Abstract
OBJECTIVE
Physical inactivity is a risk factor for type 2 diabetes and may be more detrimental in first-degree relative (FDR) subjects, unmasking underlying defects of metabolism. Using a positive family history of type 2 diabetes as a marker of increased genetic risk, the aim of this study was to investigate the impact of physical inactivity on adipose tissue (AT) metabolism in FDR subjects.
RESEARCH DESIGN AND METHODS
A total of 13 FDR and 20 control (CON) subjects participated in the study. All were studied before and after 10 days of bed rest using the glucose clamp technique combined with measurements of glucose uptake, lipolysis, and lactate release from subcutaneous abdominal (SCAAT) and femoral (SCFAT) adipose tissue by the microdialysis technique. Additionally, mRNA expression of lipases was determined in biopsies from SCAAT.
RESULTS
Before bed rest, the FDR subjects revealed significantly increased glucose uptake in SCAAT. Furthermore, mRNA expression of lipases was significantly decreased in the SCAAT of FDR subjects. Bed rest significantly decreased lipolysis and tended to increase glucose uptake in the SCFAT of both CON and FDR subjects. In response to bed rest, SCAAT glucose uptake significantly increased in CON subjects but not in FDR subjects.
CONCLUSIONS
FDR subjects exhibit an abnormal AT metabolism including increased glucose uptake prior to bed rest. However, the differences between FDR and CON subjects in AT metabolism were attenuated during bed rest due to relatively more adverse changes in CON subjects compared with FDR subjects. Physical inactivity per se is not more deleterious in FDR subjects as compared with CON subjects with respect to derangements in AT metabolism.
Type 2 diabetes is the product of a complex interplay between genetic susceptibility and environmental factors. The best known environmental modifiable risk factors for type 2 diabetes are obesity and a low level of habitual physical activity (1).
Even though there is substantial evidence that a change toward a healthy lifestyle halts the progression of type 2 diabetes (2), certain groups, including first-degree relatives (FDRs) of patients with type 2 diabetes, are at increased risk of developing the disease (3). Type 2 diabetes has a major hereditary component (4), and FDR subjects show multiple abnormalities in intermediary metabolism and pancreatic islet cell function, displaying insulin resistance despite normal glucose tolerance (5). The metabolic defects include insufficient insulin secretion (6), decreased peripheral glucose uptake (7), and the impaired antilipolytic effect of insulin in subcutaneous adipose tissue (AT) (8).
AT is an active compartment in the lipid and glucose metabolism of humans, but the role of AT metabolism in the development of type 2 diabetes is not clarified. AT, along with skeletal muscle, is a site of peripheral insulin resistance in type 2 diabetes (9). The role of AT in the pathophysiology of insulin resistance can partly be attributed to lipolytic activity resulting in the mobilization of free fatty acids (FFAs), which are deleterious for glucose utilization and insulin action (10). However, AT may play a more direct role since it is a site of deranged glucose metabolism. Although AT is of minor quantitative importance for whole-body glucose disposal, the tissue produces lactate (11), which functions as a gluconeogenic precursor in the liver (12). Previous studies have demonstrated increased plasma lactate (13) and lactate release from adipocytes (14) in FDR subjects.
In this study, we investigated in vivo AT glucose uptake, lipolysis, and lactate release, as well as subcutaneous abdominal adipose tissue (SCAAT) mRNA expression of lipases and GLUT-4 in FDR and control (CON) subjects prior to and after 10 days of bed rest. We hypothesized that FDR subjects would show abnormalities in baseline AT metabolism and be more sensitive to the unhealthy effects of physical inactivity.
RESEARCH DESIGN AND METHODS
The data presented in this article are part of a larger study on the influence of physical inactivity in healthy and pre-diabetic subjects as initiated and funded within the framework program of the European Union's EXGENESIS Consortium.
Thirty-three young healthy men participated in the study, which included 13 FDR of patients with type 2 diabetes and 20 CON subjects without any family history of diabetes. All subjects were from singleton pregnancies born at term (FDR: 40 ± 0.3 week; CON: 40 ± 0.1 week). During inclusion, the groups were matched with respect to age, BMI, and cardiorespiratory fitness. FDRs were recruited via their type 2 diabetic parent attending the outpatient clinic at the Steno Diabetes Center, Gentofte, Denmark, and CON subjects were recruited from the Danish National Birth Registry. CON and FDR subjects had birth weights between the 50th and 75th percentile (CON: 3,827 ± 49 g; FDR: 3,500 ± 150 g, P > 0.05). The purpose and potential risk of the study were explained to all subjects before they gave their written informed consent. The study was approved by the Ethical Committee of Copenhagen and Frederiksberg, Denmark (protocol no. [01]-262546), and all procedures used conformed to the Declaration of Helsinki II. All subjects had normal fasting glucose measured before entering the study. Additional inclusion criteria were male, aged 20–30 years, maximal oxygen uptake (Vo2max) 35–55 ml · kg body mass−1 · min−1 and BMI 18.5–24.9 kg per m2. An International Physical Activity Questionnaire was answered before entering the study. Subjects were Caucasian, and none took any medication. Subject characteristics are summarized in Table 1.
TABLE 1.
Subject characteristics
| Before bed rest |
After bed rest |
||||||
|---|---|---|---|---|---|---|---|
| CON | FDR | aP | CON | FDR | bP | cP | |
| Age (years) | 25 ± 0.2 | 26.1 ± 1 | ns | — | — | nm | nm |
| VO2max (ml O2/kg/min) | 43.5 ± 1.5 | 39.1 ± 1.9 | ns | 42.8 ± 1.1 | 37.5 ± 2.0 | ns | ns |
| VO2max (ml/min) | 3,659 ± 125 | 3,405 ± 162 | P < 0.05 | 3,527 ± 101 | 3,190 ± 160 | ns | ns |
| Fasting arterial glucose (mmol/l) | 5.3 ± 0.1 | 5.5 ± 0.1 | ns | 5.1 ± 0.1 | 5.2 ± 0.1 | P < 0.05 | P < 0.05 |
| Weight (kg) | 82.5 ± 2.3 | 84.0 ± 3.3 | ns | 82.2 ± 2.3 | 83.6 ± 3.2 | ns | ns |
| Height (m) | 1.85 ± 0.01 | 1.84 ± 0.02 | ns | — | — | nm | nm |
| BMI (kg/m2) | 24.1 ± 0.5 | 24.9 ± 0.9 | ns | 23.9 ± 0.5 | 24.8 ± 0.9 | ns | ns |
| Total fat mass (kg) | 14.3 ± 1.6 | 21.6 ± 2.5 | P < 0.05 | 14.7 ± 1.8 | 21.5 ± 2.7 | ns | ns |
| Total lean mass (kg) | 63.8 ± 1.1 | 58.6 ± 1.5 | P < 0.05 | 63.6 ± 1.1 | 58.4 ± 1.5 | ns | ns |
| Whole body fat percentage (%) | 17.4 ± 1.7 | 25.0 ± 2.3 | P < 0.05 | 17.1 ± 1.6 | 24.9 ± 2.5 | ns | ns |
| Trunk fat mass/total fat mass | 0.48 ± 0.1 | 0.58 ± 0.001 | P < 0.05 | 0.50 ± 0.01 | 0.58 ± 0.01 | ns | ns |
| Leg fat mass/total fat mass | 0.37 ± 0.1 | 0.29 ± 0.01 | P < 0.05 | 0.37 ± 0.01 | 0.29 ± 0.01 | ns | ns |
| Waist/hip ratio | 0.85 ± 0.01 | 0.88 ± 0.02 | ns | 0.86 ± 0.01 | 0.87 ± 0.02 | ns | ns |
| Abdominal skinfold (mm) | 21 ± 1.8 | 25 ± 3.0 | ns | 21 ± 1.6 | 23 ± 2.0 | ns | ns |
| Femoral skinfold (mm) | 16 ± 1.5 | 16 ± 2.0 | ns | 15 ± 1.4 | 16 ± 2.0 | ns | ns |
Data are mean ± SE.
