Abstract
OBJECTIVE
Obesity impairs adiponectin expression, assembly, and secretion, yet the underlying mechanisms remain elusive. The aims of this study were 1) to determine the molecular mechanisms by which obesity impairs adiponectin multimerization and stability, and 2) to determine the potential role of disulfide-bond-A oxidoreductase-like protein (DsbA-L), a recently identified adiponectin interactive protein that promotes adiponectin multimerization and stability in obesity-induced endoplasmic reticulum (ER) stress and adiponectin downregulation.
RESEARCH DESIGN AND METHODS
Tauroursodeoxycholic acid (TUDCA), a chemical chaperone that alleviates ER stress, was used to study the mechanism underlying obesity-induced adiponectin downregulation in db/db mice, high-fat diet-induced obese mice, and in ER-stressed 3T3-L1 adipocytes. The cellular levels of DsbA-L were altered by RNAi-mediated suppression or adenovirus-mediated overexpression. The protective role of DsbA-L in obesity- and ER stress–induced adiponectin downregulation was characterized.
RESULTS
Treating db/db mice and diet-induced obese mice with TUDCA increased the cellular and serum levels of adiponectin. In addition, inducing ER stress is sufficient to downregulate adiponectin levels in 3T3-L1 adipocytes, which could be protected by treating cells with the autophagy inhibitor 3-methyladenine or by overexpression of DsbA-L.
CONCLUSIONS
ER stress plays a key role in obesity-induced adiponectin downregulation. In addition, DsbA-L facilitates adiponectin folding and assembly and provides a protective effect against ER stress–mediated adiponectin downregulation in obesity.
Adiponectin is an insulin sensitizer that plays a versatile role in the regulation of energy homeostasis and insulin sensitivity. The serum adiponectin levels are significantly reduced under the conditions of obesity, insulin resistance, and type 2 diabetes (1), yet the precise underlying mechanisms remain largely unknown.
Adiponectin is synthesized as a single polypeptide of 30 kDa and is then assembled in the endoplasmic reticulum (ER), primarily into three species: trimer, hexamer, and high molecular weight (HMW) multimer (2–5). The different forms of adiponectin have been found to play distinct roles in the regulation of energy homeostasis (2–4,6). Several ER-associated proteins, including Ero1-lα, ERp44, and GPR94, have recently been found to be involved in the assembly and secretion of higher-order adiponectin complexes (5,7,8). Impairment in adiponectin multimerization affects both secretion and function of this adipokine, and is associated with diabetes and hypoadiponectinemia (2,4).
ER is a eukaryotic organelle responsible for several specialized and important functions such as protein translation, folding, and transport of membrane or secreted proteins. Numerous protein chaperones are present in the ER lumen that yield an oxidizing environment necessary for correct folding and assembly of various membrane and secretive proteins such as adiponectin. In obesity, increased demand on ER function leads to ER stress and the unfolded protein response (UPR) to ensure that normal cell function and viability are maintained (9). However, whether and how ER stress plays a role in obesity-induced adiponectin downregulation remain to be established.
We recently identified an adiponectin-interacting protein named disulfide-bond A oxidoreductase-like protein (DsbA-L) (10). DsbA-L is expressed in various mouse tissues such as liver, kidney, pancreas, and heart, but the highest expression of this protein is detected in adipose tissue where adiponectin is synthesized and secreted (10). The cellular levels of DsbA-L are significantly reduced in adipose tissues of obese mice and human subjects. Overexpression of DsbA-L promotes adiponectin multimerization while reducing DsbA-L expression by RNAi markedly, and selectively reduces adiponectin levels and secretion in 3T3-L1 adipocytes (10). However, how DsbA-L regulates adiponectin multimerization and stability remains unknown.
In the present study, we show that alleviating ER stress enhances the cellular and plasma levels of adiponectin in both db/db mice and diet-induced obese mice. In addition, we demonstrate that inducing ER stress is sufficient to downregulate the cellular levels and secretion of adiponectin in 3T3-L1 adipocytes. Furthermore, overexpression of DsbA-L suppresses the inhibitory effect of ER stress on adiponectin multimerization and stability. Our studies demonstrate that ER stress is a key factor in obesity-induced downregulation of adiponectin and that increasing the cellular levels of DsbA-L improves adiponectin assembly and stability by suppressing the negative effect of ER stress.
RESEARCH DESIGN AND METHODS
Chemicals and antibodies.
