GJ, Lang CH. Endotoxin and interferon-gamma inhibit translation in skeletal muscle cells by stimulating nitric oxide synthase activity. Shock 2009;32:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA 1990;263:3029–3034 [PubMed] [Google Scholar]
- 46.Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 1994;330:1769–1775 [DOI] [PubMed] [Google Scholar]
- 47.Morganti CM, Nelson ME, Fiatarone MA, Dallal GE, Economos CD, Crawford BM, Evans WJ. Strength improvements with 1 yr of progressive resistance training in older women. Med Sci Sports Exerc 1995;27:906–912 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.