aP, significant difference between the groups before bed rest (P < 0.05).
bP, significant difference before vs. after bed rest within CON, P < 0.05.
cP, significant difference before vs. after bed rest within FDR, P < 0.05. ns, not significant; nm, not measured.
Pre- and post–bed-rest testing.
Fasting plasma glucose was determined in capillary blood using an ABL 625 (Radiometer, Copenhagen, Denmark). Body composition was determined by dual-energy X-ray absorptiometry full body scanning (DPX-IQ, software version 4.7e; Lunar Radiation, Madison, WI), and abdominal and femoral skinfold thickness were measured using a caliper (Lafayette Instrument, Lafayette, IN). Vo2max was measured using a bicycle ergometer exercise protocol by means of an Oxycon Pro System (Jaeger, Höchberg, Germany).
All subjects participated in one experimental day ∼three weeks before ten days of strict bed rest. On the tenth day of the bed-rest period, subjects participated in a second and identical experimental day. Four days before the first experimental day, subjects were provided a standardized isocaloric diet (i.e., 55 E% [percent of energy] of carbohydrates, 15 E% of protein, and 30 of E% fat). Caloric fluids, including alcohol, were prohibited. During these 4 days, as well as during the bed-rest period, a combined accelerometer and heart rate sensor (Actiheart; Cambridge Neurotechnology, Cambridge, U.K.) registered movements and heart rate, and the energy expenditure was calculated. The Actiheart device was placed on the subject's upper left chest clipped on two standard electrocardiogram electrodes. The participants wore the Actiheart device continuously during the 4 days prior to the first experiment and throughout the entire bed- rest period. The set time resolution for measurements was 30 s during the 4 days of “free living” and 1 min during the bed-rest period. The data recorded from the Actiheart monitors were downloaded into a database via the Actiheart reader/charger interface for assessment of activity, heart rate, and calculated energy expenditure and analyzed using the Actiheart commercial program provided with the device (Actiheart version 2.2 software). Data were edited with full traceability and without compromising the integrity of the original recording. Subjects were instructed to continue their daily living activities, including physical activity habits, during the 4 days and in the 3 weeks prior to the bed-rest period and to refrain from vigorous physical activity 24 h prior to the first experimental day. During the bed-rest period, subjects were in bed all day (a maximum of 60° upper body elevation) under surveillance. A standardized isocaloric diet was provided during bed rest with adjusted reduced amounts to ensure weight stability.
Experimental day protocol.
The experimental day consisted of a 210-min baseline period followed by a 180-min hyperinsulinemic euglycemic clamp. Microdialysis catheters were inserted and dialysate was sampled during the last 60 min of baseline and the last 60 min of the clamp period.
An arterial catheter (Becton Dickinson, U.K.) was inserted in the brachial artery and blood was sampled at 180 and 210 min (basal) and at 360 and 390 min (clamp). A venous catheter (18G Venflon; Medex Medicine, U.K.) was inserted in the medial antecubital vein for infusion of insulin and glucose. Subcutaneous adipose tissue blood flow (ATBF) was measured by the local 133Xe washout method (15).
Microdialysis.
Prior to insertion of the microdialysis catheters (CMA60; CMA Microdialysis AB, Solna, Sweden), the skin was anesthetized with 0.2 ml lidocaine (Lidokain SAD, 5 mg/ml−1; Sygehus Apotekerne, Denmark). One catheter was inserted in SCAAT ∼4 cm lateral to the umbilicus, and another catheter was inserted in SCFAT of the leg adjacent to the vastus lateralis part of the quadriceps muscle 30 cm above the patella. The catheters were perfused with Ringer acetate containing 2 mmol/l glucose (Skanderborg Apotek, Skanderborg, Denmark) at 1 μl/min−1 using a high-precision syringe pump (CMA100; CMA Microdialysis AB, Solna, Sweden). Dialysate was immediately frozen and stored at −20°C.
The relative recovery over the membrane was determined using internal reference calibration (16) and was used for the calculation of interstitial metabolite concentrations. For internal reference calibration, 12 μl d-1[14C]-glucose (PerkinElmer, Boston, MA) and 1 μl 2-[3H]-glycerol (PerkinElmer, Boston, MA) was added to 10 ml of perfusate in order to achieve a specific activity of 5 kBq/ml−1 for each isotope. Mean relative recovery for glucose and glycerol were ∼45 and 55%. Relative recovery of lactate was assumed to equal the relative recovery of glucose as found previously (17). Data were excluded if relative recovery was less than 20%. The absolute rate of exchange of glucose, glycerol, and lactate was calculated using Fick's equation as described previously (17,18).
Biochemical analysis.
Blood samples for the analysis of glucose, glycerol, lactate, FFA, and triglyceride were distributed in iced tubes containing 1.5 mg ethylene-diamine-tetraacetic acid per ml blood. For the analysis of insulin, the tubes contained 500 KIE Trasylol and 1.5 mg EDTA per ml blood. The tubes were centrifuged (Hettich Labinstrument ApS, Hvidovre, Denmark) and stored at −80°C. Plasma and microdialysate glucose, glycerol, and lactate were determined by a CMA600 microdialysis analyzer (CMA Microdialysis AB, Solna, Sweden). Plasma FFA was determined using a nonesterified fatty acids C kit (Wako Chemicals, Neuss, Germany). Plasma insulin analysis was determined by enzyme-linked immunosorbent assay (ELISA) technique (DAKO ELISA, U.K.) and plasma triglyceride with Triglyceride GPO-PAP (Roche Diagnostic, Mannheim, Germany).