The following chemicals were used: thapsigargin (TG) (350–004, Alexis Biochemicals, San Diego, CA), 3-methyladenine (3-MA) (M9281, Sigma-Aldrich), tauroursodeoxycholic acid ([TUDCA] 580549, Calbiochem, Gibbstown, NJ), and G418 sulfate (30–234-CR, Cellgro, Manassas, VA). Polyclonal antiadiponectin and DsbA-L antibodies were generated as described before (10). Antibodies against β-actin, AMP-activated protein kinase (AMPK), phosphor-Thr172-AMPK, Acetyl-CoA carboxylase, phosphor-Ser79-Acetyl-CoA carboxylase, and anti-LC3 were from Cell Signaling Technology (Danvers, MA). Other antibodies used were: monoclonal antiadiponectin (MAB3608, Chemicon International), anti-β-tubulin 2.1 (T4026, Sigma-Aldrich), and anti-GADD 153/CHOP (sc-7351, Santa Cruz Biotechnology, Santa Cruz, CA).
Cell culture, differentiation and adenovirus infection.
The culture of C2C12 cells and the murine hepatocytes was described in our previous studies (11,12). The 3T3-L1 cells stably expressing enhanced green fluorescent protein (EGFP)-LC3 were generated by transfection of cells with the pEGFP-LC3 plasmid, a gift from Dr. Jean X. Jiang (University of Texas Health Science Center at San Antonio), and selection with 400 μg/ml of G418. Independent clones were selected by limited dilution. Next, C2C12 and 3T3-L1 cell differentiation were performed as described in our previous studies (10,11). Adenoviruses encoding GFP and GFP plus wild type or S16A mutant of DsbA-L were described in our previous studies (10).
Animal studies.
Male db/db mice and their lean controls (stock# 000642, 3–5 weeks old) were purchased from Jackson Laboratories (Bar Harbor, ME). The mice were group-housed in a specific-pathogen–free facility at 22 to 24°C on a 12-h light/12-h dark cycle with the lights on at 8:00 a.m. The mice were fed with standard rodent chow, and all animals had access to water ad libitum. At 8 weeks of age, the mice were intraperitoneally injected with TUDCA (250 mg/kg) or equal volumes of vehicle (saline) twice daily (at 8:00 a.m. and 8:00 p.m.) for 26 days. Mice were then killed and mouse tissues were isolated according to the procedure described in our recent studies (13). For high-fat diet (HFD) experiments, 6- to 8-week-old C57BL/6 mice (10 mice per group) were fed with regular chow diet (Research Diets #D12328: 10.5 kcal% fat, 73.1 kcal% carbohydrate, and 16.4 kcal/% protein) or HFD (Research Diets #D12330: 58.0 kcal% fat, 16.0 kcal/% protein, and 26 kcal% carbohydrate) for 5 months. During the last 3 weeks of the HFD feeding period, the mice were intraperitoneally injected with TUDCA (250 mg/kg) or equal volumes of vehicle (saline) twice daily (8:00 a.m. and 8:00 p.m.). Mice were killed, and mouse tissues were isolated according to the procedure described in our recent studies (13). All animal procedures were approved by the University of Texas Health Science Center Animal Care and Use Committee.
Insulin tolerance test.
The saline- and TUDCA-treated male db/db mice and lean control mice (8–10 mice per group) were fasted for 6 h followed by insulin injection (2 IU/kg animal body weight i.p.). Blood was obtained from the tail vein before (0 min) and after insulin injection (15, 30, 60, and 120 min). Glucose levels were measured using an automatic glucometer (Rightest GM300; Bionime Corp, San Diego, CA).
Determination of adiponectin multimers.
Adiponectin multimers were determined by gel filtration fast-protein liquid chromatography with a Superdex 200 10/30 column (GE Healthcare Bio-Sciences, Piscataway, NJ), as described in our previous study (10).
Immunofluorescence study and Western blot studies.
The immunofluorescence and Western blot studies were performed as previously described (10,14). The localization and expression of adiponectin were visualized with an Olympus IX 81 confocal microscope with a 40X, 1.35 NA oil immersion objective. Quantification of the relative change in protein levels detected by Western blot (expressed as a percentage of control protein levels, arbitrarily set as 1.0) was performed by analyzing Western blots using the Scion Image Alpha 4.0.3.2 program (Scion Corporation) and was normalized for the amount of protein loaded in each experiment.
Quantitative real-time PCR.
Adiponectin mRNA was purified mRNA from cells using TRIZOL reagent (Invitrogen). Total RNA was reverse transcribed using SuperScript III First-Strand Synthesis Supermix (Invitrogen). The cDNA samples were amplified in duplicate in 96-microtiter plates (Applied Biosystems). Each PCR reaction (20 μl total volume) contained: 10 μl of SYBR Green PCR Master Mix (Applied Biosystems), 5 pmols of each primer, and 1 μg of cDNA. The PCR primers for adiponectin were: 5′-CCC AAG GGA ACT TGT GCA GGT TGG ATG-3′ and 5′-GTT GGT ATC ATG GTA GAG AAG AAA GCC-3′. The mRNA of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was quantified as an endogenous control, using the following primers: 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′. Real-time PCR reactions were carried out in an ABI PRISM 7,700 real-time PCR apparatus. Reactions without a template or without the reverse transcriptase were used as negative controls.
Statistical analysis.
Statistical analysis of the data were performed using ANOVA. Statistical significance was set at P values of *< 0.05, **< 0.01, and ***< 0.001.
RESULTS
Reduction of ER stress increases adiponectin levels in obese mice and in cells.
Obesity induces ER stress, and ER stress is correlated with reduced adiponectin levels (15). To determine whether ER stress is involved in downregulating adiponectin expression in obesity, we examined adiponectin levels in db/db mice treated with vehicle or the ER stress–reducing chemical chaperone, TUDCA. Obesity led to a great increase in ER stress in fat tissue of the db/db mice, as demonstrated by increased C/EBP-homologous protein (CHOP) expression (Fig. 1A). Concurrent with increased ER stress, the cellular (Fig. 1B) and circulating (Fig. 1C) levels of adiponectin were significantly reduced in the db/db mice compared with that of the lean mice. Treating the db/db mice with TUDCA greatly reduced obesity-induced CHOP expression, concurrent with an increase in circulating and cellular levels of adiponectin and its interacting protein DsbA-L (Fig. 1A–D). TUDCA treatment had no significant effect on the food intake (data not shown) (16) and body weight of the db/db mice (supplementary Fig. S1A in the appendix available online at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0412/DC1, indicating that the TUDCA-mediated rescuing effect on DsbA-L and adiponectin levels was not a consequence of altered food intake/body weight. The expression levels of DsbA-L and adiponectin were also greatly reduced in WAT of HFD-induced obese mice (10) and this reduction was also rescued by TUDCA treatment (supplemental online Fig. S2). Taken together, these results suggest that ER stress may play a key role in obesity-induced adiponectin downregulation.
FIG. 1.
Suppression of ER stress improves adiponectin expression in db/db mice. A: The expression levels of adiponectin (Adpn), DsbA-L, and CHOP in white adipose tissue (WAT) and the serum adiponectin levels from saline- or TUDCA-treated lean or db/db mice were determined by Western blot with specific antibodies as indicated. β-actin or immunoglobulin (IgG) was used as a tissue or serum loading control, respectively. The relative protein levels of adiponectin in WAT (B), or in serum (C), and DsbA-L (D) were quantified. Data represent mean ± SEM. **P < 0.01, ***P < 0.001; n = 10 for the saline control mice, and n = 8 per treatment group for the db/db mice. E: Differentiated 3T3–L1 adipocytes were pretreated with or without TUDCA (1 mmol/l) for 1 h followed with TG (0.01 μmol/l) cotreatment for 24 h. The cells were lysed, and the expression levels of adiponectin, DsbA-L, CHOP, TNFα, resistin, and β-tubulin were determined by Western blot analysis with specific antibodies as indicated. Adiponectin levels in conditioned medium were determined with an antiadiponectin antibody. F: The mRNA levels of adiponectin were determined by quantitative reverse transcription PCR. The protein levels of adiponectin as shown in (E) were quantified (G). N = 3, *P < 0.05, **P < 0.01, ***P < 0.001; ns, no statistical difference; ctrl, control.
DsbA-L prevents ER stress–induced adiponectin downregulation in 3T3-L1 adipocytes.
To determine whether ER stress is sufficient to reduce adiponectin levels, we examined adiponectin expression in 3T3-L1 adipocytes treated with and without thapsigargin (TG), a chemical widely used to stimulate ER stress by inhibiting ER calcium-ATPase (17). Treating 3T3-L1 adipocytes with TG led to a marked increase in ER stress, as demonstrated by enhanced CHOP expression (Fig. 1E). TG treatment also greatly reduced the cellular levels of adiponectin and DsbA-L as well as adiponectin secretion into the medium, which could be partially prevented by pretreating the cells with TUDCA (Fig. 1E). Although inducing ER stress by TG led to an ∼25% reduction in the mRNA levels of adiponectin, treating 3T3-L1 adipocytes with TUDCA had no significant rescuing effect on the TG-induced downregulation of the mRNA levels of adiponectin (Fig. 1F). On the other hand, TG treatment led to a 60% reduction in the protein levels of adiponectin and the TG-induced downregulation of adiponectin was significantly protected by treating the cells with TUDCA (Fig. 1G), suggesting that the protective effect of DsbA-L on cellular levels of adiponectin is mainly at the protein level rather than mRNA level. TG treatment had no significant effect on the expression levels of two other adipokines, tumor necrosis factor-α (TNFα) and resistin (Fig. 1E), suggesting a selective role of this ER stress inducer in adipokine stability.