Adipose tissue blood flow.
ATBF was measured by the local 133Xe washout technique (15). Briefly, 0.5–1 MBq gaseous 133Xe (Amersham Health, Amersham, U.K.) was injected into the SCAAT and SCFAT contra lateral to the regions in which microdialysis was performed. Washout of 133Xe was registered by a Mediscint system (Oakfield Instruments, Oxford, U.K.), and ATBF was calculated as described previously (19).
Whole-body glucose uptake.
Whole-body glucose disposal was determined using the hyperinsulinemic (40 mU · min−1 · m2(−1)) (Actrapid; Novo Nordisk, Copenhagen, Denmark) euglycemic clamp technique as described previously (20). Whole-body insulin–mediated glucose uptake rates were averaged for 10 min and calculated as the mean of steady-state glucose infusion rates from t = 360 to t = 390 (M value).
Adipose tissue biopsy.
SCAAT biopsies were collected in the basal state and at the end of a 180-min hyperinsulinemic (80 mU · min−1 · m2(−1)) euglycemic clamp before bed rest and on the ninth day of the bed-rest intervention using the Bergstrom biopsy needle technique with suction under local anesthesia (1% lidocaine). Biopsies were snap-frozen in liquid nitrogen and stored at −80°C.
Determination of mRNA levels.
Total RNA was isolated using Trizol reagent (Gibco BRL; Life Technologies, Roskilde, Denmark), and cDNA was made with random hexamer primers using the GeneAmp PCR kit (Applied Biosystems, CA). Glyceraldehyde-3-phosphate dehydrogenase (GADPH) was chosen as housekeeping gene for hormone-sensitive lipase (HSL), lipoprotein lipase (LPL), and adipose triglyceride lipase (ATGL), and β-actin as housekeeping gene for GLUT4. Quantification was performed with a SYBR Green real-time PCR assay using an iCycler PCR machine (Bio-Rad Laboratories, Hercules, CA) as described previously (21).
Statistical analysis.
The computer program SAS for Windows version 9.1 (SAS Institute, Cary, NC) was used for statistical analysis. Data were analyzed using a linear mixed model. The model estimates differences between mean values. Repeated measures data, as in the present study, requires special attention to the covariance structure due to the sequential nature of the data on each individual. The covariance structure refers to variances at individual times and to correlation between measures at different times on the same subject. In the mixed model, the variation between subjects is specified by the RANDOM statement, and covariation within subjects is specified by the REPEATED statement. In the present study, we assumed compound symmetry in the model, i.e., that all measurements on the same subject were equally correlated and had the same variance. The implication is that the only aspect of the covariance between repeated measures is due to the subject contribution irrespective of proximity of time. Compound symmetric structure was fitted with the RANDOM statement. The fixed effects parameters: group, experimental day and time was included in the MODEL statement of the analysis.
The figures presented (Figs. 1–3) are absolute means, while the differences and CIs described in the results section are from the linear mixed model. If data were not normally distributed or homogeneous as assessed by residual plots of each dependent variable, log transformation data were used and significant effects described as relative (%) differences. We evaluated the effect of groups (CON and FDR), the effect of bed rest (before vs. after), and time (baseline or insulin-stimulated) and tested an eventual differential effect of bed rest between FDR and CON subjects by including interaction between groups and bed rest and the marginal effects in the model. Threshold for significance was P ≤ 0.05. The Bonferroni corrected P value that is equivalent to an uncorrected P value of 0.05 in the present study is P = 0.0028, although this is likely overly conservative. However in the interpretation of the results, the risks of type I errors are acknowledged. Data are presented as absolute means ± SE (figures) or differences and CI.
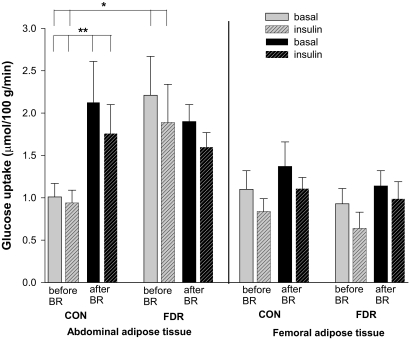
FIG. 1.
SCAAT and SCFAT glucose uptake in the basal state (no pattern) and the insulin-stimulated state (patterned) before (gray) and after (black) bed rest (BR) in CON and FDR of type 2 diabetic subjects. *Basal (P = 0.005) and insulin-stimulated state (P = 0.02) before bed rest, CON vs. FDR. **Basal (P = 0.01) and insulin-stimulated state (P = 0.02) before vs. after bed rest in CON.
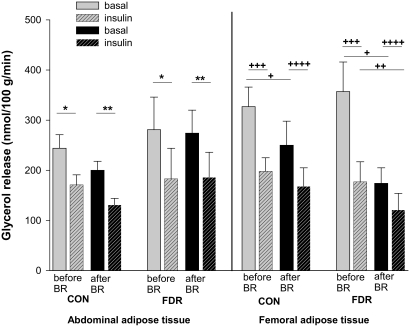
FIG. 2.
SCAAT and SCFAT lipolysis in the basal state (no pattern) and the insulin-stimulated state (patterned) before (gray) and after (black) bed rest (BR) in CON and FDR of type 2 diabetic subjects. *Basal vs. insulin-stimulated state before bed rest in CON (P = 0.01) and FDR (P = 0.006). **Basal vs. insulin-stimulated state after BR in CON (P = 0.0009) and FDR (P = 0.004). +Basal state before vs. after BR in CON (P = 0.02) and in FDR (P = 0.0006). ++Insulin-stimulated state before vs. after BR in FDR (P = 0.009). +++Basal vs. insulin-stimulated state before BR in CON (P = 0.0001) and in FDR (P = 0.0004). ++++Basal vs. insulin-stimulated state after BR in CON (P = 0.02) and in FDR (P = 0.02).
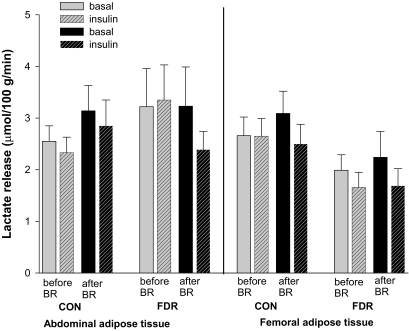
FIG. 3.
SCAAT and SCFAT lactate release in the basal state (no pattern) and the insulin-stimulated state (patterned) before (gray) and after (black) bed rest (BR) in CON and FDR of type 2 diabetic subjects.