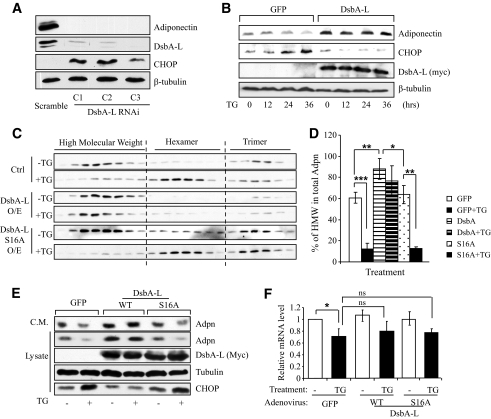
Since DsbA-L levels are negatively associated with obesity (10) and ER stress (Fig. 1), we were interested in determining whether reducing the expression levels of DsbA-L contributes to ER stress–induced adiponectin downregulation. To test this, we examined UPR, which is induced by ER stress, in scramble and three independent DsbA-L–suppressed 3T3-L1 cells. In agreement with our previous findings (10), the cellular levels of adiponectin were greatly suppressed in the DsbA-L–suppressed 3T3-L1 adipocytes (Fig. 2A, top panel). Interestingly, UPR was markedly increased in the DsbA-L–suppressed cells, as demonstrated by increased expression of the UPR marker CHOP (Fig. 2A, third panel). Taken together with our previous finding that suppressing DsbA-L expression in 3T3-L1 adipocytes greatly reduced the protein, but not the mRNA, levels of adiponectin (10), these results suggest that downregulation of DsbA-L may lead to accumulation of incorrectly folded proteins that in turn induces UPR. Consistent with this, overexpression of DsbA-L–suppressed CHOP expression and enhanced resistance to ER stress–induced adiponectin downregulation in 3T3-L1 CAR adipocytes (Fig. 2B).
FIG. 2.
DsbA-L suppresses ER stress and improves adiponectin expression and multimerization. A: The expression levels of adiponectin, DsbA-L, and CHOP in DsbA-L–suppressed or scramble control 3T3–L1 adipocytes were determined by Western blot with specific antibodies. β-tubulin was used as a loading control. The data represent three independent experiments with similar results. B: The 3T3–L1-CAR adipocytes were infected with adenovirus encoding DsbA-L or GFP for 24 h. The cells were then treated with or without TG (0.01 μmol/l) for various times as indicated. The expression levels of adiponectin, CHOP, and myc-DsbA-L in cell lysates were determined by Western blot with specific antibodies as indicated. β-tubulin was used as a loading control. The data represent three independent experiments with similar results. C: The 3T3–L1-CAR adipocytes were infected with adenovirus encoding GFP, DsbA-L, or the S16A mutant of DsbA-L for 24 h. The cells were then treated with or without TG (0.01 μmol/l) for 24 h as indicated. Adiponectin multimers in cell lysates were separated by gel filtration and determined by Western blot with an antiadiponectin antibody. The percentages of HMW form in total adiponectin (D) were quantified (n = 3, *P < 0.05, **P < 0.01, ***P < 0.001). E: The 3T3–L1-CAR adipocytes were infected with adenoviruses encoding myc-tagged wild-type (WT) or the S16A mutant of DsbA-L for 24 h. The cells were then treated with or without TG (0.01 μmol/l) for 24 h. The adiponectin levels in conditioned medium (C.M.) and the expression levels of adiponectin, myc-DsbA-L or DsbA-LS16A, and CHOP were determined by Western blot with specific antibodies as indicated. β-tubulin was used as a loading control. F: The mRNA levels of adiponectin were determined by quantitative reverse transcription PCR. N = 3, *P < 0.05; ns, no statistical difference.
To determine the role of the DsbA-L in ER stress–induced dysregulation of adiponectin multimerization, we studied adiponectin multimer distribution by gel filtration in 3T3-L1-CAR adipocytes. To better compare the effects of TG on the relative distributions of various adiponectin multimers, equal amounts of adiponectin from TG-treated and nontreated 3T3-L1-CAR cells were analyzed by Western blot. In 3T3-L1-CAR adipocytes overexpressing GFP control, the majority of the adiponectin multimers is the HMW form (Fig. 2C, top panel, and D). Induction of ER stress by TG treatment led to marked reduction of the HMW form of adiponectin, which was alleviated by overexpression of wild-type but not the S16A mutant of DsbA-L (Fig. 2C and D). Overexpression of the S16A mutant of DsbA-L failed to protect ER stress–induced downregulation of cellular and secreted adiponectin (Fig. 2E). Since the DsbA-LS16A mutant is unable to bind to adiponectin (10), these findings suggest that the interaction with adiponectin is critical for the protective effect of DsbA-L. Our results also demonstrated that overexpression of either wild-type or the S16A mutant of DsbA-L had no significant protecting effect on the mRNA levels of adiponectin (Fig. 2F). On the other hand, the protein levels of adiponectin were significantly increased in cells overexpressing wild-type DsbA-L (Fig. 2E). Taken together, these results provide further evidence that the rescuing effect of DsbA-L on cellular levels of adiponectin is mainly at the post-translational level.
Adiponectin is downregulated in DsbA-L–suppressed cells via autophagy-dependent degradation.
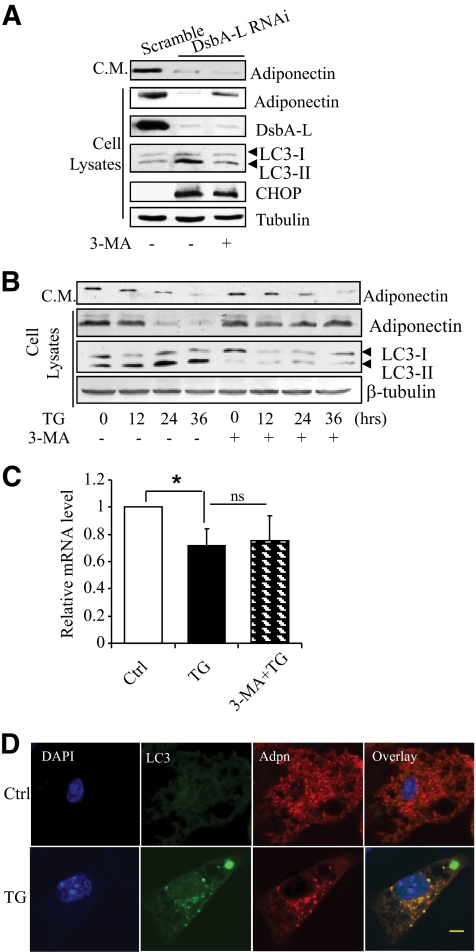
We recently found that autophagy plays a critical role in ER stress–induced insulin receptor downregulation (18). Since ER stress is induced in DsbA-L–suppressed cells (Fig. 2A), we asked whether autophagy is involved in the downregulation of adiponectin expression in these cells. The levels of LC3-II, an autophagy marker protein, were noticeably increased in the DsbA-L–suppressed 3T3-L1 adipocytes compared with the scramble control cells (Fig. 3A). Pretreating the DsbA-L–suppressed adipocytes with the autophagy inhibitor 3-MA, but not the protesome inhibitor MG-132, greatly suppressed LC3 expression and significantly blocked DsbA-L deficiency-induced adiponectin protein downregulation (Fig. 3A and data not shown). A rescuing effect of 3MA on the total levels of adiponectin was also observed in TG-treated 3T3-L1 adipocytes (Fig. 3B). However, despite elevated cellular adiponectin levels, adiponectin secretion was not significantly increased by the 3MA treatment (Fig. 3A and B, top panel). One possible explanation for these results is that most of the rescuing adiponectin molecules were not correctly folded in the DsbA-L–deficient cells and thus unable to pass the ER-Golgi quality control system for secretion. The 3-MA treatment had no significant rescuing effect on adiponectin mRNA levels (Fig. 3C), consistent with an inhibitory role of 3MA in autophagy-induced protein degradation. Immunofluorescence studies revealed that ER stress promoted adiponectin translocation to the autophagosome, which is evident by increased colocalization of the adiponectin signal with the punctate LC3 staining (Fig. 3D, bottom panel). No major punctate LC3 fluorescent autophagosome signal could be detected in the control cells (Fig. 3D, top panels). These results provide further evidence on the involvement of an autophagy-dependent pathway in ER stress–induced adiponectin degradation.
FIG. 3.
ER stress or DsbA-L knockdown suppresses adiponectin levels by inducing autophagy-dependent degradation. A: DsbA-L-suppressed or scramble control 3T3–L1 adipocytes were treated with 3-MA (5 μmol/l) or vehicle for 24 h as indicated. The protein levels of adiponectin, DsbA-L, and LC3 in cell lysates were determined by Western blot using specific antibodies as indicated. β-tubulin was used as a loading control. Adiponectin levels in conditioned medium (C.M.) were determined with an antiadiponectin antibody. The data represent three independent experiments with similar results. B: The 3T3–L1 adipocytes were pretreated with (+) or without (−) 3-MA (5 μmol/l) for 1 h, followed by TG (0.01 μmol/l) for different times as indicated. The protein levels of adiponectin, LC3, and β-tubulin were determined by Western blot analysis with specific antibodies as indicated. Adiponectin levels in conditioned medium (C.M.) were determined with an antiadiponectin antibody. The mRNA levels of adiponectin (C) were determined by quantitative reverse transcription PCR; N = 3, *P < 0.05; ns, no statistical difference. D: Differentiated 3T3–L1 adipocytes were treated with TG (0.01 μmol/l) or vehicle for 36 h, and then fixed. The cellular localization of adiponectin (Adpn, red) was determined by immunofluorescence-staining with a specific antibody to the protein. The cellular localization of GFP-LC3 was observed by green fluorescent protein fluorescence. In each experiment, more than 100 cells from each group were checked, and ∼70% of the cells showed a similar pattern. The data represent three independent experiments with similar results. (A high-quality digital representation of this figure is available in the online issue.)