RESULTS
FDR subjects were characterized by increased body fat percentage and abdominal adiposity compared with CON subjects, but similar BMI and waist-to-hip ratio (Table 1). In response to bed rest, no changes in anthropometrics were observed. Total, resting, and activity-related energy expenditure and the physical activity score (Actiheart recordings) did not differ between groups during daily living but decreased significantly in both groups during bed rest with no difference between groups (Table 2). Vo2max expressed in absolute units (ml/min) was slightly lower in FDR subjects compared with CON subjects before bed rest (P = 0.035) but when expressing Vo2max per kg body weight, there was no difference between groups. Vo2max did not change significantly in response to bed rest in FDR subjects or CON subjects (Table 1).
TABLE 2.
Energy expenditure and physical activity level before and during bed rest
| Daily living |
During bed rest |
||||||
|---|---|---|---|---|---|---|---|
| CON | FDR | aP | CON | FDR | bP | cP | |
| Resting energy expenditure (REE) (kJ/day/kg) | 99 ± 1.0 | 96 ± 2.7 | ns | 101 ± 1.4 | 97 ± 2.4 | ns | ns |
| Activity energy expenditure (AEE) (kJ/day/kg) | 83 ± 7 | 102 ± 8 | ns | 26 ± 4 | 27 ± 3 | P < 0.0001 | P < 0.0001 |
| Total energy expenditure (TEE) (kJ/day/kg) | 207 ± 8 | 218 ± 12 | ns | 146 ± 5 | 138 ± 4 | P < 0.0001 | P < 0.0001 |
| Daily physical activity level (PAL) (TEE/REE) | 2.1 ± 0.1 | 2.3 ± 0.1 | ns | 1.5 ± 0.05 | 1.4 ± 0.03 | P < 0.0001 | P < 0.0001 |
| Sitting (hours/day) | 7.2 ± 0.6 | 6.5 ± 0.9 | ns | — | — | — | — |
Data are mean ± SE.
aP, significant difference between the groups before bed rest.
bP, significant difference before vs. after bed rest within CON.
cP, significant difference before vs. after bed rest within FDR. ns, nonsignificant. REE, AEE, TEE, and PAL based on Actiheart recordings during 4 days of daily living 3 weeks before bed rest. Hours sitting per day based on International Physical Activity Questionnaire.
Subcutaneous adipose tissue glucose uptake
Abdominal.
The interstitial glucose concentration in SCAAT was significantly lower than plasma water glucose indicating that glucose is taken up in SCAAT in both groups (Table 3). Before bed rest, basal and insulin-stimulated SCAAT glucose uptake was higher in FDR subjects compared with CON subjects (difference basal: 1.21 nmol · 100 g−1 · min−1, 95% CI 0.39–2.02, P = 0.005; difference insulin-stimulated: 0.94 nmol · 100 g−1 · min−1, 95% CI 0.17–1.72, P = 0.02) with no differences between groups after bed rest (Fig. 1). The difference disappeared as glucose uptake increased only in CON in response to bed rest (difference basal: 1.1 nmol · 100 g−1 · min−1, 95% CI 0.3–1.9, P = 0.01; difference insulin-stimulated: 0.7 nmol · 100 g−1 · min−1, 95% CI 0.1–1.4, P = 0.02). Test for interaction between groups and bed rest was significant in the basal state (P = 0.02) and during insulin stimulation (P = 0.047). Insulin did not stimulate SCAAT glucose uptake in either of the groups (Fig. 1).
TABLE 3.
Basal subcutaneous adipose tissue interstitial and plasma water concentrations of metabolites
| CON |
FDR |
|||
|---|---|---|---|---|
| Before BR | After BR | Before BR | After BR | |
| Interstitial glucose abdominal (μM) | 4.4 ± 0.2 (n = 19)* | 3.6 ± 0.4 (n = 18)* | 4.0 ± 0.5 (n = 11)* | 4.5 ± 0.3 (n = 13)* |
| Interstitial glucose femoral (μM) | 4.7 ± 0.2 (n = 19)* | 4.0 ± 0.2 (n = 19)* | 5.7 ± 0.4 (n = 11)* | 4.3 ± 0.3 (n = 11)* |
| Arterial plasma water glucose (μM) | 5.3 ± 0.1 (n = 20) | 5.1 ± 0.1 (n = 20) | 5.9 ± 0.1 (n = 13) | 5.6 ± 0.1 (n = 13) |
| Interstitial glycerol abdominal (μM) | 235 ± 29 (n = 20) | 172 ± 13 (n = 20)* | 228 ± 40 (n = 13)* | 241 ± 39 (n = 12)* |
| Interstitial glycerol femoral (μM) | 309 ± 38 (n = 20) | 232 ± 34 (n = 19)* | 355 ± 49 (n = 11)* | 205 ± 29 (n = 12)* |
| Arterial plasma water glycerol (μM) | 36 ± 3 (n = 20) | 33 ± 2 (n = 20) | 42 ± 4 (n = 13) | 36 ± 3 (n = 13) |
| Interstitial lactate abdominal (μM) | 2.7 ± 0.2 (n = 19)* | 3.0 ± 0.3 (n = 18)* | 3.1 ± 0.5 (n = 10)* | 3.0 ± 0.3 (n = 13)* |
| Interstitial lactate femoral (μM) | 2.8 ± 0.3 (n = 19)* | 3.1 ± 0.3 (n = 19)* | 2.8 ± 0.2 (n = 10)* | 2.6 ± 0.3 (n = 12)* |
| Arterial plasma water lactate (μM) | 0.8 ± 0.06 (n = 20) | 0.8 ± 0.05 (n = 20) | 0.8 ± 0.08 (n = 13) | 0.8 ± 0.05 (n = 13) |
Data are mean ± SE.
*Significant difference interstitial vs. arterial plasma water concentration, P < 0.05. BR, bed rest.
Femoral.
Also in SCFAT, the interstitial glucose concentration was significantly lower than the arterial plasma water concentration indicating that glucose is taken up in SCFAT in both groups (Table 3). SCFAT glucose uptake did not differ between groups before or after bed rest. There was a nonsignificant tendency to increased SCFAT glucose uptake in both groups after bed rest (P = 0.06). Insulin did not stimulate SCFAT glucose uptake in any of the groups (Fig. 1).
Subcutaneous adipose tissue lipolysis
Abdominal.