Alleviating ER stress improves AMPK signaling and insulin sensitivity in db/db mice.
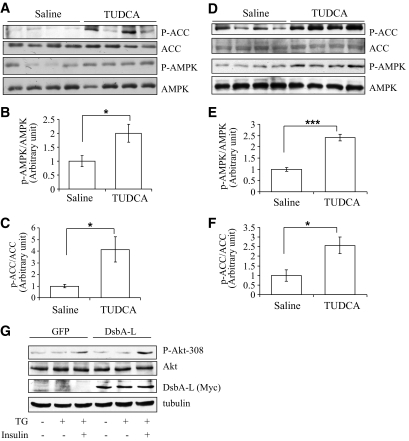
Adiponectin activates AMPK, which in turn phosphorylates and inactivates acetyl-CoA carboxylase (ACC) to regulate fatty acid oxidation (19). Based on the finding that alleviating ER stress increased adiponectin expression levels and secretion in db/db mice (Fig. 1), we examined the effect of TUDCA on AMPK and ACC phosphorylation. We found that AMPK activation, as indicated by Thr172 phosphorylation, was significantly increased in the skeletal muscle of the TUDCA-treated db/db mice (Fig. 4A and B). Suppressing ER stress by TUDCA also led to a great increase in ACC phosphorylation at the AMPK-mediated phosphorylation site (Ser79) (Fig. 4A and C). A similar increase in AMPK activation was observed in the liver of TUDCA treated db/db mice (Fig. 4D–F). To determine whether DsbA-L has an effect on insulin signaling, we infected 3T3-L1-CAR adipocytes with adenoviruses encoding GFP or GFP plus DsbA-L. We found that overexpression of DsbA-L increased the cellular levels and secretion of adiponectin and enhanced resistance to TG-induced ER stress (Fig. 2B) and insulin-stimulated Akt phosphorylation (Fig. 4G). Consistent with the result that suppressing ER stress increased insulin signaling, insulin tolerance test experiments showed that TUDCA treatment increased insulin sensitivity in db/db mice (online supplemental Fig S3A). TUDCA treatment did not stimulate AMPK activation in cultured C2C12 myotubes and murine hepatocytes (online supplemental Fig. S4), suggesting that the increased AMPK/ACC phosphorylation and insulin sensitivity in vivo is not caused by a direct stimulatory effect of this chemical chaperone.
FIG. 4.
Inhibition of ER stress improves adiponectin signaling in db/db mice. A: The expression levels and phosphorylation of AMPK (Thr172) and ACC (Ser79) in skeletal muscle of TUDCA- or saline-treated db/db mice were determined by Western blot with specific antibodies as indicated. The relative AMPK (B) and ACC (C) phosphorylation levels were quantified and analyzed (*P < 0.05, n = 4). D: The expression levels and phosphorylation of AMPK and ACC in the liver of TUDCA- or saline-treated db/db mice were determined by Western blot with specific antibodies as indicated. The relative AMPK (E) and ACC (F) phosphorylation levels were quantified and analyzed (*P < 0.05, ***P < 0.001, n = 4). G: 3T3–L1-CAR adipocytes were infected with adenoviruses encoding GFP or GFP plus myc-DsbA-L for 24 h. The cells were then pretreated with or without TG (0.01 μmol/l) for 24 h as indicated, followed with or without 10 nmol/l insulin stimulation for 5 min. The expression levels of myc-tagged DsbA-L, Akt, and phosphor-Akt (Thr308) in cell lysates were determined by Western blot with specific antibodies as indicated. β-tubulin was used as a loading control. The experiment represents three independent experiments with similar results.
DISCUSSION
It is well documented that the cellular and serum levels of adiponectin are negatively correlated with obesity. However, the precise underlying mechanisms remain unclear. Obesity leads to a low-grade chronic inflammatory state accompanied by increased production of proinflammatory cytokines such as TNF-α and interleukin (IL)-6, which have been shown to reduce adiponectin gene expression (15,20,21). Obesity also results in a hypoxic microenvironment (22,23), leading to increased ER stress and attenuated adiponectin promoter activity (15). In the present study, we show that inducing ER stress is sufficient to decrease the protein levels of adiponectin in 3T3-L1 adipocytes (Fig. 2), suggesting a causative role of ER stress in downregulating the cellular levels of adiponectin. Consistent with this view, inhibiting ER stress by the chemical chaperone TUDCA partially restores adiponectin levels in both db/db mice (Fig. 1) and HFD-induced obese mice (online supplemental Fig. S2). Our results are in agreement with an earlier finding that treating the db/db mice with peroxisome proliferator–activated receptor (PPAR)α/γ dual agonist macelignan, which reduces ER stress, increases adiponectin expression in adipose tissue of db/db mice (24).
Obesity reduces the cellular levels of PPARγ, which has been shown to downregulate adiponectin transcription (25). However, a recent study showed that activation of PPARγ by pioglitazone treatment increased plasma levels of adiponectin in human subjects, but had no effect on adiponectin expression in adipose tissue (26), suggesting that the primary effect of PPARγ may be to promote adiponectin assembly and secretion, rather than transcription. Consistent with this notion, treating adipocytes with PPARγ agonists increased the synthesis and secretion of HMW adiponectin without affecting mRNA expression and protein synthesis of this adipokine (5,27). A likely mechanism by which PPARγ enhances adiponectin cellular levels and secretion is to increase the expression levels of ER chaperones such as Ero1-lα, which have been shown to promote adiponectin assembly and secretion in mature adipocytes and in mice (5,7). Interestingly, we recently found that treating 3T3-L1 adipocytes with rosiglitazone increases the expression levels of DsbA-L (10). Since suppressing DsbA-L leads to ER stress and adiponectin downregulation (Fig. 2A), these results suggest that DsbA-L may enhance adiponectin stability and secretion by enhancing ER function.
In obesity, the cellular levels of DsbA-L are reduced (10), which may contribute to increased adiponectin misfolding in the ER and blockage of this adipokine to form HMW multimers necessary for secretion. The obesity-induced accumulation of misfolded molecules in the ER may result in ER stress and UPR, which in turn lead to degradation of incorrectly folded proteins by either the proteasome- or autophagy-associated protein degradation pathway (28,29). We found that treating the ER-stressed 3T3-L1 cells with 3MA, but not MG132, greatly rescued adiponectin levels (Fig. 3), suggesting that ER stress–induced adiponectin downregulation is mediated by an autophagy-dependent mechanism. Our data showed that the expression levels of endogenous DsbA-L were reduced in TG-treated cells (Fig. 1E), suggesting that DsbA-L reduction is a consequence of ER stress. This result is consistent with our previous finding that DsbA-L expression levels are negatively correlated with obesity in both obese human subjects and animal models of obesity (10). Interestingly, we also found that ER stress is increased in DsbA-L–suppressed cells (Figs. 2A and 3A). One possible explanation for these seemly contradictory results is that DsbA-L plays a critical role in the maintenance of cell homeostasis, probably functioning as a chaperone to facilitate correct folding and assembly of adiponectin and potentially other macromolecules in the ER, where transmembrane and secreted proteins are synthesized, assembled, and/or modified. Thus, reducing its expression levels may lead to overloading of incorrectly-folded proteins, resulting in ER stress and subsequent activation of an autophagy-dependent protein-degradation pathway. Thus, the negative effect of obesity on adiponectin levels may be twofold: obesity leads to overloading of misfolded adiponectin in the ER, which may induce ER stress–induced degradation. Obesity also downregulates DsbA-L, thus accelerating adiponectin misfolding and degradation by diminishing the protective chaperone activity of DsbA-L. Consistent with this model, overexpression of DsbA-L promotes the assembly of the HMW form of adiponectin and prevents ER stress–induced adiponectin downregulation (Fig. 2B and C).
In conclusion, we have presented evidence showing that inducing ER stress is sufficient to cause adiponectin degradation in 3T3-L1 adipocytes. In addition, we show that DsbA-L has a protective role in ER stress–induced adiponectin downregulation. Since DsbA-L levels are reduced in obese human subjects and animal models of obesity (10), reduction of DsbA-L could be a mechanism by which obesity downregulates adiponectin levels and secretion, contributing to increased insulin resistance. We previously showed that the cellular levels of DsbA-L are stimulated by the PPARγ agonist rosiglutazone (10), suggesting that increasing the expression levels of this protein could be a promising approach to increase adiponectin multimerization and insulin sensitivity.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health RO1 grants DK -76902 (to F.L.) and DK-69930 (to L.Q.D.).
No potential conflicts of interest relevant to this article were reported.
L.Z. designed the research, performed research, analyzed data, and wrote the manuscript. M.L., J.Z., and H.C. performed research. L.Q.D. analyzed data. F.L. designed the research, analyzed data, and wrote the manuscript.