The concentration of glycerol was significantly higher in the interstitial fluid than in the arterial plasma water indicating glycerol release from and thus lipolysis in SCAAT in both groups (Table 3). SCAAT lipolysis did not differ between groups before or after bed rest and did not influence SCAAT lipolysis in either of the groups (Fig. 2). Insulin inhibited SCAAT lipolysis in both groups before (FDR: P = 0.006, CON: P = 0.01) and after bed rest (FDR: P = 0.004, CON: P = 0.0009).
Femoral.
Also in SCFAT, the concentration of glycerol was significantly higher in the interstitial fluid than in the arterial plasma water indicating lipolysis in the tissue in both groups (Table 3). Lipolysis did not differ between groups before or after bed rest (Fig. 2). However after bed rest, basal lipolysis was decreased in both FDR (difference before vs. after bed rest: 43%, 95% CI 29–64, P = 0.0006) and CON (difference before vs. after bed rest: 62%, 95% CI 42–91, P = 0.02). In FDR, insulin-inhibited lipolysis was also decreased after bed rest (difference before vs. after bed rest: 50%, 95% CI 31–81, P = 0.009). Insulin inhibited SCFAT lipolysis in both groups before (FDR: P = 0.0004, CON: P < 0.0001) and after bed rest (FDR: P = 0.02, CON: P = 0.02).
Subcutaneous adipose tissue lactate release
Abdominal.
Lactate concentrations were significantly higher in the interstitial space than in the arterial plasma water indicating that lactate is released from SCAAT in both groups (Table 3). SCAAT lactate release did not differ between the groups before or after bed rest and did not change in response to bed rest or insulin stimulation in either of the groups (Fig. 3).
Femoral.
Also in SCFAT, lactate concentrations were significantly higher in the interstitial space than in the arterial plasma water indicating lactate release in both groups (Table 3). Before bed rest, there was a nonsignificant tendency to lower SCFAT lactate release in FDR compared with CON (P = 0.06), while lactate release was similar after bed rest (Fig. 3). Bed rest and insulin did not influence SCFAT lactate release in either of the groups.
Whole-body insulin sensitivity.
Before bed rest, whole-body insulin sensitivity (M value) was lower (P < 0.05) in FDR compared with CON (4.3 ± 0.5 vs. 6.8 ± 0.5 mg · min−1· kg−1), and bed rest decreased the M value in both FDR (P = 0.007) and CON (P = 0.0001) although less in FDR compared with CON (28 ± 1% vs. 37 ± 4%, P < 0.05). After bed rest, the M value was still lower in FDR compared with CON (3.1 ± 0.3 vs. 4.3 ± 0.3 mg · min−1· kg−1, P < 0.05).
Subcutaneous adipose tissue blood flow.
SCAAT blood flow did not differ between groups before or after bed rest and did not change in response to bed rest or insulin stimulation in either of the groups (data not shown). Before bed rest, SCFAT blood flow was lower in FDR compared with CON during insulin stimulation (1.03 ml · 100 g−1 · min−1, 95% CI 0.39–1.69, P = 0.003). SCFAT blood flow did not change in response to bed rest or insulin stimulation in any of the groups (data not shown).
Arterial plasma glucose, glycerol, lactate, FFA, triglyceride, and insulin.
Fasting plasma glucose was higher (P = 0.002) in FDR compared with CON before and after bed rest and decreased in both groups after bed rest (P < 0.0001) (Table 4). Fasting plasma glycerol did not differ between CON and FDR before or after bed rest and did not change in response to bed rest in any of the groups but decreased (P < 0.0001) during insulin stimulation in both groups. Plasma lactate was similar between the groups before bed rest but was lower in FDR compared with CON after bed rest during insulin stimulation (P = 0.03). Plasma lactate increased during insulin stimulation in CON before bed rest (P = 0.007) and decreased in FDR (P = 0.03) after bed rest. Before bed rest, fasting (P = 0.045) as well as insulin-stimulated (P = 0.008) plasma FFA were higher in FDR, but after bed rest only insulin-stimulated concentrations remained different between groups (P = 0.03). Bed rest did not influence plasma FFA in any of the groups while plasma FFA decreased during insulin stimulation in both groups (P < 0.01). Basal plasma triglyceride concentrations were higher in FDR before (P = 0.04) and after bed rest (P = 0.03) and increased in response to bed rest in FDR (P = 0.03) but not in CON (P = 0.7). Testing for interaction between groups and bed rest was nonsignificant (P = 0.32) and thus does not support a differential effect of bed rest between the groups but a main effect. Thus, plasma triglyceride concentrations increased in both groups in response to bed rest (P = 0.04). Insulin inhibited plasma triglyceride concentrations in both groups (P < 0.02). Fasting plasma insulin was elevated in FDR compared with CON before (P = 0.02) and after bed rest (P < 0.04) and did not change in response to bed rest in any of the groups.
TABLE 4.
Plasma glucose, glycerol, lactate, FFA, triglyceride, and insulin
| CON |
FDR |
||||
|---|---|---|---|---|---|
| Before bed rest | After bed rest | Before bed rest | After bed rest | ||
| n | 20 | 20 | 13 | 13 | |
| P glucose (mM) | Basal | 5.1 ± 0.06*†‡ | 4.8 ± 0.05*† | 5.4 ± 0.1*† | 5.2 ± 0.1*† |
| P glucose (mM) | Insulin stimulated | 4.9 ± 0.07* | 4.8 ± 0.07† | 5.3 ± 0.1*† | 55.1 ± 0.09* |
| P glycerol (μM)** | Basal | 34.6 ± 2.7‡ | 31.2 ± 2.1‡ | 38.5 ± 3.7‡ | 34.2 ± 2.9‡ |
| P glycerol (μM)** | Insulin stimulated | 14.5 ± 0.9 | 13.9 ± 0.9 | 14.7 ± 1.2 | 13.0 ± 1.0 |
| P lactate (mM) | Basal | 0.7 ± 0.05‡ | 0.7 ± 0.05 | 0.7 ± 0.07 | 0.8 ± 0.05‡ |
| P lactate (mM) | Insulin stimulated | 0.9 ± 0.05 | 0.8 ± 0.04* | 0.7 ± 0.06 | 0.6 ± 0.03* |
| P FFA (μM) | Basal | 264 ± 30*‡ | 282 ± 30‡ | 369 ± 46*‡ | 307 ± 36‡ |
| P FFA (μM) | Insulin stimulated | 13 ± 2* | 11 ± 2 | 26 ± 5* | 20 ± 3 |
| P triglyceride (mM) | Basal | 0.9 ± 0.1*‡ | 1.0 ± 0.1*‡ | 1.1 ± 0.1*†‡ | 1.3 ± 0.1*†‡ |
| P triglyceride (mM) | Insulin stimulated | 0.5 ± 0.05 | 0.6 ± 0.1‡ | 0.7 ± 0.1 | 1.1 ± 0.1‡ |
| P insulin (pM) | Basal | 35 ± 3* | 35 ± 3* | 50 ± 8* | 47 ± 5* |
| P insulin (pM) | Insulin stimulated | 294 ± 10 | 272 ± 13 | 287 ± 26 | 308 ± 22 |
Data are presented as mean ± SE.