The authors are grateful to Derong Hu, Department of Pharmacology, University of Texas Health Science Center at San Antonio, for excellent technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Shetty S, Kusminski CM, Scherer PE. Adiponectin in health and disease: evaluation of adiponectin-targeted drug development strategies. Trends Pharmacol Sci 2009;30:234–239 [DOI] [PubMed] [Google Scholar]
- 2.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem 2003;278:9073–9085 [DOI] [PubMed] [Google Scholar]
- 3.Tsao TS, Murrey HE, Hug C, Lee DH, Lodish HF. Oligomerization state-dependent activation of NF-κ B signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30). J Biol Chem 2002;277:29359–29362 [DOI] [PubMed] [Google Scholar]
- 4.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem 2003;278:40352–40363 [DOI] [PubMed] [Google Scholar]
- 5.Qiang L, Wang H, Farmer SR. Adiponectin secretion is regulated by SIRT1 and the ER oxidoreductase Ero1-Lα. Mol Cell Biol 2007;27:4698–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang ZV, Scherer PE. DsbA-L is a versatile player in adiponectin secretion. Proc Natl Acad Sci U S A 2008;105:18077–18078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang ZV, Schraw TD, Kim JY, Khan T, Rajala MW, Follenzi A, Scherer PE. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol 2007;27:3716–3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie L, Boyle D, Sanford D, Scherer PE, Pessin JE, Mora S. Intracellular trafficking and secretion of adiponectin is dependent on GGA-coated vesicles. J Biol Chem 2006;281:7253–7259 [DOI] [PubMed] [Google Scholar]
- 9.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev 2006;86:1133–1149 [DOI] [PubMed] [Google Scholar]
- 10.Liu M, Zhou L, Xu A, Lam KS, Wetzel MD, Xiang R, Zhang J, Xin X, Dong LQ, Liu F. A disulfide-bond A oxidoreductase-like protein (DsbA-L) regulates adiponectin multimerization. Proc Natl Acad Sci U S A 2008;105:18302–18307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol 2006;8:516–523 [DOI] [PubMed] [Google Scholar]
- 12.Riojas RA, Kikani CK, Wang C, Mao X, Zhou L, Langlais PR, Hu D, Roberts JL, Dong LQ, Liu F. Fine-tuning PDK1 activity by phosphorylation at Ser163. J Biol Chem 2006;281:21588–21593 [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Balas B, Christ-Roberts CY, Kim RY, Ramos FJ, Kikani CK, Li C, Deng C, Reyna S, Musi N, Dong LQ, Defronzo RA, Liu F. Peripheral disruption of Grb10 gene enhances insulin signaling and sensitivity in vivo. Mol Cell Biol 2007;18:6497–6505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim MA, Kikani C, Wick MJ, Dong LQ. Nuclear localization of PDK1: a potential novel regulatory mechanism. Proc Natl Acad Sci U S A 2003;100:14006–14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007;56:901–911 [DOI] [PubMed] [Google Scholar]
- 16.Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 2009;9:35–51 [DOI] [PubMed] [Google Scholar]
- 17.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004;306:457–461 [DOI] [PubMed] [Google Scholar]
- 18.Zhou L, Zhang J, Fang Q, Liu M, Liu X, Jia W, Dong LQ, Liu F. Autophagy-mediated insulin receptor down-regulation contributes to ER stress-induced insulin resistance. Mol Pharmacol 2009;76:596–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 2002;8:1288–1295 [DOI] [PubMed] [Google Scholar]
- 20.Chen B, Lam KS, Wang Y, Wu D, Lam MC, Shen J, Wong L, Hoo RL, Zhang J, Xu A. Hypoxia dysregulates the production of adiponectin and plasminogen activator inhibitor-1 independent of reactive oxygen species in adipocytes. Biochem Biophys Res Commun 2006;341:549–556 [DOI] [PubMed] [Google Scholar]
- 21.Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y. PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 2001;50:2094–2099 [DOI] [PubMed] [Google Scholar]
- 22.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–867 [DOI] [PubMed] [Google Scholar]
- 23.Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond) 2009;33:54–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han KL, Choi JS, Lee JY, Song J, Joe MK, Jung MH, Hwang JK. Therapeutic potential of peroxisome proliferators–activated receptor-α/γ dual agonist with alleviation of endoplasmic reticulum stress for the treatment of diabetes. Diabetes 2008;57:737–745 [DOI] [PubMed] [Google Scholar]
- 25.Liu M, Liu F. Transcriptional and post-translational regulation of adiponectin. Biochem J 2010;425:41–52 [DOI] [PubMed] [Google Scholar]
- 26.Rasouli N, Yao-Borengasser A, Miles LM, Elbein SC, Kern PA. Increased plasma adiponectin in response to pioglitazone does not result from increased gene expression. Am J Physiol Endocrinol Metab 2006;290:E42–E46 [DOI] [PubMed] [Google Scholar]
- 27.Banga A, Unal R, Tripathi P, Pokrovskaya I, Owens RJ, Kern PA, Ranganathan G. Adiponectin translation is increased by the PPARγ agonists pioglitazone and omega-3 fatty acids. Am J Physiol Endocrinol Metab 2009;296:E480–E89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 2006;26:9220–9231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem 2006;281:30299–30304 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.