*Significant difference CON vs. FDR, P < 0.05.
†Significant difference before vs. after bed rest within the group; P < 0.05.
‡Significant difference basal vs. insulin-stimulated state within the group on the specified day, P < 0.05.
**Data were log transformed before statistical test.
ATGL, HSL, LPL, and GLUT4 mRNA expression in SCAAT.
Basal SCAAT ATGL expression was lower (P = 0.05) in FDR compared with CON before bed rest and did not change in response to bed rest in any of the groups (Table 5). In FDR, ATGL expression increased in response to insulin stimulation before and after bed rest (P = 0.04). Basal HSL expression was lower (P < 0.0001) in FDR compared with CON before and after bed rest and did not change in response to bed rest in any of the groups (Table 5). In FDR, HSL expression increased in response to insulin stimulation before (P = 0.01) and after bed rest (P = 0.006). Basal LPL expression was lower (P < 0.0001) in FDR compared with CON before and after bed rest. In CON, but not in FDR, basal LPL expression increased (P = 0.02) markedly in response to bed rest, and the interaction between group and bed rest (P = 0.017) supported this. During insulin stimulation, LPL expression increased in FDR before (P = 0.03) and after bed rest (P = 0.006). GLUT-4 expression did not differ between CON and FDR and did not change in response to bed rest in either of the groups. In both groups, insulin stimulation increased GLUT-4 expression after bed rest (FDR: P = 0.03; CON: P = 0.02), but not before bed rest.
TABLE 5.
ATGL, HSL, LPL mRNA expression relative to GADPH, and GLUT-4 mRNA expression relative to β-actin in SCAAT
| CON |
FDR |
||||
|---|---|---|---|---|---|
| Before BR rest (basal n = 16; insulin stimulation n = 14) | After BR (basal n = 16; insulin stimulation n = 16) | Before BR (basal n = 10; insulin stimulation n = 11) | After BR rest (basal n = 10; insulin stimulation n = 11) | ||
| ATGL** | Basal | 0.10 ± 0.02* | 0.10 ± 0.01 | 0.04 ± 0.02*‡ | 0.06 ± 0.04 |
| ATGL** | Insulin stimulation | 0.12 ± 0.03 | 0.11 ± 0.02 | 0.08 ± 0.02 | 0.07 ± 0.01 |
| HSL** | Basal | 0.10 ± 0.02* | 0.15 ± 0.04* | 0.02 ± 0.01*‡ | 0.01 ± 0.01*‡ |
| HSL** | Insulin stimulation | 0.08 ± 0.01 | 0.09 ± 0.02 | 0.09 ± 0.03 | 0.06 ± 0.02 |
| LPL** | Basal | 1.2 ± 0.2*† | 2.3 ± 0.4*† | 0.4 ± 0.1*‡ | 0.3 ± 0.2*‡ |
| LPL** | Insulin stimulation | 1.8 ± 0.4 | 1.7 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.3 |
| GLUT 4** | Basal | 0.9 ± 0.3 | 0.6 ± 0.1‡ | 1.0 ± 0.3 | 1.0 ± 0.3‡ |
| GLUT 4** | Insulin stimulation | 0.8 ± 0.2 | 1.2 ± 0.2 | 0.6 ± 0.2 | 2.3 ± 0.8 |
Data are mean ± SE.
*Significant difference CON vs. FDR, P < 0.05.
†Significant difference before vs. after BR within the group; P < 0.05.
‡Significant difference basal vs. insulin-stimulated state within the group on the specified day, P < 0.05.
**Data were log transformed before statistical test.
DISCUSSION
The major findings were that individuals with a family history of type 2 diabetes (FDR) have a significantly higher glucose uptake in SCAAT compared with individuals not predisposed (CON), and physical inactivity in individuals not predisposed to type 2 diabetes increases SCAAT glucose uptake to the same level as in predisposed individuals (Fig. 1). These differences were not found in SCFAT. However, in SCFAT, but not in SCAAT, lipolysis decreased in response to physical inactivity in both predisposed individuals and those individuals not predisposed (Fig. 2). SCAAT mRNA expression of ATGL, HSL, and LPL was consistently lower in FDR compared with CON (Table 5) despite similar SCAAT lipolysis in the two groups.
At the whole-body level, FDR subjects in the present and several other studies (5,22) are clearly insulin resistant. Whole-body insulin sensitivity, as determined by the hyperinsulinemic-euglycemic clamp technique, is primarily a measure of insulin-stimulated glucose uptake in skeletal muscle, and we previously found insulin resistance in skeletal muscle of FDR subjects as evidenced by decreased insulin-stimulated forearm blood flow and glucose clearance (23). In another study, we found increased glucose production in FDR subjects indicating hepatic insulin resistance in these individuals (24). In a recent study (25) as well as in the present study, we found higher glucose uptake in SCAAT in FDR indicating redirection of glucose uptake to AT promoted by a combination of muscle insulin resistance and an increased rate of hepatic glucose production as shown previously (24). Mice with muscle-specific inactivation of the insulin receptor gene (MIRKO) and, as a consequence, insulin resistance in skeletal muscle, likewise show increased glucose uptake in AT (26). Furthermore, MIRKO mice exhibit increased fat mass, serum triglyceride, and FFA levels in face of normoglycemia and normal body weight as also seen in the FDR subjects of the present study. Thus, in the pre-diabetic state, insulin resistance confined to skeletal muscle can induce dyslipidemia, increased AT glucose uptake, and increased fat mass. We speculate that insulin resistance will develop also in AT during the further pathophysiological development toward type 2 diabetes, possibly in response to a further increase in fat mass. Interestingly, the shunting of glucose seems to be site-specific as glucose uptake was increased in SCAAT but not in SCFAT, which might explain the preferential localization of fat in abdominal depots of the FDR subjects.
Glucose may be used for reesterification of FFA via glycerol-3-phosphate or oxidized, stored as triglyceride or degraded to lactate. Lactate release in SCAAT was not different between groups indicating that the extra glucose taken up in SCAAT of FDR subjects takes other metabolic pathways. A likely pathway is conversion to glycerol-3-phosphate, which is a key substrate for lipid storage in adipocytes. Also the tendency to decreased lactate release in SCFAT of FDR combined with normal SCFAT glucose uptake is leaving extra glucose for lipid storage.
Our study revealed that lipolysis in subcutaneous AT was similar in predisposed individuals and those individuals not predisposed. Despite normal lipolysis in FDR subjects, we found increased plasma FFA concentrations, which probably reflect an increased amount of AT in FDR subjects. The reduced expression of ATGL and HSL found in FDR subjects could contribute to the relatively high abdominal fat mass. The low expression of LPL in FDR could potentially increase plasma triglyceride, which we did find in the FDR subjects underpinning a metabolic abnormal condition. AT lipolysis is very sensitive to the action of insulin (27), and failure to adequately turn off lipolysis could induce skeletal muscle and liver insulin resistance through excessive availability of FFA (28). The coexistence of normal SCAAT lipolysis and low mRNA expression of lipases in FDR subjects could be explained by the increased fasting plasma insulin levels in FDR subjects and indicates a state of insulin resistance seen as the failure to turn off lipolysis in AT of FDR subjects. The low mRNA expression of AT lipases suggests that a primary defect in the development of insulin resistance relates to an impaired ability to regulate the activity of AT lipases, which is important for the balance of triglyceride storage and the mobilization of FFA.
CON subjects responded to bed rest by increasing SCAAT glucose uptake to the same level as seen in FDR subjects prior to bed rest. Bed rest induced whole-body insulin resistance in both FDR and CON subjects, although the decrease in insulin sensitivity was relatively less in FDR subjects (29). When skeletal muscle reduces its utilization of glucose as a consequence of physical inactivity, glucose is shunted to AT as also seen in the insulin resistant FDR subjects before BR. Also, SCAAT LPL mRNA expression was upregulated after bed rest in CON but not in FDR subjects. Increased FFA and glucose uptake in SCAAT would tend to increase fat mass after bed rest, which we did not find. This may relate to the short time frame and the fact that we aimed at a constant body weight during the bed-rest period. However, a high AT substrate uptake caused by a low level of physical activity would in the long term contribute to increased fat mass. The fact that CON and FDR subjects had similar AT glucose uptake after bed rest does to some extent reject our a priori hypothesis that FDR subjects are more sensitive to the unhealthy effects of physical inactivity than CON subjects. However, it is worth noting that the abnormalities of whole-body and AT metabolism seen in FDR subjects in their “native” normal activity state are comparable to the metabolic abnormalities induced by 10 days of bed rest in healthy CON subjects without any family history of type 2 diabetes. Shifting to a sedentary lifestyle causes less need for energy supply, especially to skeletal muscle, wherefore lipolysis is expected to decrease, which we did find in SCFAT of both groups.
Interestingly, SCAAT GLUT-4 mRNA expression was markedly upregulated by insulin in both groups after bed rest, thus indicating that physical inactivity increases the capacity of AT to respond to insulin stimulation and supporting the finding of increased AT glucose uptake after bed rest. Chronic hyperinsulinemia in rats induces insulin resistance in skeletal muscle but a hyper-response to insulin in AT, which is associated with an increase in AT GLUT-4 mRNA and protein (30). Our study seems to confirm that hyperinsulinemia per se can produce divergent effects in AT and skeletal muscle.
In the present study, increased plasma insulin (∼300 pmol/l) during the hyperinsulinemic euglycemic clamp did not stimulate AT glucose uptake, lactate release, or blood flow. In support of our findings, previous studies have revealed that even “supraphysiological” insulin concentrations (∼1,600 pmol/l) barely stimulate glucose uptake in AT (17,25) and that physiological concentrations of insulin do not stimulate ATBF (17,25).
A multiple comparisons procedure was applied in the present study, which introduces a risk of type I errors wherefore caution is needed in the interpretation of the results revealing variance heterogeneity. Furthermore, noninherited factors, e.g., dietary habits and habitual physical activity level, might differ between FDR and CON subjects, which potentially could explain some of the between-group differences before and in response to bed rest.
In conclusion, FDR subjects show abnormalities in subcutaneous AT metabolism prior to bed rest by displaying increased glucose uptake in SCAAT and decreased SCAAT mRNA expression of lipases. Ten days of bed rest decreases lipolysis and tends to increase glucose uptake in SCFAT in both FDR and CON subjects, and SCAAT glucose uptake is increased by bed rest in CON subjects to the same level as in FDR subjects. Thus, with respect to AT metabolism, FDR subjects are not more sensitive than CON subjects to the unhealthy effects of physical inactivity, but the abnormalities of whole-body and AT metabolism seen in FDR subjects prior to bed rest are comparable with the metabolic abnormalities induced by 10 days of bed rest in healthy CON subjects without any family history of type 2 diabetes.
ACKNOWLEDGMENTS
Financial support from a European Union Grant (6th framework LSHM-CT-2004–005272, EXGENESIS) and the Nordea Foundation are gratefully acknowledged. L.H. was granted a PhD scholarship from the Ministry of Science, Technology and Innovation, Copenhagen, Denmark.
No potential conflicts of interest relevant to this article were reported.
L.H. wrote the manuscript and researched data. M.P.S. researched data and reviewed/edited the manuscript. A.C.A. researched data and reviewed/edited the manuscript. F.D. contributed to the discussion and reviewed/edited the manuscript. A.V. contributed to the discussion and reviewed/edited the manuscript. J.MB. reviewed/edited the manuscript. K.B.C. researched data and reviewed/edited the manuscript. B.S. wrote the manuscript.
We thank Regitze Kraunsøe, Jeppe Bach, Thomas Beck, Lenette Pedersen, and Pia Hornbek, as well as the laboratory at the Steno Diabetes Center, for technical assistance. We also thank the staff at the metabolic kitchen at the Steno Diabetes Hospital for providing and managing the diet. The Steno Diabetes Center is also thanked for providing the location for the bed rest.
Footnotes
REFERENCES
- 1.Schulze MB, Hu FB. Primary prevention of diabetes: what can be done and how much can be prevented? Annu Rev Public Health 2005;26:445–467 [DOI] [PubMed] [Google Scholar]
- 2.Eriksson J, Lindstrom J, Valle T, et al. Prevention of type II diabetes in subjects with impaired glucose tolerance: the Diabetes Prevention Study (DPS) in Finland. Study design and 1-year interim report on the feasibility of the lifestyle intervention programme. Diabetologia 1999;42:793–801 [DOI] [PubMed] [Google Scholar]
- 3.Nauck MA, Meier JJ, Wolfersdorff AV, Tillil H, Creutzfeldt W, Köbberling J. A 25-year follow-up study of glucose tolerance in first-degree relatives of type 2 diabetic patients: association of impaired or diabetic glucose tolerance with other components of the metabolic syndrome. Acta Diabetol 2003;40:163–172 [DOI] [PubMed] [Google Scholar]
- 4.McCarthy MI, Zeggini E. Genome-wide association studies in type 2 diabetes. Curr Diab Rep 2009;9:164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaag A, Henriksen JE, Beck-Nielsen H. Decreased insulin activation of glycogen synthase in skeletal muscles in young nonobese Caucasian first-degree relatives of patients with non-insulin-dependent diabetes mellitus. J Clin Invest 1992;89:782–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pimenta W, Korytkowski M, Mitrakou A, Jenssen T, Yki-Jarvinen H, Evron W, Dailey G, Gerich J. Pancreatic beta-cell dysfunction as the primary genetic lesion in NIDDM: evidence from studies in normal glucose-tolerant individuals with a first-degree NIDDM relative. JAMA 1995;273:1855–1861 [PubMed] [Google Scholar]
- 7.Eriksson J, Franssila-Kallunki A, Ekstrand A, Saloranta C, Widén E, Schalin C, Groop L. Early metabolic defects in persons at increased risk for non-insulin-dependent diabetes mellitus. N Engl J Med 1989;321:337–343 [DOI] [PubMed] [Google Scholar]
- 8.Eriksson JW, Smith U, Waagstein F, Wysocki M, Jansson PA. Glucose turnover and adipose tissue lipolysis are insulin-resistant in healthy relatives of type 2 diabetes patients: is cellular insulin resistance a secondary phenomenon? Diabetes 1999;48:1572–1578 [DOI] [PubMed] [Google Scholar]
- 9.Ciaraldi TP, Kolterman OG, Scarlett JA, Kao M, Olefsky JM. Role of glucose transport in the postreceptor defect of non-insulin-dependent diabetes mellitus. Diabetes 1982;31:1016–1022 [DOI] [PubMed] [Google Scholar]
- 10.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 2002;32(Suppl. 3):14–23 [DOI] [PubMed] [Google Scholar]
- 11.Jansson PA, Smith U, Lönnroth P. Evidence for lactate production by human adipose tissue in vivo. Diabetologia 1990;33:253–256 [DOI] [PubMed] [Google Scholar]
- 12.Consoli A, Nurjhan N, Reilly JJ, Jr, Bier DM, Gerich JE. Mechanism of increased gluconeogenesis in noninsulin-dependent diabetes mellitus: role of alterations in systemic, hepatic, and muscle lactate and alanine metabolism. J Clin Invest 1990;86:2038–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnett AH, Spiliopoulos AJ, Pyke DA, Stubbs WA, Burrin J, Alberti KG. Metabolic studies in unaffected co-twins of non-insulin-dependent diabetics. Br Med J (Clin Res Ed) 1981;282:1656–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandqvist MM, Eriksson JW, Jansson PA. Increased lactate release per fat cell in normoglycemic first-degree relatives of individuals with type 2 diabetes. Diabetes 2001;50:2344–2348 [DOI] [PubMed] [Google Scholar]
- 15.Larsen OA, Lassen NA, Quaade F. Blood flow through human adipose tissue determined with radioactive xenon. Acta Physiol Scand 1966;66:337–345 [DOI] [PubMed] [Google Scholar]
- 16.Lönnroth P, Strindberg L. Validation of the “internal reference technique” for calibrating microdialysis catheters in situ. Acta Physiol Scand 1995;153:375–380 [DOI] [PubMed] [Google Scholar]
- 17.Stallknecht B, Larsen JJ, Mikines KJ, Simonsen L, Bülow J, Galbo H. Effect of training on insulin sensitivity of glucose uptake and lipolysis in human adipose tissue. Am J Physiol Endocrinol Metab 2000;279:E376–E385 [DOI] [PubMed] [Google Scholar]
- 18.Stallknecht B, Simonsen L, Bulow J, Vinten J, Galbo H. Effect of training on epinephrine-stimulated lipolysis determined by microdialysis in human adipose tissue. Am J Physiol 1995;269(Pt 1):E1059–E1066 [DOI] [PubMed] [Google Scholar]
- 19.Bülow J. Measurement of adipose tissue blood flow. Methods Mol Biol 2001;155:281–293 [DOI] [PubMed] [Google Scholar]
- 20.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 21.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab 2006;290:E961–E967 [DOI] [PubMed] [Google Scholar]
- 22.Perseghin G, Ghosh S, Gerow K, Shulman GI. Metabolic defects in lean nondiabetic offspring of NIDDM parents: a cross-sectional study. Diabetes 1997;46:1001–1009 [DOI] [PubMed] [Google Scholar]
- 23.Sonne MP, Højbjerre L, Alibegovic AA, Vaag A, Stallknecht B, Dela F. Impaired endothelial function and insulin action in first-degree relatives of patients with type 2 diabetes mellitus. Metabolism 2009;58:93–101 [DOI] [PubMed] [Google Scholar]
- 24.Alibegovic AC, Højbjerre L, Sonne MP, van Hall G, Stallknecht B, Dela F, Vaag A. Impact of 9 days of bed rest on hepatic and peripheral insulin action, insulin secretion, and whole-body lipolysis in healthy young male offspring of patients with type 2 diabetes. Diabetes 2009;58:2749–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dela F, Stallknecht B. Effect of physical training on insulin secretion and action in skeletal muscle and adipose tissue of first degree relatives to type 2 diabetic patients. Am J Physiol Endocrinol Metab 2010;20:E80–E91 [DOI] [PubMed] [Google Scholar]
- 26.Kim JK, Michael MD, Previs SF, Peroni OD, Mauvais-Jarvis F, Neschen S, Kahn BB, Kahn CR, Shulman GI. Redistribution of substrates to adipose tissue promotes obesity in mice with selective insulin resistance in muscle. J Clin Invest 2000;105:1791–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langin D. Control of fatty acid and glycerol release in adipose tissue lipolysis. C R Biol 2006;329:598–607 [DOI] [PubMed] [Google Scholar]
- 28.Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA. Effect of fatty acids on glucose production and utilization in man. J Clin Invest 1983;72:1737–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonne MP, Alibegovic AC, Højbjerre L, Vaag A, Stallknecht B, Dela F. Effect of 10 days of bedrest on metabolic and vascular insulin action: a study in individuals at risk for type 2 diabetes. J Appl Physiol 2010;108:830–837 [DOI] [PubMed] [Google Scholar]
- 30.Cusin I, Terrettaz J, Rohner-Jeanrenaud F, Zarjevski N, Assimacopoulos-Jeannet F, Jeanrenaud B. Hyperinsulinemia increases the amount of GLUT4 mRNA in white adipose tissue and decreases that of muscles: a clue for increased fat depot and insulin resistance. Endocrinology 1990;127:3246–3248 [DOI] [PubMed] [Google Scholar]